Abstract
Activated coagulation and fibrinolytic system in cancer patients is associated with tumor stroma formation and metastasis in different cancer types. The aim of this study is to explore the correlation of blood coagulation assays for various clinicopathologic factors in breast cancer patients. A total of 123 female breast cancer patients were enrolled into the study. All the patients were treatment naïve. Pretreatment blood coagulation tests including PT, APTT, PTA, INR, D-dimer, fibrinogen levels, and platelet counts were evaluated. Median age of diagnosis was 51 years old (range 26–82). Twenty-two percent of the group consisted of metastatic breast cancer patients. The plasma level of all coagulation tests revealed statistically significant difference between patient and control group except for PT (p < 0.001 for all variables except for PT; p = 0.08). Elderly age (>50 years) was associated with higher D-dimer levels (p = 0.003). Metastatic patients exhibited significantly higher D-dimer values when compared with early breast cancer patients (p = 0.049). Advanced tumor stage (T3 and T4) was associated with higher INR (p = 0.05) and lower PTA (p = 0.025). In conclusion, coagulation tests show significant differences in patients with breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biology of cancer is a complicated process involving profound changes in different physiological systems such as hemostasis and vascular endothelial functions conferring increased risk of thrombosis [1]. These alterations trigger thromboembolic events resulting in morbidity and mortality [2, 3].
The relationship between cancer and coagulation is not solely an issue of increased thromboembolic complications. Several reports have reinforced the concept of a connection between activated coagulation and angiogenesis in human cancer. Tumor stroma formation, an essential step of tumoriogenesis, is particularly contributed by activation of coagulation in the perivascular region [4]. Patients with tumors of lung, pancreas, and gastrointestinal tract are supposed to be more prone to hypercoagulable state [5]. Previous studies with breast cancer patients have also demonstrated a hypercoagulable state with elevated markers of coagulation such as fibrinogen, tissue factor, and thrombin-antithrombin (TAT) complex [6–8]. Secretion of procoagulant factors by tumor cells triggers the deposition of fibrin, promoting angiogenesis and tumor cell growth. Fibrin deposition surrounding the tumor is assumed to be the result of a series of events initiated by the development of leaky tumor-associated blood vessels.
Homeostasis within this exaggerated coagulation process is always accompanied by fibrinolysis [9]. D-dimer is formed as a product of degraded fibrinogen during fibrinolysis and is elevated in various solid tumors including colorectal, ovarian, lung, and breast cancer [10–12]. In patients with colorectal cancer, D-dimer levels have been shown to correlate with depth of tumor invasion at the time of surgical excision [10]. D-dimer levels may also be elevated at the time of diagnosis and decrease after adjuvant anthracycline-based chemotherapy in early stage breast cancer patients [13–15]. Prognostic implications of coagulation tests for various tumors have been reported so far, but the relationship between coagulation parameters and clinicopathologic characteristics of breast cancer has not been outlined clearly. The aims of the current study are to confirm whether some coagulation abnormalities are more frequently encountered in patients with breast cancer and to delineate the correlation of these coagulation tests with other clinical and laboratory variables.
Patients and methods
A total of 123 female breast cancer patients admitted to Istanbul University, Institute of Oncology were enrolled into the study. Diagnosis of breast cancer was histologically proved either with tru-cut biopsy of the primary lesion or metastatic site and staged according to the sixth edition of AJCC. All the patients were treatment naïve for at least 3 months before accrual, and those who received oral/parenteral anticoagulants or those with history of thrombosis within the last 3 months were excluded from the study.
For histologic evaluation, tissue sections (2 mm) were deparaffinized and stained using H&E. Grading of tumors was established according to the modified Bloom-Richardson grading system [16]. Estrogen receptor (ER), progesterone receptor (PR), and HER-2 status were evaluated in the sample sections using appropriate antibodies. The immunohistochemical staining was assessed upon visual inspection with an optical microscope and considered as positive if the percentage of cells staining positive were more than 5 %. In case of 2(+) staining by IHC, HER-2 gene amplification was analyzed by fluorescent in situ hybridization (FISH).
Treatment
All patients were treated with the multidisciplinary approach. Overall, 99 (83 %) patients received an anthracycline-based regimen, 19 (16 %) received hormone therapy either as aromatase inhibitor or tamoxifen ± LHRH analogues and two patients were followed up without any treatment due to early stage (<1 cm). Seventy-four patients received treatment in adjuvant and 16 patients in the neoadjuvant setting. The response to treatment was evaluated according to revised RECIST criteria version 1.1. Institutional review board approval was obtained from each subject prior to the commencement of the study.
Blood collection and tests
Blood samples in patients with operated patients were obtained at least 3 weeks after surgery and during the first visit of metastatic patients in the clinics. The blood samples of the control group which consisted of 26 female subjects without any history of malignancy or anticoagulant usage were collected at the beginning of study. Five milliliters of peripheral blood was drawn into citrated tubes for the measurement of D-dimer, fibrinogen, prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR) and stored for a maximum of 2 days at −20 °C. D-dimer values were determined by Microparticle Enzyme Immunoassay (MEIA) using AxSYM analyzer following the manufacturer’s instructions. Commercially available reagents provided by a kinetic nephelometric detection system using Diagon Dia Timer 4 were employed for PT, APTT, and fibrinogen measurement.
Statistical analysis
Comparisons of continuous variables including D-dimer, fibrinogen, PT, and APTT in different subgroups were performed with Mann-Whitney U test. Relationships between categorical variables were compared using a chi-square test. Survival was calculated from the date of accrual to death resulting from any cause or to last contact with the patient or any family member. The Kaplan-Meier method was used for estimation of survival distribution; univariate survival curve differences were assessed by the log-rank test. A p value < 0.05 was considered significant. Statistical analysis was carried out using SPSS 16.0 software.
Results
Between February 2010 and June 2011, 123 consecutive female patients with breast cancer were enrolled into the study. Median age of diagnosis was 51 years old (range 26 to 82) (Table 1). Twenty-two percent of the group consisted of metastatic breast cancer patients where the most frequent site of metastasis was bone (78 %). Majority of the patients received anthracycline-based chemotherapy; 19 patients were treated only with hormone therapy.
Comparison of coagulation tests between patients and healthy controls
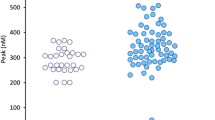
The control group comprised age and sex-matched individuals without history of malignancy. Comparison of coagulation tests between patients and controls including D-dimer, fibrinogen (F), activated partial thromboplastin time (APTT), prothrombin activity (PTA), international normalized ratio (INR), and platelet counts revealed a significant difference between the two groups (p < 0.001) (Table 2). Additionally, there was a tendency toward increased PT in the patient group, but it was not statistically significant (p = 0.08).
Correlation of coagulation tests with clinical and laboratory variables
Elderly age (>50 years) was associated with higher D-dimer levels (median 174 vs 345 U/ml, p = 0.003) (Table 3). Similarly, metastatic patients exhibited significantly higher D-dimer values when compared with early breast cancer patients (p = 0.049). Advanced tumor stage (T3 and T4 vs T1 and T2) was associated with higher INR (median 1.15 vs 1.09, p = 0.05) and concordantly lower prothrombin activity (82 vs 70 %, p = 0.025). Nodal involvement did not seem to have an influence on coagulation parameters. However, histological grade of tumor had a correlation with prothrombin time; grade 3 tumors were associated with prolonged PT (15 vs 14.4 s, p = 0.014).
Higher erythrocyte sedimentation rates (ESR) (>40 mm/h) reflecting inflammatory response were associated with high fibrinogen levels (358 vs 297 mg/dl, p = 0.005). Besides, elevated ESR was clearly correlated with prolonged PT (p = 0.025) and INR (p = 0.045), low prothrombin activity (p = 0.034), and higher platelet counts (p = 0.022).
Receptor status including ER, PR, and HER-2 did not affect coagulation parameters evidently (Table 4). When patients were analyzed according to the tumors’ receptor status such as triple negative or hormone negative (ER −, PR −, and HER-2 positive or negative) or triple positive (ER +, PR +, HER-2 +) groups, no significant relationship was detected with respect to coagulation tests.
Survival analysis
Median follow-up time was 9.1 months (range 2–56 months). During follow-up, five (4 %) patients were dead due to disease-related events. Univariate analysis of survival revealed that advanced tumor stage and leukocytosis were indicators of poorer overall survival (Table 5). Since numbers of events were scarce during follow-up due to high overall survival rates among breast cancer patients, none of the coagulation assays was determined as a prognostic factor on survival.
Discussion
The processes of hemostasis/fibrinolysis and angiogenesis are closely linked physiological systems that remain silent under resting conditions, but in case of malignancy, these highly activated mechanisms may be involved in tumor expansion and invasive behavior.
The fibrinolytic system includes a broad spectrum of proteolytic enzymes with pathophysiological functions in several processes such as tumor invasion, angiogenesis, and reproduction. The main enzyme of the plasminogen activator system, plasmin is responsible for degradation of fibrin into soluble degradation products. Plasminogen activators (PA) urokinase-type plasminogen activator (uPA), and tissue type plasminogen activator (PAIs) are serine proteases that catalyze the cleavage of inactive plasminogen to plasmin [17]; PAs are secreted by many cells including tumor cells [18]. Through the proteolytic activity of malignant cells, the extracellular matrix and basal membrane of tumor stroma are degraded, enabling invasion of adjacent tissues and migration of tumor cells [19]. Thus, products of the induced fibrinolytic process such as D-dimer have been investigated as a marker for distinguishing benign disorders from malignancies and evaluating invasiveness in various tumors [15, 20–22]. Our results are consistent with the previous data reflecting the significant difference between patient and control group concerning coagulation parameters which involve fibrinogen, aPTT, and INR in addition to D-dimer levels [23, 24].
Elevated D-dimer levels as a predictor of tumor overload have been confirmed in different studies for lung cancer and gynecologic cancer types previously [25, 26]. Similarly, preoperative D-dimer levels have provided support for the role of increased levels in predicting vascular invasion, advanced tumor stage, and poor postoperative survival in colorectal cancer patients [27]. Among breast cancer patients, plasma D-dimer levels were increased in progressive metastatic disease correlating with tumor load, number of metastatic sites, and progression kinetics [12]. Our study confirmed the utility of elevated D-dimer levels in predicting extent of disease, since there was a statistically significant difference between metastatic and early breast cancer patients (p = 0.049). Besides, advanced tumor stage correlated with elevated INR (p = 0.025) and reduced prothrombin activity (p = 0.05), indirectly reflecting an ongoing consumption of coagulation assays through the exaggerated fibrinolytic process. Although previously a few reports have mentioned a relationship between elevated D-dimer levels and involved axillary lymph nodes [20, 28], we did not find such a correlation in our study group. In concordance with our results, lymphonodal status was not defined as a factor affecting D-dimer values in the other study involving hormone receptor negative breast cancer patients [24]. However, we were able to demonstrate a relationship between histological grade and PT; grade 3 tumors were associated with prolonged PT (p = 0.014). So far, to our knowledge no association between histological grade and coagulation tests has been described.
Hormone receptor status is one of the most relevant biological factors determining prognosis. Particularly, regardless of tumor size or nodal status, triple negativity is depicted as an independent negative prognostic factor, reflecting the aggressive nature of this tumor (TNBC-2). The current study also aimed to define the effect of hormone receptor status on coagulation parameters. When patients were analyzed according to their receptor status as triple negative, triple positive, hormone receptor positive (ER +, PR +, HER-2 −) or hormone receptor negative (ER −, PR −, HER-2 −/+) subtypes, no significant difference was detected according to variation of coagulation parameters. Comparison of D-dimer values of triple-negative and non-triple-negative breast cancer patients had not yielded a significant difference in the previous study, in agreement with our findings [24]. Furthermore, in a recent study investigating the relationship between fibrinolytic cascade and clinicopathologic variables, increased levels of D-dimer were not shown to be related with histological subtypes and hormone receptor status [12]. Probably, receptor status is an irrelevant factor for tumor progression kinetics via the fibrinolytic system.
In conclusion, almost all coagulation tests show significant difference in patients with breast cancer. The current study supports the significance of increased plasma D-dimer as a predictor for advanced stage disease. Since fibrin degradation products reflect an ongoing fibrinogen metabolism within the actively remodeled tumor stroma, considering the amount of these products as a marker for disease load seems justified. As D-dimer tests are neither time consuming nor expensive, this proposal appears to be suitable for daily practices deserving further investigation.
References
Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Semin Thromb Haemost. 1999;25:173–82.
Clahsen PC, van de Velde CJ, Julien JP, Floiras JL, Mignolet FY. Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: a European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group study. J Clin Oncol. 1994;12:1266–71.
Weiss RB, Tormey DC, Holland JF, Weinberg VE. Venous thrombosis during multimodal treatment of primary breast carcinoma. Cancer Treat Rep. 1981;65:677–9.
Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–56.
Dvorak HF. Thrombosis and cancer. Hum Pathol. 1987;18:275–84.
Miller B, Heilmann L. Hemorheologic variables in breast cancer patients at the time of diagnosis and during treatment. Cancer. 1988;62:350–4.
Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br J Cancer. 2000;83:164–70.
Falanga A, Levine MN, Consonni E, et al. The effect of very-low-dose warfarin on markers of hypercoagulation in metastatic breast cancer: results from a randomized trial. Thromb Haemost. 1998;79:23–7.
Murray JJ. Coagulation and cancer. Br J Cancer. 1991;64:422–4.
Oya M, Akiyama Y, Yanagida T, Akao S, Ishikawa H. Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker. Surg Today. 1998;28:373–8.
den Ouden M, Ubachs JMH, Stoot JEGM, van Wersch JWJ. Thrombin-antithrombin III and D-dimer plasma levels with benign or malignant ovarian tumors. Scand J Clin Lab Invest. 1998;58:555–60.
Dirix LY, Salgado R, Weytjens R, et al. Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer. Br J Cancer. 2002;86:389–95.
McCulloch P, Douglas J, Lowe GD, Murray G, George WD. In vivo measurement of fibrin formation and fibrinolysis in operable breast cancer. Thromb Haemost. 1989;61:318–21.
Mitter CG, Zielinski C. Plasma levels of D-dimer: a cross-linked fibrin-degradation product in female breast cancer. J Cancer Res Clin Oncol. 1991;117:259–62.
von Tempelhoff GF, Dietrich M, Hommel G, Heilmann L. Blood coagulation during adjuvant epirubicin/cyclophosphamide chemotherapy in patients with operable breast cancer. J Clin Oncol. 1996;14:2560–8.
Pereira H, Pinder SE, Sibbering DM, et al. Pathological prognostic factors in breast cancer. IV: should you be a typer or grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology. 1995;27:219–26.
Mayer M. Biochemical and biological aspects of the plasminogen activator system. Clin Biochem. 1990;23:197–211.
Schmitt M, Jänicke F, Moniwa N, et al. Tumor-associated urokinase-type plasminogen activator; biological and clinical significance. Biol Chem Hoppe Seyler. 1992;373:611–22.
Zorio E, Gilabert-Estellés J, Espaňa F, et al. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. 2008;15:923–9.
Kim HK, Song KS, Lee KR, et al. Comparison of plasma D-dimer and thrombus precursor in patients with operable breast cancer as a potential predictor of metastasis. Blood Coagul Fibrinolysis. 2004;15:9–13.
Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31:388–94.
Taguchi O, Gabazza EC, Yasui H, et al. Prognostic significance of plasma D-dimer levels in patient with lung cancer. Thorax. 1997;52:563–5.
Kirwan CC, McDowell G, McCollum CN, Kumar S, Byrne GJ. Early changes in the haemostatic and procoagulant systems after chemotherapy for breast cancer. Br J Cancer. 2008;99:1000–6.
Batschauer APB, Figueiredo CP, Bueno EC, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21:1267–72.
Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of haemostatic parameters in patients with lung cancer. Respir Med. 2004;98:93–8.
Rella C, Coviello M, Frenza ND, et al. Plasma D-dimer measurement as a marker of gynecological tumors: comparison with CA 125. Tumori. 1993;79:347–51.
Kilic M, Yoldas O, Keskek M, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis. 2008;10:238–41.
Blackwell K, Haroon Z, Broadwater G, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18:600–8.
Conflicts of interest
None
Role of the funding source
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tas, F., Kilic, L. & Duranyildiz, D. Coagulation tests show significant differences in patients with breast cancer. Tumor Biol. 35, 5985–5992 (2014). https://doi.org/10.1007/s13277-014-1793-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1793-4