Abstract
The objective of this study is to investigate the expression level of periostin in cancer stem cells as well as in the glioma tissues and the relationship between periostin expression and clinical and pathological characteristics and prognosis of gliomas. ESA+/CD133+/lin− tumor cells were selected by flow cytometry from glioma tissues, and the periostin expression in ESA+/CD133+/lin− tumor cells and non-ESA+/CD133+/lin− tumor cells was detected by quantitative real-time polymerase chain reaction (RT-PCR) and Western blot analysis. The expression status of periostin in glioma tissues was analyzed by immunohistochemistry staining, and the relationship between periostin and clinicopathological parameters of gliomas was determined. It showed that periostin is expressed higher in ESA+/CD133+/lin− tumor cells compared to non-ESA+/CD133+/lin− tumor cells in both mRNA and protein levels. One hundred eighteen (37.82 %) glioma patients were observed with highly expressed periostin protein in immunohistochemistry. Moreover, we observed that the expression of periostin protein was related to Karnofsky performance scale score (KPS), extent of resection, Ki67, and WHO grade of gliomas in universal analysis (P = 0.008, 0.045, 0.001, and 0.001, respectively). However, only WHO grade was identified to be related to periostin expression in gliomas after multivariate analysis. After survival analysis, the cases with highly expressed periostin protein attained a significantly poorer postoperative disease-specific survival and distant metastasis than those with none/low expressed periostin protein (P = 0.001 and 0.002). In the Cox regression test, KPS, extent of resection, Ki67, WHO grade, and periostin were detected as the independent prognostic factors (P = 0.008, 0.007, 0.032, 0.001, and 0.001, respectively). Periostin can be an important prognostic marker for gliomas, which may present a new therapeutic target for glioma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, some scholars have put forward cancer stem cell theory regarding tumorigenesis [1, 2]. This theory suggests that a minority of stem-like cells exist in quantity and have the potency for self-renewal and differentiation, as well as tumor formation [3, 4]. These cells can divide asymmetrically to produce massive proliferative cells. Thus, they are important in the process of tumor occurrence, development, and metastasis. Indeed, researchers have confirmed that they play a significant role in many tumors, including leukemia, breast cancer, brain neoplasms, retinoblastoma, liver cancer, metastatic germ cell tumor, prostate cancer, embryonal carcinoma, and ovarian cancer, among others [5–7].
In some studies, CD133, CD15, A2B5, nestin, Musashi-1, BMI1, SOX2, Id1, and Oct-4 have been suggested to be closely related to cancer stem cell properties in gliomas [8–10]. CD133 is a 5-TM glycoprotein located in the membrane of human hematopoietic cells and in neural progenitor cells [8]. Singh et al. showed that only 100 CD133-positive (CD133+) cells were required to produce a tumor in mice similar to the original patient tumor. In contrast, 105 CD133-negative (CD133−) cells were unable to produce tumors, suggesting that CD133+ cells have cancer stem cell properties [11].
Following the better understanding of cancer stem cell theory, stem cell-related genes in malignant tumors gain more academic attention [12]. Recently, Malanchi et al. found that periostin is required to allow cancer stem cell maintenance, and blocking its function prevents metastasis [13]. To be excited, blocking periostin protein rarely caused side effects in mice. In another study, Kyutoku et al. reported that periostin plays a pivotal role in the progression and metastasis of breast cancer [14]. Since administration of periostin antibody prolonged cell survival through inhibition of the progression and metastasis of 4T1 cells, further development of the periostin antibody such as generation of a humanized antibody may provide a new therapeutic agent against breast cancer.
Periostin is a kind of bone adhesion molecule regulating osteoblast adhesion and differentiation, classified in the extracellular matrix (ECM) proteins [12]. It presents as part of the extracellular matrix in natural conditions and plays an important role in fetal development. In adults, only some specific organs such as the breast, bone, skin, and intestine have periostin activity [15]. Periostin is reported to be involved in tumor EMT, extracellular matrix degradation, tumor invasion, and distant metastasis, but the mechanism is still unclear [15]. At present, the periostin expression status in glioma stem cells and the clinical implication in gliomas are still unclear. In the study herein, we try to investigate the expression status of periostin in glioma stem cells and the clinical implications of periostin in gliomas in order to lay a foundation for glioma management.
Materials and methods
Patients and tissue specimens
Paraffin-embedded tumor tissues and matched normal tissues were taken from 312 glioma patients who had histologically confirmed gliomas and who underwent operations in the Tumor Hospital and Cancer Institute of Liaoning Province and Harbin Medical University between Jan 2002 and Jan 2008; the patients were enrolled for immunohistochemical staining as well as prognostic analysis. The mean age was 48.52 ± 7.08 years (ranging from 35 to 79 years). All the enrolled cases were followed up every 6 months after surgery, and the median length of follow-up was 31 months (ranging from 1 to 108 months). The study protocol was approved by the Ethics Committee of Liaoning Medical College. All patients signed the informed consents to allow molecular research on specimens obtained during the surgical operation. In addition, the eight fresh specimens were selected for ESA+/CD133+/lin− tumor cell selection by flow cytometry.
ESA+/CD133+/lin− tumor cell sorting by flow cytometry
The clinical specimens were digested into single tumor cells using collagenase III. The tumor cells were suspended in 100 μl/106 cells of HBSS with 2 % HICS. The samples were then washed twice with HBSS/2 % HICS and suspended. Antibodies, including anti-CD2, anti-CD3, anti-CD10, anti-CD16, anti-CD18, anti-CD31, and CD326 were added and incubated for 20 min on ice and then washed twice with HBSS/2 % HICS. Lineage+ cells were first eliminated by anti-CD2, anti-CD3, anti-CD10, anti-CD16, anti-CD18, anti-CD31, and CD326 during flow cytometry. Dead cells were eliminated using the viability dye 7AAD. Second, CD133+ tumor cells were sorted by CD133 in flow cytometry. Cells to be injected were then suspended in RPMI1640/Matrigel mix (1:1 volume) and injected into the appropriate area of the fat pad. When maintained in the stem cell culturing media, the ESA+/CD133+/lin− cells remained undifferentiated, grew in clusters (Fig. 1a), and demonstrated a much higher tumor-initiating potential than ESA+/CD133−/lin− cells and the unsorted cells in a limiting dilution injection experiment (Fig. 1b).
RT-PCR
Total RNA was isolated using Trizole according to manufacturer’s instruction. Complementary DNAs (cDNAs) were synthesized using RevertAidTM first cDNA synthesis kit (Fermentas). Quantitative real-time PCR was performed using SYBR Green PCR Master Mix on Applied Biosystems 7500 Fast Real-Time PCR System. Primers used in real-time PCR are as follows: forward 5′-CACCACAACGCAGCGCTATT-3′ and reverse 5′-TGGAAGTTTCTCAAAAGCCT-3′. The reaction conditions are as follows: 95 °C for 5 min, followed by 40 cycles of 94 °C for 15 s, and 55.5 or 55.2 °C for 20 s. Melting temperature curve analyses were performed after PCR.
Western blot analysis
For Western blot analysis, cells were lysed with the buffer [0.1 % SDS, 50 mmol/l Tris-HCl (pH 7.6), 1 % NP-40, 150 mmol/l NaCl, 2 mg/ml aprotinin, 2 mg/ml leupeptin, and 7 mg/ml PMSF]. The protein concentrations were determined using the BCA Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Thirty micrograms of protein was separated on 10 % SDS-PAGE gels and transferred to a PVDF membrane. After blocking, the membrane was incubated with anti-periostin antibody (1:1,000, R&D Systems, Minneapolis, MN, USA) at 4 °C overnight. After washing, the membrane was incubated with a secondary antibody at a dilution of 1:3,000 at room temperature for 1 h. Proteins were detected with the ECL kit (Varsal Instruments, Beijing, China), and anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA) was used as loading control. Densitometry was performed by Gel-Pro Analyzer software (Media Cybernetics, Silver Spring, MD, USA).
Immunohistochemical staining and evaluation
Thin slices of tumor tissue of all cases received in our histopathology unit were fixed in 4 % formaldehyde solution (pH 7.0) for periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding, and 4-μm-thick sections were cut and placed on glass slides coated with 3-aminopropyl triethoxysilane for immunohistochemistry. Tissue samples were stained with hematoxylin and eosin to determine histological type and grade of tumors.
Briefly, glioma tissues were cut at a thickness of 4 μm using a cryostat. The sections were mounted on microscope slides, air-dried, and then fixed in a mixture of 50 % acetone and 50 % methanol. The sections were then de-waxed with xylene, gradually hydrated with gradient alcohol, and washed with phosphate-buffered saline (PBS). Sections were incubated for 60 min with the primary antibody. Following washings with PBS, sections were incubated for 30 min in the secondary biotinylated antibody (Multilink Swine anti-goat/mouse/rabbit immunoglobulin; Dako Inc.). Following washings, avidin-biotin complex (1:1,000 dilution, Vector Laboratories Ltd) was then applied to the sections for 30–60 min at room temperature. The immunoreactive products were visualized by catalysis of 3,3-diaminobenzidine (DAB) by horseradish peroxidase in the presence of H2O2, following extensive washings. Sections were then counterstained in Gill’s hematoxylin and dehydrated in ascending grades of methanol before clearing in xylene and mounting under a coverslip.
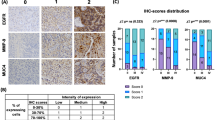
To score periostin as immunopositive staining, the positive cells are shown as a yellow to brown color of the nucleus and/or cytoplasm. Periostin expression was classified semi-quantitatively according to the following criteria: 0 if <1 % of neoplastic cells discretely expressed periostin, 1+ if ≥1 and <10 % of morphologically unequivocal neoplastic cells discretely expressed periostin, and 2+ if ≥10 % of morphologically unequivocal neoplastic cells discretely expressed periostin. Samples scored as 1+ or 2+ were considered positive.
Statistical analysis
Statistical analysis was performed using the SPSS Statistical Software (SPSS 13.0, Chicago, IL, USA). The difference between periostin expression in tumor tissues and normal tissues was determined using unpaired Student’s t test. The correlation between periostin expression and clinicopathological characteristics was analyzed using a chi-square test and Spearman’s correlation analysis. Survival analysis was conducted by the Kaplan-Meier curves; P < 0.05 was considered statistically significant.
Results
The percentage of CD133+ tumor cells in gliomas
To investigate the clinical implications of the CD133+ tumor cell ratio in Chinese glioma cancer patients, we applied fluorescent immunohistochemical staining to quantify the proportion of CD133+ tumor cells in each of the glioma clinical specimens. The mean average CD133+ tumor cell ratio in clinical specimens was 2.84 % (ranging from 0.58 to 7.22 %).
After 7 days of culture, single-cell suspensions of ESA+/CD133+/lin− tumor cells separated from the solid tumors produced viable mammospheres (20 to 100 μm) which could be passaged further, while no mammosphere was produced by CD133− tumor cells in the same culture condition.
Periostin expression in human glioma tissues at mRNA and protein levels
Periostin messenger RNA (mRNA) was expressed higher in ESA+/CD133+/lin− tumor cells than in non-ESA+/CD133+/lin− tumor cells (0.42 vs. 0.24 normalized expression to GAPDH). Real-time polymerase chain reaction (RT-PCR) analysis of periostin mRNA expression in high-grade (WHO III–IV) and low-grade (WHO II) tumor tissues showed that periostin mRNA was upregulated in high-grade glioma cancer tissues when compared to low-grade tumor tissues (P = 0.01, Fig. 1). Furthermore, in the Western blot analysis, the periostin protein was observed to be expressed higher in ESA+/CD133+/lin− tumor cells than in non-ESA+/CD133+/lin− tumor cells and in high-grade glioma cancer tissues than in low-grade glioma cancer tissues (P = 0.001, Fig. 2).
The expression of stem cell gene periostin in glioma patients and the relationship between periostin expression and clinicopathological characteristics
It was shown that periostin was located in the cytoplasm and membrane of glioma cells. One hundered eighteen (37.82 %) glioma patients were observed with highly expressed periostin protein in immunohistochemistry (Fig. 3). Moreover, we observed that the expression of periostin protein was related to Karnofsky performance scale score (KPS), extent of resection, Ki67, and WHO grade of gliomas in universal analysis (P = 0.008, 0.045, 0.001, and 0.001, respectively) (Table 1). However, only WHO grade was identified to be related to periostin expression in gliomas after multivariate analysis (Table 2).
Prognostic analysis
After survival analysis, the cases with highly expressed periostin protein attained a significantly poorer postoperative disease-specific survival than those with none/low expressed periostin protein in both low-grade gliomas and high-grade gliomas (P = 0.001 and 0.001) (Fig. 4). In the Cox regression test, KPS, extent of resection, Ki67, WHO grade, and periostin were detected as the independent prognostic factors (P = 0.008, 0.007, 0.032, 0.001, and 0.001, respectively) (Table 3).
Discussion
Gliomas make up more than 30 % of all brain and central nervous system tumors and 80 % of all malignant brain tumors [16]. Although surgery-based comprehensive therapy has greatly improved the treatment of gliomas, the prognosis of high-level gliomas remains poor [17]. At present, the reasons for glioma recurrence and poor postoperative prognosis were still unclear [10]. The tumor stem cell theory has provided novel hypotheses for the further understanding of gliomas and related treatments.
With further studies of tumor stem cells in breast tumors, the researchers speculated that these stem cells may also exist in brain cancers. In 2003, Singh et al. were the first to identify a cell subpopulation with an unlimited proliferative potential and specific differentiation potential in medulloblastoma and glioblastoma multiforme [11]. These cells possessed different molecular genetics and cell biology characteristics compared with common brain tumor cells since they expressed neural stem cell markers, such as nestin, Musashi-1, BMI1, and CD133. Additionally, these cells had an increased self-renewal and proliferation ability compared with neural stem cells. These cells were able to differentiate into tumor cells with the same phenotype as the original tumor in vitro and form tumors in vivo following transplantation. Tumor stem cells in the brain are resistant to radiotherapy and chemotherapy, and consequently, an increasing amount of glioma stem cell research has been carried out. However, there is controversy with regard to the sorting and identification criteria of brain tumor stem cells [18, 19]. CD133 is a transmembrane protein with a relative molecular weight of 120,000 Da and was initially used as a hematopoietic stem cell marker. CD133 is a common marker in neural stem cells, rather than a specific marker of brain tumor stem cells [20]. However, CD133 may be useful for identifying brain tumor stem cell-specific markers and is considered to be important for the separation and purification of brain tumor stem cells, as well as in tumor development and prognosis. In a study, Liu et al. aimed to conduct CD133+/− cell selection in the glioblastoma tissues of eight individuals from Northern China and to analyze the biological characteristics of the two cell subtypes through in vivo and in vitro observations [21]. The results verified the high tumorigenicity and invasiveness of CD133+ tumor cells and demonstrated the limitations and deficiencies of using CD133 alone as a marker to distinguish tumor stem cells.
Pallini et al. investigated the expression of CD133 in 44 glioblastoma multiformes (GBMs) and showed that more than 2 % CD133+ cells and the presence of CD133/Ki67 co-expression were associated with a poor outcome [22]. In multivariate analysis, patients with less than 2 % CD133+ cells had a better progression-free survival (PFS) and overall survival (OS). The difference increased when CD133/Ki67 co-expression was evaluated; patients with CD133−/Ki67+ cells had a median OS of 12.3 months compared to 6.8 months in patients with CD133+/Ki67+ cells.
We further studied the clinical implications of periostin in glioma patients. It was found that 37.82 % of glioma patients highly expressed periostin protein. Moreover, we observed that the expression of periostin protein was related to WHO grade in multivariate analysis. Finally, cases with highly expressed periostin protein attained a significantly poor postoperative disease-specific survival. In the Cox regression test, periostin was detected as the independent prognostic factor. After investigating the periostin expression in 71 glioma patients, Wang et al. reported that periostin protein was related to the high WHO grade of gliomas [23]. However, there was still no study investigating the relationship between periostin expression and distant metastasis of gliomas. We observed that the tumors with high periostin protein expression will suffer a higher distant metastasis rate compared to the others, and the most likely metastastic organ is the bone.
Conclusions
In summary, we demonstrated the overexpression of periostin in glioma tissues, and overexpression of periostin protein predicted a worse prognosis for glioma patients. These results suggest that periostin can be an important prognostic marker for gliomas and a predictor for distant metastasis of gliomas, which may present a new therapeutic target for glioma patients. Of course, further study is needed.
References
Ulukaya E, Frame FM, Cevatemre B, Pellacani D, Walker H, Mann VM, et al. Differential cytotoxic activity of a novel palladium-based compound on prostate cell lines, primary prostate epithelial cells and prostate stem cells. PLoS One. 2013;8(5):e64278.
Ren F, Sheng WQ, Du X. CD133: a cancer stem cells marker, is used in colorectal cancers. World J Gastroenterol. 2013;19(17):2603–11.
Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V. Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells. 2013;2(1):52–61.
Liao WY, Liaw CC, Huang YC, Han HY, Hsu HW, Hwang SM, et al. Cyclohexylmethyl flavonoids suppress propagation of breast cancer stem cells via downregulation of NANOG. Evid Based Complement Alternat Med. 2013;2013:170261.
Chen SF, Lin YS, Jao SW, Chang YC, Liu CL, Lin YJ, et al. Pulmonary adenocarcinoma in malignant pleural effusion enriches cancer stem cell properties during metastatic cascade. PLoS One. 2013;8(5):e54659.
Nakada M, Nambu E, Furuyama N, Yoshida Y, Takino T, Hayashi Y, et al. Integrin α3 is overexpressed in glioma stem-like cells and promotes invasion. Br J Cancer. 2013;108(12):2516–24.
Tu SM. Cancer: a “stem-cell” disease? Cancer Cell Int. 2013;13(1):40.
Wei Y, Jiang Y, Zou F, Liu Y, Wang S, Xu N, et al. Activation of PI3K/Akt pathway by CD133-p85 interaction promotes tumorigenic capacity of glioma stem cells. Proc Natl Acad Sci U S A. 2013;110(17):6829–34.
Jin X, Jin X, Jung JE, Beck S, Kim H. Cell surface Nestin is a biomarker for glioma stem cells. Biochem Biophys Res Commun. 2013;433(4):496–501.
Dahlrot RH, Hermansen SK, Hansen S, Kristensen BW. What is the clinical value of cancer stem cell markers in gliomas? Int J Clin Exp Pathol. 2013;6(3):334–48.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8.
Liu CG, Lu Y, Wang BB, Zhang YJ, Zhang RS, Lu Y, et al. Clinical implications of stem cell gene Oct-4 expression in breast cancer. Ann Surg. 2011;253(6):1165–71.
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481(7379):85.
Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Iekushi K, et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28(2):181–6.
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H, et al. Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS One. 2012;7(10):e46670.
Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–6.
Caruso C, Carcaterra M, Donato V. Role of radiotherapy for high grade gliomas management. J Neurosurg Sci. 2013;57(2):163–9.
Ahmed AU, Auffinger B, Lesniak MS. Understanding glioma stem cells: rationale, clinical relevance and therapeutic strategies. Expert Rev Neurother. 2013;13(5):545–55.
Rath P, Lal B, Ajala O, Li Y, Xia S, Kim J, et al. In vivo c-Met pathway inhibition depletes human glioma xenografts of tumor-propagating stem-like cells. Transl Oncol. 2013;6(2):104–11.
Lehnus KS, Donovan LK, Huang X, Zhao N, Warr TJ, Pilkington GJ, et al. CD133 glycosylation is enhanced by hypoxia in cultured glioma stem cells. Int J Oncol. 2013;42(3):1011–7.
Liu X, Chen L, Jiang Z, Wang J, Su Z, Li G, et al. Malignant behavioral characteristics of CD133(+/−) glioblastoma cells from a Northern Chinese population. Exp Ther Med. 2013;5(1):65–72.
Pallini R, Ricci-Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(24):8205–12.
Wang H, Wang Y, Jiang C. Stromal protein periostin identified as a progression associated and prognostic biomarker in glioma via inducing an invasive and proliferative phenotype. Int J Oncol. 2013;42(5):1716–24.
Acknowledgments
This work was supported by the Science and Technology Development Project of Liaoning Province in China (Grant No. 2012225019IL-1).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, B., Zhang, Y. & Zhang, J. Periostin is a new potential prognostic biomarker for glioma. Tumor Biol. 35, 5877–5883 (2014). https://doi.org/10.1007/s13277-014-1778-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1778-3