Abstract
Increasing scientific evidences suggest that aerobic exercise may improve cancer-related fatigue in breast cancer patients, but many existing studies have yielded inconclusive results. This meta-analysis aimed to derive a more precise estimation of the effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy. The PubMed, CISCOM, CINAHL, Web of Science, Google Scholar, EBSCO, Cochrane Library, and CBM databases were searched from inception through July 1, 2013 without language restrictions. Crude standardized mean difference (SMD) with 95 % confidence interval (CI) was calculated. Twelve comparative studies were assessed with a total of 1,014 breast cancer patients receiving chemotherapy, including 522 patients in the aerobic exercise group (intervention group) and 492 patients in the usual care group (control group). The meta-analysis results revealed that the Revised Piper Fatigue Scale (RPFS) scores of breast cancer patients in the intervention group were significantly lower than those in the control group (SMD = −0.82, 95% CI = −1.04 ∼ −0.60, P < 0.001). However, there was no significant difference in the Functional Assessment of Chronic Illness Treatment-Fatigue scale (FACIT-F) scores between the intervention and control groups (SMD = 0.09, 95% CI = −0.07 ∼ 0.25, P = 0.224). Subgroup analysis by ethnicity indicated that there were significant differences in RPFS and FACIT-F scores between the intervention and control groups among Asian populations (RPFS: SMD = −1.08, 95% CI = −1.35 ∼ −0.82, P < 0.001; FACIT-F: SMD = 1.20, 95 % CI = 0.70 ∼ 1.71, P < 0.001), but not among Caucasian populations (all P > 0.05). The current meta-analysis indicates that aerobic exercise may improve cancer-related fatigue in breast cancer patients receiving chemotherapy, especially among Asian populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Breast cancer is one of the most common cancers in the world and also the leading cause of cancer death among women, which accounts for 23 % of total cancer cases and 14 % of cancer deaths [1]. However, because of earlier detection and advances in treatment, death rates of breast cancer continue to decrease accounting for 34 % of the total decline in women [2]. New effective chemotherapy, hormone therapy, and biological agents are used to integrate systemic therapy, surgery, and radiation therapy for better treatment of breast cancer [3]. Although these effective therapies improve overall survival, they are also associated with several side effects, such as decreased cardiac function, muscle wasting, reductions in physical and cognitive functioning, and especially cancer-related fatigue (CRF) [4, 5]. Previous studies suggested that regular physical activity especially aerobic exercise can reduce CRF presented after breast cancer diagnosis and treatment [6, 7]. Therefore, exercise intervention may play an important role in improving CRF in patients with breast cancer.
Physical activity has generally been proposed as a potentially appealing intervention that could mitigate sequelae related to cancer and assist patients in returning to the health status they had before treatment, which may improve physiological and psychological effects simultaneously [8–10]. In the past decades, there were plenty of randomized controlled trials focused on the effects of physical activity on cancers [11]. Most of the studies have reached a conclusion that exercise is a rehabilitation treatment which can help cancer patients with common complaints such as CRF, nausea, loss of strength and flexibility, and weight gain [12, 13]. Some studies have suggested that physical activity has significant effects on CRF, body composition, physical functions, psychological outcomes, and quality of life in patients after treatment for breast cancer [14, 15]. The various types of physical activities involved in these studies can mainly be classified into occupational, resistance exercise, recreational, and aerobic exercise [16], among which the efficacy of aerobic exercise on the management of CRF in breast cancer patients has attracted an ever-increasing interest. Aerobic exercise is defined as the rhythmic contraction and relaxation of large muscle groups over a prolonged time [17]. A growing body of scientific evidence supports that moderately intense aerobic exercise may be an effective tool for reducing the progression of CRF in breast cancer patients receiving chemotherapy [18]. Nevertheless, other studies indicated that aerobic exercise could not alleviate CRF in breast cancer patients receiving chemotherapy [11]. The inconsistent conclusions may be due to the limitations in sample size; the inadequate statistical power in genetic studies of complex traits, such as age, ethnicity, and gender; the differentiation on tumor stage; and research methodology. Therefore, we performed a meta-analysis of all eligible case–control studies to reveal the effects of aerobic exercise on CRF in breast cancer patients receiving chemotherapy.
Methods
Search strategy
The PubMed, CISCOM, CINAHL, Web of Science, Google Scholar, EBSCO, Cochrane Library, and CBM databases were searched from inception through July 1, 2013 without language restrictions. The following keywords and MeSH terms were used: [“cancer-related fatigue” or “CRF” or “chemotherapy-related fatigue” or “treatment-related fatigue”], [“breast cancer” or “breast neoplasms” or “breast tumor” or “breast carcinoma” or “mammary cancer” or “mammary carcinoma”], and [“aerobic exercise” or “aerobic sports” or “aerobic training”]. We also performed a manual search to find other potential articles.
Selection criteria
The diagnostic criteria for cancer-related fatigue was defined as a common disease that refers to a continuous stressful feeling of physical or psychological tiredness which could affect functional level as well as quality of life (QOL) and is not possibly to be relieved by rest or sleep. To be included in the meta-analysis, these studies must meet the following criteria: (1) The type of study should be a clinical comparative study, (2) The study must be focused on the effects between the aerobic exercise and CRF in breast cancer patients receiving chemotherapy, (3) All patients diagnosed with breast cancer should be confirmed by pathohistological examinations, (4) Fatigue scores should be capable of extraction, and (5) The publication should be in English or Chinese. If the study failed the inclusion criteria, it was excluded. When authors published several studies using the same subjects, either the one most recently published or with the largest sample size was included.
Data extraction
Two independent authors extracted data from eligible studies using a standardized form. The following information were collected: surname of first author, year of publication, source of publication, country of origin, ethnicity, language of publication, study type, total number of subjects, exercise time, and fatigue scores. In cases of conflicting evaluations, disagreements on inconsistent data from the eligible studies were resolved through discussions and careful reexaminations of the full text by the authors.
Quality assessment
Methodological quality was independently assessed by two researchers according to the Newcastle-Ottawa Scale (NOS) criteria [19]. The NOS criteria includes three dimensions scored within the following ranges: (1) subject selection 0 ∼ 4, (2) comparability of subject 0 ∼ 2, and (3) clinical outcome 0 ∼ 3. NOS scores range from 0 to 9 with a score of ≥7 indicating good quality.
Statistical analysis
We calculated crude odds ratios (OR) with their 95 % confidence intervals (95% CI) to evaluate their relationships under five genetic models. The Z test was used to determine inclusive OR significance. The Cochran’s Q-statistic and I 2 test were used to evaluate potential heterogeneity between studies [20]. The I 2 test was also conducted to quantify the heterogeneity (ranges from 0 to 100 %) [21]. If the Q test showed a P < 0.05 or I 2 test exhibits >50 %, indicating significant heterogeneity, the random effects model was conducted; otherwise, the fixed-effects model was used. We also performed subgroup and meta-regression analyses to explore potential sources of heterogeneity. Sensitivity analysis was performed by omitting each study in turn to evaluate the influence of single studies on the overall estimate. Begger’s funnel plots and Egger’s linear regression test were conducted to investigate publication bias [22]. All analyses were calculated using the STATA software, version 12.0 (Stata Corp, College Station, TX, USA).
Results
Baseline characteristics of included studies
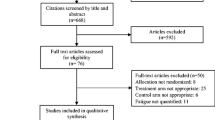
A total of 126 articles relevant to the searched keywords were initially identified. Of these articles, 74 were excluded after a review of their titles and abstracts; then, full texts and data integrity were reviewed, and another 40 papers were excluded. Twelve comparative studies met our inclusion criteria for this meta-analysis [11, 23–33]. Publication years of the eligible studies ranged from 2005 to 2012. The flow chart of the study selection process is shown in Fig. 1. The distribution of the number of topic-related literatures in the electronic database during the last decade was showed in Fig. 2. A total of 1,014 breast cancer patients receiving chemotherapy were involved in this meta-analysis, including 522 patients in the aerobic exercise group (intervention group) and 492 patients in the usual care group (control group). Overall, four studies were conducted in Asian populations; the other eight studies were conducted in Caucasian populations. The Revised Piper Fatigue Scale (RPFS) was used in six studies, while the Functional Assessment of Chronic Illness Treatment-Fatigue scale (FACIT-F) was performed in the other six studies. The characteristics and methodological quality of the included studies are summarized in Table 1.
Quantitative data synthesis
Since heterogeneity obviously existed (all P < 0.05), which could be a result of differences in ethnicity and exercise time, the random effects model was conducted. The meta-analysis results revealed that the RPFS scores of breast cancer patients in the intervention group were significantly lower than those in the control group (SMD = −0.82, 95% CI = −1.04 ∼ −0.60, P < 0.001). However, there was no significant difference in FACIT-F scores between the intervention and control groups (SMD = 0.09, 95% CI = −0.07 ∼ 0.25, P = 0.224) (Fig. 3). Subgroup analysis by ethnicity indicated that there were significant differences in RPFS and FACIT-F scores between the intervention and control groups among Asian populations (RPFS: SMD = −1.08, 95% CI = −1.35 ∼ −0.82, P < 0.001; FACIT-F: SMD = 1.20, 95% CI = 0.70 ∼ 1.71, P < 0.001), but not among Caucasian populations (all P > 0.05) (Fig. 4). Further subgroup analysis based on exercise time showed significant difference of RPFS scores between the intervention and control groups in the ≤8-week subgroup (SMD = −0.87, 95% CI = −1.10 ∼ −0.64, P < 0.001) (Fig. 5). Nevertheless, we observed no difference of RPFS scores between the intervention and control groups in the >8-week subgroup (SMD = −0.41, 95% CI = −1.09 ∼ 0.27, P = 0.240). Furthermore, no evidence for any difference in FACIT-F scores was found between the intervention and control groups in both the ≤8-week and >8-week subgroups (all P > 0.05).
Meta-regression and sensitivity analyses
Univariate and multivariate meta-regression analyses were used to explore possible sources of heterogeneity among studies. The results showed that no factor could explain the source of heterogeneity (as shown in Table 2). Sensitivity analysis was performed to assess the influence of each individual study on the pooled SMD by omitting individual studies. The analysis results suggested that no individual studies significantly affected the pooled SMD, which indicated statistically robust results (Fig. 6).
Publication bias evaluation
Begger’s funnel plots and Egger’s linear regression test were used to assess potential publication bias in the included studies. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Fig. 7). Egger’s test also did not display strong statistical evidence for publication bias (RPFS: t = −1.13, P = 0.320; FACIT-F: t = −1.43, P = 0.225).
Discussion
CRF is one of the most common and stressful side effects affecting up to 70 to 100 % of cancer patients undergoing treatments such as chemotherapy and radiation therapy [34, 35]. Theoretically, CRF occurs in connection with oncological disease, characterized by feelings of fatigue and lack of energy, and even after successful primary treatment of the underlying disease, as an independent event in impairment of the health and QOL as well as the ability to work, can lead to vocational disability [36]. Due to the improved management of the previous dominant side effects such as pain, nausea, and vomiting, CRF is increasingly identified as the most burdensome and distinct cancer-related symptom [37]. Nowadays, the exact mechanisms involved in CRF pathophysiology are poorly understood owing to its characteristics as a complex and multifactorial phenomenon which could be induced by a variety of causes and contributing factors [38–40]. Breast cancer is one of the most malignant female death-related cancers worldwide [1]. New effective chemotherapy, hormone therapy, and biological agents are used to integrate systemic therapy, surgery, and radiation therapy for better treatment of breast cancer [3]. However, it is reported in the studies that up to 99 % of breast cancer patients experience some level of fatigue during the course of radiation therapy and chemotherapy, and more than 60 % rate the level of their fatigue as moderate to severe [41]. Evidence has accumulated indicating that CRF may last for months or even years after completion of breast cancer treatment, particularly among patients who have received adjuvant chemotherapy [42]. Previous studies have reported that various methods such as relaxation therapy, group psychotherapy, sleep, and physical exercise have been adopted to effectively manage the influences resulting from the CRF in patients with breast cancer [43–45]. Recent studies have revealed that physical exercise is safe, feasible, and well tolerated by breast cancer patients and owns beneficial effects on improving their QOL, body composition, fitness, and diminishing the side effects of therapy including symptoms of CRF [10]. Among them, aerobic exercise seems to be a preferred intervention to CRF. Aerobic exercise is strongly associated with improved pulmonary function, cardiovascular fitness, and self-esteem, helping patients reduce the feelings of anxiety and depression and facilitating all aspects of health that are typically diminished in radiotherapy patients, to recover to a relatively stable level [33]. Mock et al. reported that adherence to a home-based moderate-intensity walking exercise program could mitigate high levels of CRF in treatment of breast cancer [46]. Nevertheless, there are few clinical studies to examine the clinical values of aerobic exercise on CRF for breast cancer. Therefore, our meta-analysis aims to provide insight into the effects of aerobic exercise on CRF in breast cancer patients undergoing chemotherapy.
In the present meta-analysis, twelve clinical comparative studies were included with a total of 522 patients in the aerobic exercise group and 492 patients in the usual care group. When all the eligible studies were pooled into the meta-analysis, the results showed that the RPFS scores of breast cancer patients in the aerobic exercise group were significantly lower than those in the usual care group, which indicated that aerobic exercise may improve cancer-related fatigue in breast cancer patients receiving chemotherapy. However, there was no significant difference in FACIT-F scores between the aerobic exercise and usual care groups. One possible reason for these results could be the difficulty to determine what frequency, intensity, and duration may be most effective and safe in improving CRF for breast cancer patients, thereby possibly explaining inter-individual difference in improving CRF [47, 48]. In the subgroup analysis by ethnicity , it indicated that there were significant differences in RPFS and FACIT-F scores between the aerobic exercise and usual care groups among Asian populations, but not among Caucasian populations. A possible reason for the ethnic difference could be that large differences in aerobic exercise which improve CRF are mostly due to cultural factors and natural selection. Previous studies have reported that various methods such as relaxation therapy, group psychotherapy, sleep, and physical exercise have been adopted to effectively manage the influences resulting from the CRF in patients with breast cancer. Our findings are partially consistent with the previous studies, suggesting that aerobic exercise may improve CRF in breast cancer patients receiving chemotherapy.
In interpreting our current meta-analysis results, some limitations need to be addressed. First, the sample size is still relatively small and may not provide sufficient statistical power to estimate the effects of aerobic exercise on CRF in breast cancer patients receiving chemotherapy. Therefore, more studies with larger sample size are needed to accurately provide a more representative statistical analysis. Second, as a retrospective study, a meta-analysis may encounter recall or selection bias, possibly influencing the reliability of our results [49]. Third, our lack of access to the original data from the included studies limited further evaluation of potential effects of aerobic exercise on QOL and clinical outcomes of breast cancer patients receiving chemotherapy. Finally, although all included studies were well defined with similar inclusion criteria, there may be other potential factors that were not taken into account that might have influenced our results.
In conclusion, this meta-analysis provides strong evidence that aerobic exercise may improve CRF in breast cancer patients receiving chemotherapy, especially among Asian populations. Based on the limitations mentioned above, detailed studies are still needed to confirm our findings. Further studies are still needed to warrant and validate the effects of aerobic exercise on QOL and clinical outcomes of breast cancer patients receiving chemotherapy.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22.
Truong PT, Olivotto IA, Whelan TJ, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. CMAJ. 2004;170(8):1263–73.
Grimison PS, Stockler MR. Quality of life and adjuvant systemic therapy for early-stage breast cancer. Expert Rev Anticancer Ther. 2007;7(8):1123–34.
Courneya KS, Mackey JR, McKenzie DC. Exercise for breast cancer survivors: research evidence and clinical guidelines. Phys Sportsmed. 2002;30(8):33–42.
Short CE, James EL, Girgis A, McElduff P, Plotnikoff RC. Move more for life: the protocol for a randomised efficacy trial of a tailored-print physical activity intervention for post-treatment breast cancer survivors. BMC Cancer. 2012;12:172.
Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70.
Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–42.
McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–404.
Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–9.
Courneya KS, Vallance JK, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51(3):249–61.
Goodwin P, Esplen MJ, Butler K, Winocur J, Pritchard K, Brazel S, et al. Multidisciplinary weight management in locoregional breast cancer: results of a phase ii study. Breast Cancer Res Treat. 1998;48(1):53–64.
Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008(2):CD006145.
Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42(8):636–47.
Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108(2):343–8.
Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Zintzaras E, Ioannidis JP. Hegesma: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3.
Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–5.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–80.
Xu Y. Intervention of aerobic exercise on the cancer-related fatigue in breast cancer patients undergoing chemotherapy in outpatient department. Hainan Med J. 2012;23(19):145–7.
Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs. 2011;34(2):E1–E13.
Yao MC, Liu ZP. Effects of aerobic exercises on post-chemotherapy fatigue in patients with breast cancer. Today Nurse. 2010;7:95–6.
Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol. 2009;7(5):158–67.
Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18(4):360–8.
Zhao J, Liu HP, Chen JL, Liu Y. The effect of home-based aerobic exercise on breast cancer patients with cancer-related fatigue in outpatient clinics receiving chemotherapy. Chin J Nursing. 2008;43(7):585–8.
Yuen HK, Sword D. Home-based exercise to alleviate fatigue and improve functional capacity among breast cancer survivors. J Allied Health. 2007;36(4):e257–75.
Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517.
Moadel AB, Shah C, Wylie-Rosett J, Harris MS, Patel SR, Hall CB, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387–95.
Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–21.
Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur J Oncol Nurs. 2005;9(1):56–63.
Smets EM, Visser MR, Willems-Groot AF, Garssen B, Oldenburger F, van Tienhoven G, et al. Fatigue and radiotherapy: (a) experience in patients undergoing treatment. Br J Cancer. 1998;78(7):899–906.
Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18(4):233–42.
Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park). 2000;14(11A):151–61.
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the fatigue coalition. Oncologist. 2000;5(5):353–60.
Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–50.
Gutstein HB. The biologic basis of fatigue. Cancer. 2001;92(6 Suppl):1678–83.
Morrow GR, Hickok JT, Andrews PL, Stern RM. Reduction in serum cortisol after platinum based chemotherapy for cancer: a role for the hpa axis in treatment-related nausea? Psychophysiology. 2002;39(4):491–5.
Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17(5):367–78.
Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21(1):1–18.
Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000.
Decker TW, Cline-Elsen J, Gallagher M. Relaxation therapy as an adjunct in radiation oncology. J Clin Psychol. 1992;48(3):388–93.
Forester B, Kornfeld DS, Fleiss JL, Thompson S. Group psychotherapy during radiotherapy: effects on emotional and physical distress. Am J Psychiatry. 1993;150(11):1700–6.
Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14(6):464–77.
Butt Z, Lai JS, Rao D, Heinemann AW, Bill A, Cella D. Measurement of fatigue in cancer, stroke, and HIV using the Functional Assessment of Chronic Illness Therapy-Fatigue (Facit-F) scale. J Psychosom Res. 2013;74(1):64–8.
Carayol M, Bernard P, Boiche J, Riou F, Mercier B, Cousson-Gelie F, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed? Ann Oncol. 2013;24(2):291–300.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (Moose) group. JAMA. 2000;283(15):2008–12.
Acknowledgments
This was funded by the Department of Science and Technology Project of Liaoning Province (2013225305). All authors read and approved the final manuscript. We would like to acknowledge the helpful comments on this paper received from our reviewers.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, LY., Yang, L., He, XL. et al. Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Tumor Biol. 35, 5659–5667 (2014). https://doi.org/10.1007/s13277-014-1749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-1749-8