Abstract
Recently, telomerase-targeted therapy was studied intensively; however, many studies have verified the existence of alternative lengthening mechanisms of telomeres in vitro. In the present work, we explored the expression characteristic of the two kinds of telomere-prolonging mechanisms in the breast cancer tissues per se. Furthermore, we studied the relationship between Her-2 expression and ALT pathway. Ninety samples of breast cancer tissues were examined in this research. RT-PCR was used for the detection of the expression of human telomerase reverse transcriptase (hTERT); IHC was used for the detection of the expression of promyelocytic leukemia body (PML) bodies; the co-expression of PML bodies and hTERT was detected using the QDs-based immunofluorescence. The co-expression of PML body and hTERT was found in the same cell in breast cancer tissues, and ten samples expressed neither PML bodies nor hTERT. Additionally, the expression of PML bodies and Her-2 was statistically co-related (P = 0.047). The two kinds of mechanisms of telomere extension can co-exist in the same cell in beast cancer tissues, and there may be other mechanisms of telomere extension functioning in human breast carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a kind of malignant tumor which occurs more frequently in women and seriously threatens the quality of women's lives [1, 2]. The death rate caused by breast cancer was in the first place of the female cancer incidence. More and more attention focuses on the targeted therapy of breast cancer, and the telomerase-targeted therapy becomes a hot spot.
It had been reported that telomerase activity can be detected in more than 90 % of breast cancer cases, and telomerase-targeted therapies became a hot topic [3]. However, researchers had found an alternative pathway (alternative lengthening of telomeres, ALT) which can be detected in about 10 % of breast cancer besides telomerase activity [4].
Some researches confirmed that the telomerase pathway and telomere alternative pathway worked synergically to lengthen telomeres in vitro [5]; however, mutual inhibition was not observed between two pathways. Our hypothesis was to find out that whether these two mechanisms exist simultaneously in the breast cancer tissues.
It was generally agreed in recent researches that ALT-related human leukemia promyelocytic bodies (PML) were the place where telomeres were lengthened in the mode of telomere recombination in telomere alternative pathway, and PML bodies, expressed or not, may represent if the telomere alternative pathway was active or not. Based on the evidence mentioned above, the expression of ALT-related PML bodies was designed to represent the telomere alternative mechanisms in this experiment.
Materials and methods
Patients and tumor specimens
Tissue samples of 90 patients who underwent surgical resections from January 2009 to December 2010 were obtained from Zhongnan Hospital (Wuhan, Hubei, China). The data of patient's characteristics were displayed in Table 1. Histological grade was scored using the Nottingham system. Estrogen receptor (ER), progesterone receptor (PR), and Her-2 statuses were determined on the basis of immunohistochemical (IHC) staining. Hormone receptors (ER and PR) were considered positive if at least 10 % of tumor cells nuclei were stained. Tumors were considered Her-2 positive if they were scored as 3+ using IHC.
Ethical issues
The ethical issues involved in the study were applied to ethics committee of Zhongnan Hospital, Wuhan University, Wuhan, China and were approved; informed consent of the patients was taken in the form without signature.
Antibody
PML monoclonal antibody (PG-M3, sc-966, 200 μg/ml) was purchased from Santa Cruz Company; human telomerase reverse transcriptase (hTERT) monoclonal antibody (ab32020, 100 μl) was purchased from Abcam Company.
Immunohistochemical assay
All frozen tissue samples were fixed, dehydrated, dipped, and wax-embedded into paraffin blocks. The paraffin was sliced to about 4 μm. Immunohistochemistry was carried out by streptavidin–biotin complex method. Antigen retrieval was performed with a steamer for 15 min. Then the sections were blocked with 5–10 % normal goat serum; 10 min later, the sections were incubated with first antibodies for 1–2 h at 37 °C. After, the sections were incubated with biotinylated secondary antibody (1:800) for 10–30 min in 37 °C. Then sections were incubated with streptavidin–alkaline phosphatase for 10–30 min in 37 °C. Using 3,3′-diaminobenzidine (DAB) as the chromogenic agent, the section was developed for 3–10 min then washed with distilled water for 3–5 min; hematoxylin counter staining and dehydration were done, then the samples were cleared and sealed. Phosphate-buffered solution (PBS) was used to wash the sections for 5 min per step for three times.
IHC staining was scored according to the following criteria: three fields were randomly selected at high magnification (×400) of each slice; 100 tumor cells were counted in each field; when the number of positive cells is less than 20 %, the sample was consider to be negative; on the opposite, when the number of positive cells is more than 20 %, the sample was positive.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from frozen tissue samples; RNA isolation was performed using a two-step process; the total RNA was reversely transcribed in a 25-μl reaction mixture containing 8.5 μl 5× of RT buffer, 2 μl of RT Enzyme Mix, 2 μl of Primer Mix, 12.5 μl of nuclease-free water at 42 °C for 1 h. cDNA was amplified using specific primers (β-actin: 5′-CGTACCACTGGCATCGTGAT-3′,5′-GTGTTGGCGTACAGGTCTTTG-3′, 462 bp; hTERT: 5′-CAGCTCCCATTTCATCAGCAA-3′,5′-GCGACATCCCTGCGTTCTT-3′, 103 bp). The PCR mixture was first denatured at 94 °C for 2 min then amplified for 30 cycles (hTERT: 94 °C, 30 s; 57 °C, 30 s; 72 °C, 1 min; β-actin: 94 °C, 30 s; 60 °C, 30 s; 72 °C, 1 min) using an authorized thermal cycler (Eppendorf, Hamburg, Germany). After amplification, 10 μl of each PCR product and 2 μl of 6× loading buffer were mixed and electrophoresed on 1.5 % agarose gel in 0.5× Tris-boric acid–EDTA containing 0.5 μg/ml ethidium bromide. The gels were scanned and photographed.
Quantum dots double-label immunofluorescence
All frozen tissue specimens were fixed, dehydrated, dipped, and wax-embedded into paraffin blocks. The paraffin was sliced to about 3 μm, and the slices were incubated with 3 % H2O2 at room temperature for 5–10 min.
For the formation of hTERT–primary antibody–biotinylated secondary antibody–QDs545-SA complex, antigen retrieval was performed with steamer for 15 min. Then the sections were blocked with 5–10 % normal goat serum for 30 min then were washed in TBS twice, then the sections were incubated with first antibodies diluted with TBS overnight at 4 °C. After, Tris-buffered saline and Tween 20 (TBS-T) was used to wash the sections three times for 3 min, then the sections were blocked with 5–10 % normal goat serum for 10 min at 37 °C. The sections were then incubated with biotinylated secondary antibody (1:800) for 30 min at 37 °C. TBS-T was used to wash the sections at 3 min for three times, then blocked with 5–10 % normal goat serum for 20 min at 37 °C. Then sections were incubated with QDs545-SA (1:200) for 30 min at 37 °C. After, TBS-T was used to wash the sections twice for 3 min and then TBS to wash the sections twice for 3 min. Then the sections were mounted with glycerol carbonate buffer.
The PML bodies were connected to QDs605-SA using the similar method.
Because of the different emission wavelength of the two kinds of quantum dots, QDs545 and QDs605, when stimulated, a green and red fluorescence was seen. hTERT which was connected to QDs545 was seen in the green light when stimulated and the PML bodies which were connected to QDs605 were seen in the red light when stimulated. The OLYMPUS BX-51 inverted fluorescence microscope was used to observe the expression of the two complexes in tumor tissues. Three fields were randomly selected at high magnification (×400) of each slice; 100 tumor cells were counted in each field. When the number of positive cells is less than 20 %, the sample was consider to be negative; on the opposite, when the number of positive cells is more than 20 %, the sample was positive [6].
Statistical analysis
The associations between PML bodies, hTERT expression levels, and clinicopathological parameters were evaluated using Chi-squared test. The level of significance was set at P < 0.05. All statistical tests were performed using the Software Package SPSS, Version 20.0, Chicago, IL, USA.
Results
IHC for the detection of expression of PML bodies
The expression of ALT-related PML bodies was detected by IHC, and the results showed that ten samples were positive for PML bodies as displayed in Fig. 1a.
a The expression of PML bodies. IHC was used for the detection; the negative control was achieved using PBS instead of first antibody, and the cells in the breast carcinoma nests were blue in cell nucleus (a, ×200), the cells in the breast carcinoma nests were blue in cell nucleus in the negative expression of PML bodies (b, ×400), the cells in the breast carcinoma nests were brown in cell nucleus in the positive expression of PML bodies (c, ×200; b, ×400) b The co-expression of hTERT and PML bodies. QDs immunofluorescence was used for the detection. The cells in the breast carcinoma nests were green in the cell nucleus in the positive expression of hTERT (a, ×400). The cells in the breast carcinoma nests were green and red in cell nucleus in the co-expression of PML bodies and hTERT (b, ×400)
QDs immunofluorescence for the detection of co-expression of PML bodies and hTERT
The two different sizes of quantum dots, QDs605 and QDs545, were used to label the PML bodies and hTERT, respectively. Based on the different optical properties, these two quantum dots emit green or red fluorescence respectively after being excited without affecting each other. Thus, the quantum dots can be used to label and detect different antigens in the same tissue sample; in this experiment, quantum dot QDs605 was used to mark hTERT and the green light could be seen in the positive expression of hTERT, quantum dot QDs545 was used to mark PML and the red light could be seen in the positive expression of the PML bodies. With blue background color, the co-expression of hTERT and PML bodies was displayed in Fig. 1b, and the co-expression of PML bodies and hTERT was also seen in the same cell. The expression data of PML bodies and hTERT were shown in Table 2.
RT-PCR for the detection of expression of hTERT
The expression characteristic of telomerase subunit hTERT in the breast tissue samples was detected by RT-PCR. The results showed that 80 samples had hTERT mRNA expression as shown in Fig. 2.
a The expression of β-actin. The bands in the agarose gel electrophoresis of the first sample hole was the DNA marker, and the four bands were successive on behalf of 750, 500, 250, and 100 bp, and the other sample holes were β-actin, and the bands were located at the band around the 100 bp. b The expression of hTERT mRNA. The bands in the agarose gel electrophoresis of the first sample hole was the DNA marker, and the four bands were successive on behalf of 750, 500, 250, and 100 bp, and the other sample holes were hTERT mRNA, and the bands were located at the band between the 250 and the 500 bp
PML bodies expression, hTERT expression, and the clinical features of breast cancer patients
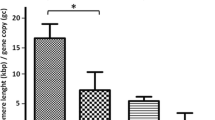
After cross-checking with the clinical stage, tumor size, nodal status of 90 cases of breast cancer tissues, and expression of PML bodies or hTERT, statistical analysis results showed that the expression of PML bodies was positively related with the histology (P = 0.002) and tumor size (P = 0.035). Moreover, the PML bodies' expression and the clinical and pathological data of patients with breast cancer were shown in Table 3.
Her-2-positive phenotype was considered as a poor prognosis factor for breast cancer, and the co-relations between PML bodies and Her-2 expression were investigated. In 90 cases of breast cancer tissues, with 29 Her-2-positive cases, 6 cases were both positive for PML bodies and Her-2. The statistical analysis showed that they were positively correlated (P = 0.047). The expression data of PML bodies and Her-2 were shown in Table 3.
Since ER and PR were all breast cancer prognosis-related factors, this experiment also explored the correlations of the expression of ER, PR, and PML bodies. Despite that there were 45 positive cases for estrogen receptor and 26 positive cases for progesterone receptors in 90 breast cancer tissues, statistical analysis showed no correlation. The expression data of PML bodies and ER and PR were shown in Table 3.
Discussion
Telomeres are specialized structures that comprised extended arrays of repeated G- and C-rich hexanucleotides and their binding proteins which are located at the ends of eukaryotic chromosomes [7]. They play important roles in protecting the chromosome ends from recombination, fusion, and degradation, and regulating cellular lifespan. In general, telomeres shorten alongside the cell when it divides; after 50–70 division cycles (a number known as the Hayflick limit, after its discoverer), a chromosome cannot grow shorter and the cell where it dwells in can no longer divide. Thus, the cell begins to age, followed by death [8]. Telomerase is a large ribonucleoprotein complex responsible for the maintenance of telomeres and continuous proliferation of germ line, stem, and neoplastic cells [9]. Telomerase is composed of the human telomerase RNA (hTR), the telomerase-associated protein 1 (TP1), and the human telomerase reverse transcriptase (hTERT) [10]; meanwhile, telomerase activity was detectable in more than 85 % of the malignant cells [11]. Telomerase was activated to extend the telomere when the length of telomere reached the H-Flick limit, so the cells continue to split and avoid apoptosis. Despite of this, telomerase activity was not detectable in 10–15 % of the malignant tumors, the length of telomeres was increased by an alternative mechanism [12], and ALT-associated PML bodies play an important role in the pathway.
The mature PML bodies were composed of sumoylated PML and were spherical. PML formed the outer shell of the bodies and their partners were usually inside. The internal region was the functional area, and lots of SUMO proteins were recruited to the mature PML bodies to be functional, including DAXX and SP100 [13, 14]. The mature PML bodies acted as a platform to extend telomere in the mode of recombination in the ALT pathway [15]. The restructuring-related proteins, telomere, as well as DNA damage repair proteins were recruited and played key roles in ALT pathway. Another study [16] showed that the formation of PML body depended on the process mediated by telomere via ALT pathway, which substantiated PML bodies as an important characteristic and participants of the ALT pathway as well.
In addition, the ALT pathway can be activated when the telomerase activity was inhibited in telomerase-positive cells. Some researchers also found that the telomeres in some cells which preliminarily were extended by telomerase can still be extended when the telomerase activity was inhibited, and telomere alternative mechanism was detectable in the cells, under which circumstances the cells survived because of the ALT pathway to avoid apopsis [17].
The expression characteristic of PML bodies of 90 cases of breast cancer tissues in this experiment was detected by immunohistochemical assay, and the expression characteristic of telomerase subunit hTERT was detected by RT-PCR. Ten cases exhibited the expression of PML bodies, and 80 cases exhibited hTERT expression; 10 cases were double positive for PML bodies and hTERT, no case was single positive for PML body, 70 cases showed single-positive hTERT expression, and 10 cases were double negative for hTERT and PML body.
To explore the expression characteristic of the ten double-positive cases, double immunofluorescence-labeled quantum dots were used, and the results showed that the PML bodies and hTERT co-expression in ten samples of breast cancer tissues could be detected in the same cells, suggesting that these two telomere elongation pathways can co-exist in the same cell in human breast cancer tissues. For the first time, the co-expression of hTERT and PML bodies were shown in one cell in breast cancer tissues intuitively and clearly, and the results were consistent with the Cerone's research which was carried out in cultured cells in vitro [5]. Based on these findings, the inhibition of telomerase activity alone in targeted therapy of cancer treatment may not be able to inhibit the tumor growth, and the drugs designed to inhibit telomerase activity must be against both telomerase and telomere alternative pathways.
The expression of hTERT and PML bodies was not detectable in ten cases, suggesting that there may be other pathways to maintain telomere length in malignant tumors, in addition to telomerase and telomere alternative pathways. Some researchers had reported that other telomere length-maintaining mechanisms named palindrome-dependent mechanism (PAL) [18], and inherited the long telomere mechanism (ILT) [19] in yeast. These two different mechanisms of telomere extension still remained elusive and required further study, for it was uncertain that two mechanisms exist in human tissues or telomere can rely on these two mechanisms to immortalize.
It is known that the expression levels of estrogen receptor and progesterone receptor were very critical in choosing treatment strategies for breast cancer patients. The correlations among the estrogen and progesterone receptors, PML bodies, and hTERT were analyzed in this study. However, there was no statistical relation, suggesting that the expression of PML body was not connected with the expression of estrogen and progesterone receptors. However, due to the insufficient number of cases involved in this study, further study is needed to confirm whether there was a negative correlation between them.
The expression levels of Her-2 were also very important in choosing treatment strategies for breast cancer patients. The correlations between Her-2 and PML bodies and hTERT were also analyzed in the present study. The statistical analysis showed there was a relation between the expression of PML bodies and Her-2 (P = 0.047), which is supported by another research in which Subhawong [20] reported. The ALT phenotype was identified in 3 out of 21 Her-2-positive cases, but none in the other 50 cases (P = 0.023). The correlation of the ALT phenotype with Her-2 positivity suggests that Her-2 expression is significantly correlated with the PML bodies; however, further comprehensive study is required to verify whether there was a positive correlation between them.
References
Chen W-q, Zheng R-s, Zeng H-m, Zhang S-w, Li G-l, Wu L-y, et al. Incidence and mortality of breast cancer in China, 2008. Thorac Cancer. 2013;4:59–65.
Zeng H, Zheng R, Zhang S, Zou X, Chen W. Incidence and mortality of female breast cancer in China, 2009. Thoracic Cancer. 2013;4:400–4.
Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91.
Reddel RR, Bryan TM, Colgin LM, Perrem KT, Yeager TR. Alternative lengthening of telomeres in human cells. Radiat Res. 2001;155:194–200.
Cerone MA, Londono-Vallejo JA, Bacchetti S. Telomere maintenance by telomerase and by recombination can coexist in human cells. Hum Mol Genet. 2001;10:1945–52.
Fan Y, Yao Y, Li L, Wu Z, Xu F, Hou M, et al. Nm23-h1 gene driven by htert promoter induces inhibition of invasive phenotype and metastasis of lung cancer xenograft in mice. Thoracic Cancer. 2013;4:41–52.
Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73.
Rubin H. The disparity between human cell senescence in vitro and lifelong replication in vivo. Nat Biotechnol. 2002;20:675–81.
Gehmert S, Gehmert S, Prantl L, Vykoukal J, Alt E, Song YH. Breast cancer cells attract the migration of adipose tissue-derived stem cells via the pdgf-bb/pdgfr-beta signaling pathway. Biochem Biophys Res Commun. 2010;398:601–5.
Hayflick L. The establishment of a line (wish) of human amnion cells in continuous cultivation. Exp Cell Res. 1961;23:14–20.
Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10–8.
Henson JD, Hannay JA, McCarthy SW, Royds JA, Yeager TR, Robinson RA, et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer Res :Off J Am Assoc Cancer Res. 2005;11:217–25.
Mukhopadhyay D, Matunis MJ. Summoning daxx-mediated repression. Mol Cell. 2011;42:4–5.
Lallemand-Breitenbach V, de The H. Pml nuclear bodies. Cold Spring Harbor Perspectives in Biology. 2010;2:a000661.
Draskovic I, Arnoult N, Steiner V, Bacchetti S, Lomonte P, Londono-Vallejo A. Probing pml body function in alt cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci U S A. 2009;106:15726–31.
Brouwer AK, Schimmel J, Wiegant JC, Vertegaal AC, Tanke HJ, Dirks RW. Telomeric DNA mediates de novo pml body formation. Mol Biol Cell. 2009;20:4804–15.
Grobelny JV, Kulp-McEliece M, Broccoli D. Effects of reconstitution of telomerase activity on telomere maintenance by the alternative lengthening of telomeres (alt) pathway. Hum Mol Genet. 2001;10:1953–61.
Maringele L, Lydall D. Telomerase- and recombination-independent immortalization of budding yeast. Genes Dev. 2004;18:2663–75.
Grandin N, Charbonneau M. Telomerase- and rad52-independent immortalization of budding yeast by an inherited-long-telomere pathway of telomeric repeat amplification. Mol Cell Biol. 2009;29:965–85.
Subhawong AP, Heaphy CM, Argani P, Konishi Y, Kouprina N, Nassar H, et al. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with her-2 overexpression. Mod Pathol Off J US Can Acad Pathol Inc. 2009;22:1423–31.
Acknowledgements
This work was supported in part by the National Nature Science Foundation (No.81102024 and No.81372407) and the Nature Science Foundation of Hubei Province (No.2013CFB234).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bin Xu and Min Peng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xu, B., Peng, M. & Song, Q. The co-expression of telomerase and ALT pathway in human breast cancer tissues. Tumor Biol. 35, 4087–4093 (2014). https://doi.org/10.1007/s13277-013-1534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1534-0