Abstract
Dysregulation of hedgehog signaling has been involved in esophageal squamous cell carcinoma (ESCC) by the mechanisms that are not fully understood. The receptor for activated protein kinase C (RACK1) is involved in the progression of multiple cancers. However, its expression and function in ESCC have not been investigated. Here, we found that the expression of RACK1 was upregulated in ESCC clinical samples. Moreover, over-expression of RACK1 in ESCC cells promoted cell proliferation and migration, while downregulation of RACK1 impaired the proliferation and migration of ESCC cells in vitro and in vivo. Mechanistically, RACK1 promoted the proliferation and migration of ESCC cells by activating hedgehog signaling. Taken together, our study suggested RACK1 might be an important therapeutic target in ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal carcinoma is one of the most frequent causes of cancer-related death in the world [1]. In Asian countries, esophageal squamous cell carcinoma (ESCC) accounts for 90 % of the esophageal carcinomas. Although advance has been achieved in surgical techniques and perioperative management, the prognosis remains poor. Therefore, it is very urgent to find molecular therapeutic targets for ESCC treatment.
The hedgehog (Hh) signaling is involved in embryonic development, cell proliferation and carcinogenesis [2]. The binding of hedgehog protein to its receptor human patched 1 homologue (PTCH1) induces the activation of smoothened (Smo), which activates the transcriptional activity of Gli1 [3]. Activation of hedgehog signaling has been reported in various cancers [4]. The components and the target genes of hedgehog pathway were found in gastrointestinal cancer, prostate cancer and pancreatic cancer [5, 6]. Previous reports have showed the expression of sonic hedgehog (Shh) and its target genes was elevated in several esophageal cancer cell lines and clinical samples and activation of hedgehog signaling was associated with poor prognosis [7]. However, the molecular mechanism for the activation of hedgehog signaling in ESCC remains largely unknown.
Receptor for activated protein kinase C (RACK1) is an adaptor protein with seven WD40 repeats [8]. Through binding a large number of signaling proteins [9–11], RACK1 has been involved in multiple intracellular signal pathways. With regard to its role in carcinogenesis, RACK1 coordinated cell growth and movement [12, 13]. However, some contradictory results existed in its oncogenic property [8–12]. The function of RACK1 in ESCC has not been explored.
In this study, we found that the expression of RACK1 was upregulated in the ESCC clinical samples. Overexpression of RACK1 in the ESCC cells significantly promoted cell growth and migration, while knockdown the expression of RACK1 inhibited the growth and migration of ESCC cells in vitro and in vivo. Mechanically, it showed that RACK1 activated hedgehog signaling in ESCC cells. Taken together, our study revealed the oncogenic role of RACK1 in ESCC and RACK1 might be an important therapeutic target in ESCC.
Materials and methods
Primary ESCC samples
Primary tissues were collected from patients who received surgery for ESCC at Xinhua Hospital, Shanghai Jiao Tong University. All patients had given informed consent. Dissected samples were frozen immediately after surgery and stored at −80 °C until needed.
Immunohistochemistry
The 5-μm-thick consecutive sections of ESCC tissues and paired normal tissues were cut and mounted on glass slides. After deparaffinizing, rehydrating, antigen retrieval and blocking endogenous peroxidases, the sections were washed thrice in 0.01 mol/l PBS (8 mmol/l Na2HPO4, 2 mmol/l NaH2PO4 and 150 mmol/l NaCl) for 5 min each, blocked for 1 h in 0.01 mol/l PBS supplemented with 0.3 % Triton X-100 and 5 % normal goat serum, followed by addition of anti-RACK1 (1:300) antibody at 4 °C overnight. After brief washes in 0.01 mol/l PBS, sections were exposed for 2 h to 0.01 mol/l PBS containing horseradish peroxidase-conjugated mouse anti-mouse IgG (1:1000), followed by development with 0.003 % H2O2 and 0.03 % 3,30-diaminobenzidine in 0.05 mol/l Tris–HCl (pH 7.6). Immunohistochemistry for each sample was performed at least three times, and all sections were counterstained with hematoxylin.
Cell culture
Immortalized esophageal epithelial cell line SHEE and ESCC cell lines KYSE180, Eca109 and Caes17 were obtained from ATCC (American Typical Culture Center) and cultured in DMEM medium (Invitrogen) with 10 % fetal bovine serum, 10 units/ml penicillin-G, and 10 mg/ml streptomycin. Cells were incubated at 37 °C in 5 % CO2 humidified air.
Western blot analysis
Western blot analysis was performed as previously described [12]. Primary antibodies to RACK1 and Patch were purchased from Santa Cruz Biotechnology, and antibodies to GAPDH and Gli1 were purchased from Sigma. Rabbit anti-mouse IgG (Sigma) and goat anti-rabbit IgG (Cell Signaling Technology) were used at a dilution of 1:1500. Primary antibodies were diluted in TBST containing 1 % BSA and NaN3. The immunoreactive protein bands were visualized by ECL kit (Pierce).
RNAi-mediated knockdown of RACK1
Target sequence for RACK1 small interfering RNA was as listed: 5’-CTACTACCCCGCAGTTCC-3’. The control nucleotide sequence of small interfering RNA was 5’-CTACATAGCCCCGTCCCT-3’, which was the random sequence that was not related to RACK1 mRNA. In our experiments, pSIREN-RetroQ vector, was used to produce small, double-stranded RNA (siRNA) to inhibit target gene expression in ESCC cells. To construct the hairpin siRNA expression cassette, complementary DNA oligonucleotides for siRNA of RACK1 (si RACK1) or mutated sequence of RACK1 siRNA as control (si con) were synthesized, annealed and inserted into pSIREN-RetroQ according to the manufacturer’s protocol. pSIREN-RetroQ vector with si RACK1 or si con was transfected into HEK293T cells, and the virus was harvested from culture medium. The harvested virus was purified and appropriate amounts of virus were used to infect ESCC cells. After 3 days of infection, cells were selected with puromycine. The puromycine resistant cells were pooled and examined the expression of RACK1.
Cell migration assay
Boyden chamber (8 μm pore size polycarbonate membrane) was obtained from Neuroprobe Corporation, Bethesda, MD, USA. Cells (2 × 105) in 0.05 ml medium containing 1 % FBS were placed in the upper chamber, and the lower chamber was loaded with 0.152 ml medium containing 10 % FBS. After the incubation for 12 h, cells migrated to the lower surface of filters were detected with traditional H&E staining, and five fields of each well were counted. Three wells were examined for each cell type, and the experiments were repeated for at least three times.
Crystal violet assay
For cell growth assay, equal number of control cells and experimental cells were seeded in 6-well plates and cultured in medium supplemented with 10 % FBS for 7 days. Medium was changed every other day. Cell growth was stopped after 7 days in culture by removing the medium and adding 0.5 % crystal violet solution in 20 % methanol. After staining for 5 min, the fixed cells were washed with phosphate-buffered saline (PBS), photographed and dissolved with 1 % SDS. The absorbance at 600 nm was evaluated using a micro-plate reader.
MTT assay
For cell growth analysis, equal numbers of cells were seeded in 48-well plates and cultured for various durations. Cell numbers were measured by MTT assay according to the manufacturer’s protocol (Roche Applied Science).
In vivo metastasis assay
The Eca109-Luc stable cell line (overexpressing luciferase) was established with G418 selection. Luciferase expression was determined using luciferin (Xenogen) and an in vivo imaging system (Xenogen). The luciferase-expressing Eca109/sicon cells and luciferase-expressing Eca109/siRACK1 cells (1 × 105 cells in 100 μl PBS) were injected into the left ventricle of the nude mice. The metastasis lesions were monitored every week. Before mice were anesthetized with Forane (Abbott), the aqueous solution of luciferin (200 mg/kg intraperitoneally) was injected 5 min before imaging. The animals were placed into a light-tight chamber of the CCD camera system (Xenogen), and the photons emitted from the luciferase expressing cells within the animal were quantified for 1 min, using the software program Living Image (Xenogen) as an overlay on Igor (Wavemetrics).
Results
The expression of RACK1 was upregulated in ESCC
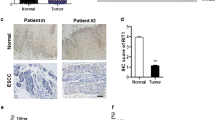
In the screening for the differentially expressed genes between the ESCC samples and paired normal tissues, RACK1 was found to be up-regulated in ESCC tissues compared with the normal tissues (data not shown). In order to investigate the expression pattern of RACK1 in ESCC samples, the mRNA level of RACK1 was examined by Real-time PCR analysis in 53 ESCC samples and paired normal tissues. Upregulation of RACK1 mRNA level was observed in ESCC samples compared with the paired normal tissues (Fig. 1a, b). In addition, the results of immunohistochemistry showed the increased protein level of RACK1 in human ESCC tissues (Fig. 1c). Moreover, up-regulation of RACK1 was observed in seven randomly chosen ESCC samples and paired normal tissues (Fig. 1d). Theses results indicated that RACK1 might play an oncogenic role in the progression of ESCC.
Upregulation of RACK1 in ESCC samples. a Relative expression of RACK1 mRNA in human ESCC samples and paired normal esophageal tissues. Semi-quantitative RT-PCR was performed on 53 paired ESCC RNA samples. The RACK1 expression was normalized to that of beta-actin. Data was calculated from triplicates. Each bar was the log2 value of the ratio of RACK1 expression levels between ESCC (T) and matched normal tissues (N) from the same patient. Because log22 = 1, bar value > 1 represents > 2-fold increase (C > N), whereas bar value < −1 represents > 2-fold decrease (C < N). (b) The expression of RACK1 was shown as box plots, with the horizontal lines representing the median; the bottom and top of the boxes representing the 25th and 75th percentiles, respectively; and the vertical bars representing the range of data. We compared the expression of RACK1 in cancer tissues and normal tissues using the t test. n = 53. *P < 0.05. c Immunohistochemical analysis of RACK1 expression in ESCC and matched normal tissues. d The protein level of RACK1 in ESCC samples and paired normal tissues
Forced expression of RACK1 promoted the growth and migration of ESCC cells
To study the roles of RACK1 in the growth of immortalized esophageal epithelia cells and ESCC cells, SHEE cells and KYSE180 cells were stably transfected with either a pcDNA3.1/RACK1 vector containing full length of RACK1 or an empty vector pcDNA3.1 as control. G418-resistant cells were pooled and confirmed the expression of exogenous RACK1 by Western blot analysis (Fig. 2a). The effect of RACK1 on cell growth was evaluated by MTT assay. It was found RACK1 promoted the growth of KYSE180 cells significantly (Fig. 2b). At the same time, we used Boyden Chamber assay to investigate the role of RACK1 in cell migration. We found that RACK1 facilitated the migration of SHEE and KYSE180 cells (Fig. 2c-d). These results indicated the oncogenic role of RACK1 in the progression of ESCC.
RACK1 promoted the growth and migration of the immortalized esophageal epithelial cells and ESCC cells. a Overexpression of RACK1 in SHEE cells and KYSE180 cells. The expression vector of RACK1 was transfected into SHEE cells and KYSE180 cells and the cells were selected with G418. The G418-resistant cells were pooled and examined for the expression of RACK1. b RACK1 promoted the growth of KYSE180 cells by MTT assay. c RACK1 promoted the migration of SHEE cells and KYSE180 cells by Boyden Chamber assay. The images showed representative density of cells that migrated. d Quantification of migrated cells in c. The results represented the mean ± SD. *P < 0.05
Knockdown of RACK1 inhibited the growth and migration of ESCC cells
In order to examine the role of endogenous RACK1 in cell growth and migration, we first knocked down the expression of RACK1 in Eca109 cells and Caes17 cells (Fig. 3a). Knockdown of RACK1 inhibited the growth of Eca109 cells as shown by MTT assay (Fig. 3b), which was consistent with the results shown in Fig. 2b. It has been reported that RACK1 played an important role in the migration of gastric cancer cells [14]. In this study, we investigated the function of RACK1 in the migration of ESCC cells using Boyden Chamber assay. It was found that knock down the expression of RACK1 dramatically inhibited cell migration of Eca109 and Caes17 cells (Fig. 3c−d). These observations indicated the potential function of RACK1 in cancer metastasis.
Knock down of RACK1 inhibited the growth and migration of ESCC cells. a Knock down the expression of RACK1 in Eca109 cells and Caes17 cells. b Knock down of RACK1 inhibited the growth of Eca109 cells by MTT assay. *P < 0.05. c Knock down of RACK1 inhibited the migration of Eca109 cells and Caes17 cells by Boyden Chamber assay. The images showed representative density of cells that migrated. d Quantification of migrated cells in c. The results represented the mean ± SD. **P < 0.01
RACK1 promoted the growth and migration of ESCC cells by activating hedgehog signaling
In order to find out the underlying mechanism responsible for the oncogenic role of RACK1 in ESCC, luciferase assay was used to identify the signal pathways regulated by RACK1 in immortalized esophageal epithelial cells and ESCC cells. We transiently overexpressed RACK1 in SHEE and KYSE180 cells and found that forced expression of RACK1 strongly activated a multimerized Gli1-binding motif (GLIBS)-luciferase reporter, suggesting RACK1 activated the hedgehog signaling in immortalized esophageal epithelial cells and ESCC cells (Fig. 4a). In the next study, we examined whether RACK1 modulated the expression of the target genes regulated by the hedgehog signaling. It was found that RACK1 upregulated the expression of Gli1 and Patch both at the protein level and mRNA level (Fig. 4b), while knockdown the expression of RACK1 greatly decreased the expression of Gli1 and Patch (Fig. 4c). We next explored whether the oncogenic effects of RACK1 were mediated by the hedgehog-Gli1 signaling pathway. It showed that knockdown the expression of Gli1 in KYSE180 cells profoundly reversed the effects of RACK1 on the growth and migration (Fig. 4d-e), and the suppressive effects on cell growth and migration induced by silencing the expression of RACK1 could be rescued by Gli1, suggesting the oncogenic effects of RACK1 were dependent, at least in part, on the hedgehog-Gli1 signaling pathway.
RACK1 promoted the growth and migration of ESCC cells through activating hedgehog signaling. a RACK1 enhanced the transcriptional activity of Gli1 by luciferase assay. *P < 0.05. b, c RACK1 regulated the expression of Gli1 and Patch, two target genes of hedgehog signaling, both at the protein and mRNA level. (d-e) Knock down of Gli1 abolished the effects of RACK1 on the migration and growth of ESCC cells, and the suppressive effects induced by silencing the expression of RACK1 could be rescued by Gli1. *P < 0.05; **P < 0.01
Silencing the expression of RACK1 inhibited the metastasis of ESCC cells in vivo
Our in vitro results suggested that knockdown of RACK1 suppressed the growth and migration of ESCC cells. Therefore, we evaluated whether modulating endogenous RACK1 expression could influence ESCC metastasis in vivo by utilizing a tumor metastasis mouse model. Consistent with our in vitro results, bioluminescent signals appeared in Eca109/sicon cells injected mice in 4 weeks and developed progressively stronger in the following weeks. In contrast, much weaker bioluminescent signals and fewer metastatic lesions were detected in mice who received Eca109/siRACK cells (Fig. 5a-b). Therefore, knockdown of RACK1 suppressed tumor metastasis in vivo.
Knock down of RACK1 inhibited the metastasis of Eca109 cells in vivo. a Monitoring metastasis of bioluminescent Eca109/sicon, Eca109/RACK1 cells. Images were obtained 5 min and 8 weeks after injection. c Mean photon counts of each group of mice were quantified and were displayed over time. Each point represented the mean ± SD. *P < 0.05; **P < 0.01
Discussion
It is known that the development of ESCC includes multiple steps through the progression of precancerous dysplastic lesions to invasive ESCC [15]. Diagnosis of cancer at later stages is a major factor for the mortality of esophageal cancer. Most of ESCC patients are diagnosed in the advanced stages. Thus, identifying gene alterations that drive the carcinogenesis process of esophageal cancer may help design novel strategies to diagnose and treat the disease.
RACK1 has been reported to be dysregulated in several cancer types, including oral squamous carcinoma, colon cancer and breast cancers. The expression of RACK1 was reported to be associated with the pathological stage and other clinical features of the cancer [16–18]. However, the expression pattern and the function of RACK1 in ESCC remained unknown. In this study, we observed that the expression of RACK1 was upregulated in ESCC and promoted the growth and migration of the ESCC cells. With regard to the molecular mechanism, it was found RACK1 promoted the growth and migration of ESCC cells partially through activation of hedgehog signaling.
Activation of hedgehog signaling is very common in the clinical samples of ESCC. Upregulation of hedgehog ligand, mutation of smoothened (Smo) and ligand-independent mechanism contributed to the activation of hedgehog signaling in ESCC [19, 20]. In lung cancer, RACK1 activated hedgehog signaling through activation of Smo[21], suggesting RACK1 activated hedgehog signaling in ESCC in a Smo-dependent manner. It has been reported that inhibition of hedgehog signaling effectively suppressed cell invasion as well as growth of ESCC cells, suggesting hedgehog signaling should be a therapeutic target in ESCC.
In this study, it has been found that RACK1 promoted the growth and migration of ESCC cells through activating hedgehog signaling. Based on the previous studies, tissue-specifically knocked down the expression of RACK1 in ESCC or applied peptide to block the interaction between RACK1 and Smo would inhibit the activation of hedgehog signaling mediated by RACK1. Therefore, RACK1 might be a therapeutic target for ESCC.
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300.
Rubin LL, Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33.
Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–22.
Xie J. Hedgehog signaling pathway: development of antagonists for cancer therapy. Curr Oncol Rep. 2008;10:107–13.
Lee SY, Han HS, Lee KY, et al. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007;17:1051–5.
Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12.
Mori Y, Okumura T, Tsunoda S, Sakai Y, Shimada Y. Gli-1 expression is associated with lymph node metastasis and tumor progression in esophageal squamous cell carcinoma. Oncology. 2006;70:378–89.
Wang Z, Zhang B, Jiang L, et al. RACK1, an excellent predictor for poor clinical outcome in oral squamous carcinoma, similar to Ki67. Eur J Cancer. 2009;45:490–6.
Slager RE, Devasure JM, Pavlik JA, et al. RACK1, a PKC targeting protein, is exclusively localized to basal airway epithelial cells. J Histochem Cytochem. 2008;56:7–14.
Doan AT, Huttenlocher A. RACK1 regulates Src activity and modulates paxillin dynamics during cell migration. Exp Cell Res. 2007;313:2667–79.
Serrels B, Sandilands E, Serrels A, et al. A complex between FAK, RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr Biol. 2010;20:1086–92.
Mamidipudi V, Dhillon NK, Parman T, et al. RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene. 2007;26:2914–24.
Wang F, Yamauchi M, Muramatsu M, et al. RACK1 Regulates VEGF/Flt1-mediated Cell Migration via Activation of a PI3K/Akt Pathway. J Biol Chem. 2011;286:9097–106.
Deng YZ, Yao F, Li JJ, et al. RACK1 Suppresses Gastric Tumorigenesis by Stabilizing beta-Catenin Destruction Complex. Gastroenterology. 2012;142:812–23.
Ma X, Chen K, Huang S, et al. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698–705.
Subauste MC, Ventura-Holman T, Du L, et al. RACK1 down-regulates levels of the pro-apoptotic protein Fem1b in apoptosis-resistant colon cancer cells. Cancer Biol Ther. 2009;8:2297–305.
Cao XX, Xu JD, Xu JW, et al. RACK1 promotes breast carcinoma proliferation and invasion/metastasis in vitro and in vivo. Breast Cancer Res Treat. 2010;123:375–86.
Wang Z, Jiang L, Huang C, et al. Comparative proteomics approach to screening of potential diagnostic and therapeutic targets for oral squamous cell carcinoma. Mol Cell Proteomics. 2008;7:1639–50.
Ishizuka T, Tanabe C, Sakamoto H, et al. Gene amplification profiling of esophageal squamous cell carcinomas by DNA array CGH. Biochem Biophys Res Commun. 2002;96:152–5.
Maesawa C, Tamura G, Iwaya T, et al. Mutations in the human homologue of the Drosophila patched gene in esophageal squamous cell carcinoma. Genes Chromosom Cancer. 1998;21:276–9.
Shi S, Deng YZ, Zhao JS, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J Biol Chem. 2012;287:7845–58.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Fengqing Hu; Zhen Tao These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Hu, F., Tao, Z., Wang, M. et al. RACK1 promoted the growth and migration of the cancer cells in the progression of esophageal squamous cell carcinoma. Tumor Biol. 34, 3893–3899 (2013). https://doi.org/10.1007/s13277-013-0977-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0977-7