Abstract
Sperm-associated antigen 9 (SPAG9) is a recently characterized oncoprotein involved in the progression of several human malignancies. The present study aims to investigate the expression pattern and biological roles of SPAG9 protein in human astrocytoma. SPAG9 expression was analyzed in 105 astrocytoma specimens by immunohistochemistry. We observed negative staining in normal astrocytes and positive staining of SPAG9 in 63 out of 105 (60 %) astrocytoma samples. Overexpression of SPAG9 correlated with tumor grade (p < 0.001). Small interfering RNA knockdown was performed in U251 and U87 cell lines with relatively high SPAG9 expression. Using methylthiazolyldiphenyl-tetrazolium bromide assay and Matrigel invasion assay, we were able to show that SPAG9 depletion in astrocytoma cell lines inhibited cell proliferation and invasion in both cell lines. In addition, mRNA and protein levels of matrix metallopeptidase 9 (MMP9) were downregulated, while the levels of tissue inhibitor of metalloproteinase 1 (TIMP1) and TIMP2 were not changed, indicating that SPAG9 might regulate invasion through MMP9. In conclusion, SPAG9 serves as an important oncoprotein in human astrocytoma by regulating cell proliferation and invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytoma arises from neural progenitor cells in the central nervous system and is the most common primary brain tumor. Despite combined treatment strategies, which include surgery, radiotherapy, and chemotherapy, the prognosis for high-grade astrocytoma remains poor [1]. So identifying of molecular targets playing a direct role in tumor invasion and cell survival is an important step toward rational design of drugs for cancer treatment [2, 3].
Cancer/testis (CT) antigens are encoded by genes that are normally expressed only in the human germ line but are also expressed in various tumors. Based on their restricted expression in cancerous tissues and in view of their immunogenicity in cancer patients, these immunogenic proteins are being vigorously pursued as targets for therapeutic cancer vaccines [4].
Sperm-associated antigen 9 (SPAG9) defines a family of scaffolding proteins that bring MAPKs and their target transcription factors together for the execution of specific signaling pathways [5–9]. Recent studies showed SPAG9 overexpression in various human cancers including renal, breast, thyroid, cervical, and colon carcinomas [10–14]. Furthermore, SPAG9 siRNA treatment inhibited tumor cell proliferation and invasion [10–14]. SPAG9 was reported to be increased during differentiation of C17.2 neural precursor cells [15]. Importantly, SPAG9 expression was also found to be associated with circulating anti-SPAG9 antibodies in early stages and in low grade of breast cancer [10] and cervical cancer patients [11], suggesting its potential use in the early detection of disease. However, the expression pattern and biological function of SPAG9 in human astrocytoma remain unclear.
In the present study, we examined SPAG9 expression in astrocytoma by immunohistochemistry. In addition, we investigated the involvement of SPAG9 in proliferation and invasion in astrocytoma cell lines and explored the possible mechanisms.
Materials and methods
Patients and specimens
The study protocol was approved by an institutional review board of the Liaoning Medical University. Primary tumor specimens were obtained from 105 patients diagnosed with astrocytoma who underwent resection in the First Affiliated Hospital of Liaoning Medical University between 2000 and 2005. The histological diagnosis was evaluated for sections stained with hematoxylin and eosin according to the World Health Organization classification guidelines. Clinical and histopathological data including histopathological diagnosis and tumor grade were extracted from medical records.
Immunohistochemistry
Four-micrometer-thick sections were prepared from the paraffin-embedded tissues. Immunostaining was performed using the two-step Elivision plus kit (MaiXin, Fuzhou, China). The sections were deparaffinized in xylene, rehydrated with graded alcohol, and then boiled in citrate buffer (pH 6.0) for 2 min with an autoclave. Next, 0.3 % hydrogen peroxide was applied to block the endogenous peroxidase activity, and the sections were incubated with normal animal serum to reduce nonspecific binding. Tissue sections were incubated with SPAG9 rabbit polyclonal antibody (1:150 dilution; Abcam) for 2 h at room temperature. Rabbit immunoglobulin (at the same concentration as for the antigen-specific antibody) was used as a negative control. The staining was followed by incubation with polymer secondary antibodies. The peroxidase reaction was developed with DAB. Counterstaining was done with hematoxylin, and the sections were dehydrated in alcohol before mounting.
We counted 400 tumor cells and calculated the percentage of positively stained cells. SPAG9 was scored positive when a specimen is showing >10 % of cancer cells stained for SPAG9 protein, as described previously [14].
Cell culture and transfection
U87 and U251 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10 % fetal calf serum (Invitrogen), 100 IU/ml penicillin (Sigma, St. Louis, MO, USA), and 100 μg/ml streptomycin (Sigma, St. Louis, MO, USA). Cells were grown on sterilized culture dishes and were passaged every 2 days with 0.25 % trypsin (Invitrogen).
The siGENOME SMARTpool SPAG9 siRNA (no. M-017462-0005) and scrambled siRNA were purchased from Dharmacon (ThermoFisher Scientific, USA). For transfections, cells were seeded in a plate 24 h before the experiment. The cells were transfected with siRNA using the DharmaFECT reagent (ThermoFisher Scientific, USA) according to the manufacturer's protocol.
Cell proliferation test
For methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay, cells were plated in 96-well plates in a medium containing 10 % fetal bovine serum (FBS) at approximately 3,000 cells per well 24 h after transfection. For quantitation of cell viability, 20 μl of 5 mg/ml MTT (thiazolyl blue) solution was added to each well and incubated for 4 h at 37 °C. The medium was removed from each well, and the resulting MTT formazan was solubilized in 150 μl of DMSO. Each solution was measured spectrophotometrically at 490 nm.
Matrigel invasion assay
Cell invasion assay was performed using the 24-well Transwell chamber with a pore size of 8 μm (Costar, Cambridge, MA). The inserts were coated with 20 μl Matrigel (1:3 dilution; BD Bioscience, San Jose, CA, USA). Forty-eight hours after the transfection, cells were trypsinized, and 3 × 105 cells in 100 μl of serum-free medium were transferred to the upper Matrigel chamber and incubated for 16 h. Medium supplemented with 10 % FBS was added to the lower chamber as the chemoattractant. After incubation, the non-invaded cells on the upper membrane surface were removed with a cotton tip, and the cells that passed through the filter were fixed with 4 % paraformaldehyde and stained with hematoxylin.
Quantitative real-time PCR
Quantitative real-time PCR was performed using the SYBR Green PCR master mix (Applied Biosystems) in a total volume of 20 μl on the 7900HT Fast Real-Time PCR System (Applied Biosystems) as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The relative levels of gene expression were represented as Δ threshold cycle (Ct) = Ct gene − Ct reference, and the fold change of gene expression was calculated using the 2−ΔΔCt method. Experiments were repeated in triplicate. The sequences of the primer pairs are as follows: β-actin forward, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′; β-actin reverse, 5′-CACCTTCTACAATGAGCTGCGTGTG-3′; matrix metallopeptidase 9 (MMP9) forward, 5′-CCTCTGGAGGTTCGACGTGA-3′; MMP9 reverse, 5′-TAGGCTTTCTCTCGGTACTGGAA-3′; SPAG9 forward, 5′-GCTTTTGATCGCAATACAGAATCTC-3′; SPAG9 reverse, 5′-AACTTCCCGACCCATTCCTAGT-3′; tissue inhibitor of metalloproteinase 1 (TIMP1) forward, 5′-CTGGCTTCTGGCATCCTGTT-3′; TIMP1 reverse, 5′-GACGAGGTCGGAATTGCAGA-3′; TIMP2 forward, 5′-CCCTCCTCGGCAGTGTGT-3′; and TIMP2 reverse, 5′-GGCCTTTCCTGCAATGAGATA-3′.
Western blot analysis
Total protein from tissue and cells was extracted in lysis buffer (Pierce, Rockford, IL) and quantified using the Bradford method. Samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) and incubated overnight at 4 °C with antibody against SPAG9 (1:1,000; Abcam) and β-actin (1:2,000; Santa Cruz). After incubation with peroxidase-coupled anti-mouse/rabbit IgG (Santa Cruz) at 37 °C for 2 h, bound proteins were visualized using ECL (Pierce) and detected using the BioImaging System (UVP, Inc., Upland, CA, USA). Relative protein levels were quantified using β-actin as a loading control.
Statistical analysis
SPSS version 16.0 for Windows was used for all analyses. The chi-squared test was used to examine possible correlations between SPAG9 expression and clinicopathologic factors. The Student's t test was used to compare other data. A p value was based on the two-sided statistical analysis, and p < 0.05 was considered to indicate a statistical significance.
Results
SPAG9 protein is overexpressed in astrocytoma and correlates with tumor grade
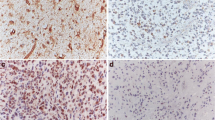
We analyzed the expression of SPAG9 in 105 astrocytoma specimens and 20 corresponding normal tissues by immunohistochemistry. In normal brain tissues examined, negative expression of SPAG9 in astrocytes and weak cytoplasmic expression in neurons were observed (Fig. 1a). No staining was observed in specimens incubated with nonimmune rabbit immunoglobulin. Positive cytoplasmic SPAG9 staining was observed in 63 of 105 (60 %) human astrocytoma specimens (Fig. 1c–f). We analyzed the correlation between SPAG9 expression and clinical factors. There was no relationship between SPAG9 expression and age or gender. As shown in Table 1, the positive rates of SPAG9 in grade I–IV astrocytoma/glioblastoma (Fig. 1c–f) were 0, 44.7, 73.1, and 75.2 %, respectively. Patients with high grade showed higher positive rate for SPAG9 expression (p < 0.001).
Expression of SPAG9 in human astrocytoma tissues. a Immunohistochemical staining of SPAG9 protein in normal brain tissue was negative in glial cells and weak in neurons. b Negative control using rabbit immunoglobulin. c Negative SPAG9 staining in pilocytic astrocytoma (grade I). d Negative SPAG9 staining in grade II glioma. e Positive cytoplasmic SPAG9 staining in grade III astrocytoma. f Positive cytoplasmic SPAG9 staining in glioblastoma (grade IV). (Magnification ×200)
SPAG9 depletion in astrocytoma cell lines inhibited proliferation and invasion
Expression of SPAG9 was checked by real-time PCR and western blot in a panel of astrocytoma cell lines (SF295, A172, TG905, U251, and U87). We found a relatively high mRNA and protein expression of SPAG9 in U251 and U87 cells (Fig. 2a). In order to explore the biological function of SPAG9 in astrocytoma cells, siRNA knockdown was performed in these two cell lines. As shown in Fig. 2b, both protein and mRNA levels of SPAG9 were significantly decreased 48 h after siRNA transfection. The role of SPAG9 on astrocytoma cell proliferation was determined by MTT assay. U251 and U87 cells treated with SPAG9 siRNA exhibited a significantly slower growth rate than that of scrambled siRNA-treated cells. The difference of 490 nm absorbance at day 5 was statistically significant for both cell lines (Fig. 3a).
SPAG9 expression and knockdown efficiency in astrocytoma cell lines. a Endogenous expression of SPAG9 was examined in five astrocytoma cell lines by western blot and real-time PCR. b Western blot and real-time PCR analysis showed that siRNA treatment of SPAG9 decreased SPAG9 levels in U251 and U87 cells in comparison with cells transfected with scrambled siRNA
SPAG9 knockdown inhibited cell proliferation in astrocytoma cell lines. a MTT assay showed that SPAG9 depletion inhibited cell proliferation. There was a time-dependent decrease in cell proliferation after SPAG9 depletion compared with control. b Matrigel invasion assay showed that SPAG9 depletion decreased cell invasion in U251 and U87 cell lines. (p < 0.05). c Real-time PCR analysis showed that siRNA knockdown of SPAG9 decreased the mRNA level of MMP9 in both cell lines. The expression of TIMP1 and TIMP2 was not changed. d Western blot analysis showed that siRNA knockdown of SPAG9 decreased the protein level of MMP9 in both cell lines. The expression of TIMP1 and TIMP2 was not changed
Matrigel invasion assay was employed to examine the role of SPAG9 on cell invasion. As shown in Fig. 3b, SPAG9 knockdown inhibited cell invasion in both U251 and U87 cell lines (U251 scrambled siRNA vs SPAG9 siRNA, 305 ± 12 vs 149 ± 17; U87 scrambled siRNA vs SPAG9 siRNA, 212 ± 12 vs 82 ± 14, p < 0.05). These results together demonstrated that SPAG9 could modulate astrocytoma proliferation and invasion.
SPAG9 regulates cell invasion via MMP9 in astrocytoma
To investigate the mechanism by which SPAG9 affected cell invasion, we used real-time PCR and western blot to analyze the expression change of MMP family which closely related to the invading ability of cancer cells. We observed a remarkable decrease in MMP9 expression at both mRNA and protein levels after siRNA treatment, while TIMP1 and TIMP2 expressions were not changed (Fig. 3c, d). These results suggest that SPAG9 could modulate MMP9 transcription, thus promoting cell invasion.
Discussion
The present study demonstrated that SPAG9 overexpression in 60 % human astrocytoma and its correlation with higher tumor grade. We also demonstrated that SPAG9 depletion in U251 and U87 astrocytoma cell lines inhibited cell growth and invasion, possibly through regulating MMP9 transcription.
SPAG9 expression has been implicated in several malignancies such as renal, breast, thyroid, cervical, and colon carcinomas, and its expression correlated with tumor stage and grade [10, 12–14, 16]. In accordance with previous studies, we found that SPAG9 was overexpressed in 60 % astrocytoma samples, with a significant correlation with tumor grade. The SPAG9 overexpression rate in astrocytoma was slightly lower than that in other cancers. It was reported that SPAG9 knockdown inhibited cell proliferation in renal, colon, and cervical carcinomas and invasion in renal and colon cancers [10, 16–19]. We employed siRNA to knockdown SPAG9 expression in both U251 and U87 cell lines which expressed high levels of SPAG9. Decreased proliferation was observed after SPAG9 knockdown, indicating that SPAG9 may stimulate malignant cell proliferation in astrocytoma cells. In addition, SPAG9 knockdown inhibited cancer cell invasion. These findings indicate that SPAG9 might play an important role in astrocytoma progression and could serve as a potential biomarker for the malignant behavior of astrocytoma cells.
The molecular pathway by which SPAG9 promotes invasion was not clear. To explore the potential mechanism of SPAG9 on cell invasion, we examined the expression of invasion-related gene and found that SPAG9 knockdown downregulated mRNA expression. The levels of TIMP1 and TIMP2 were not changed. This result suggested that SPAG9 enhanced cell invasion via MMP9 regulation. MMP9 has been suggested to be critical for the invasive and metastatic potential in cancer cells [20, 21]. Furthermore, MMP9 depletion could inhibit astrocytoma cell growth, suggesting that SPAG9 regulates cell proliferation partly through MMP9 modulation [22]. It was reported that SPAG9 participated in c-Jun N-terminal kinase (JNK) pathway activation, and JNK signaling could result in the activation of MMP9 [9, 16]. So it is possible that JNK signaling is involved in invasion-promoting function of SPAG9.
In conclusion, this study demonstrated the overexpression of SPAG9 in human astrocytoma and its correlation with tumor grade. In addition, our data showed that SPAG9 depletion could inhibit astrocytoma cell proliferation and invasion, possibly through MMP9 transcriptional regulation. Based on these findings, we conclude that SPAG9 might be a potential therapeutic target in human astrocytoma.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Krupkova Jr O, Loja T, Redova M, Neradil J, Zitterbart K, Sterba J, et al. Analysis of nuclear nestin localization in cell lines derived from neurogenic tumors. Tumour Biol. 2011;32(4):631–9.
Zadran S, Amighi A, Otiniano E, Wong K, Zadran H. ENTPD5-mediated modulation of ATP results in altered metabolism and decreased survival in gliomablastoma multiforme. Tumour Biol. 2012;33(6):2411–21.
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–25.
Ando K, Uemura K, Kuzuya A, Maesako M, Asada-Utsugi M, Kubota M, et al. N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. J Biol Chem. 2010;286(9):7619–28.
Kashef K, Lee CM, Ha JH, Reddy EP, Dhanasekaran DN. JNK-interacting leucine zipper protein is a novel scaffolding protein in the Galpha13 signaling pathway. Biochemistry. 2005;44(43):14090–6.
Lee CM, Onesime D, Reddy CD, Dhanasekaran N, Reddy EP. JLP: a scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors. Proc Natl Acad Sci U S A. 2002;99(22):14189–94.
Nguyen Q, Lee CM, Le A, Reddy EP. JLP associates with kinesin light chain 1 through a novel leucine zipper-like domain. J Biol Chem. 2005;280(34):30185–91.
Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP, et al. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175(3):383–8.
Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomark Prev. 2009;18(2):630–9.
Garg M, Kanojia D, Suri S, Suri A. Small interfering RNA-mediated down-regulation of SPAG9 inhibits cervical tumor growth. Cancer. 2009;115(24):5688–99.
Garg M, Kanojia D, Salhan S, Suri S, Gupta A, Lohiya NK, et al. Sperm-associated antigen 9 is a biomarker for early cervical carcinoma. Cancer. 2009;115(12):2671–83.
Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab. 2009;94(11):4613–8.
Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol. 2011;178(3):1009–20.
Oh JE, Bae GU, Yang YJ, Yi MJ, Lee HJ, Kim BG, et al. Cdo promotes neuronal differentiation via activation of the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23(7):2088–99.
Garg M, Kanojia D, Khosla A, Dudha N, Sati S, Chaurasiya D, et al. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res. 2008;68(20):8240–8.
Jagadish N, Rana R, Selvi R, Mishra D, Garg M, Yadav S, et al. Characterization of a novel human sperm-associated antigen 9 (SPAG9) having structural homology with c-Jun N-terminal kinase-interacting protein. Biochem J. 2005;389(Pt 1):73–82.
Gantulga D, Tuvshintugs B, Endo Y, Takino T, Sato H, Murakami S, et al. The scaffold protein c-Jun NH2-terminal kinase-associated leucine zipper protein regulates cell migration through interaction with the G protein G(alpha 13). J Biochem. 2008;144(6):693–700.
Kashef K, Radhakrishnan R, Lee CM, Reddy EP. Dhanasekaran DN Neoplastic transformation induced by the gep oncogenes involves the scaffold protein JNK-interacting leucine zipper protein. Neoplasia. 2011;13(4):358–64.
Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H, Shin SH, et al. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int J Oncol. 2005;27(3):839–46.
Dong QZ, Wang Y, Tang ZP, Fu L, Li QC, Wang ED, et al. Derlin-1 is overexpressed in non-small cell lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and MMP-9. Am J Pathol. 2013;182(3):954–64.
Sun C, Wang Q, Zhou H, Yu S, Simard AR, Kang C, et al. Antisense MMP-9 RNA inhibits malignant glioma cell growth in vitro and in vivo. Neurosci Bull. 2013;29(1):83–93.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yi, F., Ni, W., Liu, W. et al. SPAG9 is overexpressed in human astrocytoma and promotes cell proliferation and invasion. Tumor Biol. 34, 2849–2855 (2013). https://doi.org/10.1007/s13277-013-0845-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0845-5