Abstract
The aim of this study was to examine the effects of esophageal cancer-related gene 4 (ECRG4) expression levels on chemotherapeutic sensitivity of gastric cancer cells. A SGC-7901 cell system with tetracycline-inducible ECRG4 expression (SGC-7901/ECRG4) was successfully established. ECRG4 mRNA and protein expression levels were detected using quantitative reverse transcription polymerase chain reaction and Western blotting, respectively. Chemosensitivity to 5-fluorouracil (5-FU) was examined by cell proliferation assay and cell apoptosis assay. ECRG4 mRNA and protein expression levels were significantly upregulated in SGC-7901/ECRG4 cells induced with tetracycline. Compared with control cells, the growth inhibition rate of cells with ECRG4 overexpression was significantly increased when treated with 5-FU. Treatment with 5 μmol/l 5-FU resulted in 15.2 % apoptotic cells, whereas such treatment after overexpression of ECRG4 resulted in 44.5 % apoptotic cells. In conclusion, overexpression of ECRG4 enhanced the chemosensitivity of gastric cancer SGC-7901 cells to 5-FU through induction of apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite its declining incidence, gastric cancer remains the second most common cause of cancer-related mortality in Asia and worldwide [1, 2]. Surgery remains the mainstay of any curative treatment. However, even following radical surgery, the majority of gastric cancer patients develop local or distant recurrence [3]. Several meta-analyses of postoperative adjuvant trials have demonstrated a significant benefit for chemotherapy-treated patients [4]. However, certain patients have undergone expensive and potentially harmful therapy without gaining any benefit. Thus, the identification of molecular markers in resected tumor tissues that are able to predict outcomes is essential for the future development of adjuvant chemotherapy for gastric cancer patients.
Esophageal cancer-related gene 4 (ECRG4), officially called C2ORF40, was cloned and identified from normal esophageal epithelium [5]. It is localized in 2q12.2. The encoded protein (augurin) is a secretory molecule produced in endocrine tissues such as pituitary gland, adrenal gland, and choroid plexus [6]. Its actions consist in cerebrospinal fluid homeostasis, stimulation of neuroprogenitor cells after brain injury [7], and induction of cell senescence in central nervous system [8]. Even if its impact on oncogenesis is not clear, it has been described as a putative tumor suppressor gene (TSG) in several cancers including esophageal squamous cell carcinoma [9–13], prostate cancer [14], colorectal cancer, and glioma [12–15]. ECRG4 expression was associated with better survival in esophageal [10] and prostate [14] carcinomas, and with inhibition of cell proliferation and migration in esophageal cancer [11–13], colorectal cancer, and glioma [13, 15]. Recently, Wang et al. reported that aberrant ECRG4 promoter methylation may be used to monitor early gastric cancer and predict pathological staging [16]. The objective of this study has been to investigate whether ECRG4 is involved in cell sensitivity to chemotherapeutic agents in gastric cancer. To achieve this, we used the human gastric cancer cell line, SGC-7901, and established stable clones in SGC-7901 cells with a tetracycline-inducible ECRG4 expression system.
Materials and methods
Cell lines and cultures
The human gastric cancer SGC-7901 cell lines were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). All cell lines were propagated in Roswell Park Memorial Institute-1640 medium (Gibco-BRL; Carlsbad, CA, USA), supplemented with 10 % bovine serum, penicillin (100 U/ml), streptomycin (100 mg/ml), pyruvate, glutamine, and insulin at 37 °C in a water-saturated atmosphere with 5 % CO2.
Drugs
5-fluorouracil (5-FU) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were procured from Sigma, St. Louis, USA. The dilutions of all reagents were freshly prepared before each experiment. Annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detection kit was purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from SGC-7901 cell lines using the acid guanidinium–phenol–chloroform method (Trizol, Invitrogen, Carlsbad, USA), and cDNAs were synthesized using the PrimeScriptTM 1st Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, USA). Primer sequences used for amplification were designed by TaKaRa (Takara Bio Inc., Shiga, Japan) and listed as follows: forward primer 5′-GCAGCATATTCAGAAAGT TCAGA-3′; reverse primer 5′-CATTTACAGCCTAATGCCTTTACT-3′. β-Actin forward primer 5′- TGGCACCCAGCACAATGAA -3′; reverse primer 5′- CTAAGTCATAGTCCGCCTAGAAGCA -3′. Primers were used in regular polymerase chain reaction (PCR) reactions with cDNA from cells so as to evaluate their proper design and synthesis. Subsequently, the PCR products were sequenced, and their similarity to the desired nucleotide sequences was confirmed. The relative amount of mRNA was determined using the SYBR GREEN PCR Master Mix (AB, Applied Biosystems, Foster City, California, USA) with gene-specific primers for ECRG4 or β-actin. All steps were carried out according to the manufacturer’s protocol. Real-time PCR reactions were carried out on an ABI 7500 thermal cycler (Applied Biosystems, Foster City, California, USA) with the BioRad iQ5 and MyiQTM Real-time PCR Detection System (BioRad Laboratories, Hercules, California, USA). Three independent PCR tests were performed from each RT sample. The expression of ECRG4 mRNA in each sample was normalized against β-actin, and the expression level was calculated using the ΔΔCT (delta delta threshold cycle) method.
Western blot analysis
ECRG4 protein expression in cells was detected by Western blot analysis. Protein was extracted from cultured cells using lysis buffer. After a 30-min incubation on ice, the lysates were heated at 100 °C for 15 min and centrifuged at 12,000×g for 15 min at 4 °C. Lysates containing an equal amount of protein (25 μg) were dissolved in SDS sample buffer, separated on 12 % SDS slab gels, and transferred electrophoretically onto polyvinylidene difluoride membranes. Equal protein loading and transfer were confirmed by Ponceau S staining. After being blocked with 5 % nonfat dry milk in TBST, the membrane was incubated at 4 °C overnight with the appropriate primary antibodies. Following washing, horseradish peroxidase-conjugated secondary antibody was applied to the membrane. Proteins bound by the secondary antibody were visualized by ECL (Amersham Bioscience) according to the manufacturer’s instructions. The expression of GAPDH was measured as a control, and each experiment was performed in triplicate.
MTT assay
To determine cell growth, 10,000 cells were seeded in triplicate on 96-well plates and incubated overnight. 5-FU was then added so that the final concentrations were 1, 5, and 10 μmol/l, with dimethyl sulfoxide (DMSO) as a control. Cells were incubated in a humidified incubator in 5 % CO 2 at 37 °C for 24 h. After the exposure period, the medium was removed and the cells were incubated for 1 h at 37 °C with fresh growth medium containing 0.5 mg/ml MTT. The supernatant was then carefully removed, and 100 μl of DMSO was added to dissolve the formazan crystals. The absorption (A) was measured at 570 nm in a microplate reader. Growth inhibition rate (%) = (1 − A of experimental group / A of control group) × 100 %.
Apoptosis assay
The untreated SGC-7901/ECRG4 cells were treated with 5 μmol/l 5-FU or treated with 5 μmol/l 5-FU and 1 μg/ml tetracycline for 48 h, after which apoptotic cells were detected using an FITC Annexin V Apoptosis Detection kit according to the manufacturer’s instructions. Briefly, after treatment, cells were washed twice with cold phosphate buffered saline and then resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Then, 100 μl of the solution (1 × 105 cells) was transferred to a 5-ml culture tube, and 5 μl of FITC Annexin V and 5 μl propidium iodide were added to the solution. After incubation for 15 min at room temperature in the dark, 400 μl of 1× binding buffer was added to each tube. The stained cells were then analyzed using flow cytometry.
Statistical analysis
All data are presented as the mean ± standard deviation. Student’s t test was performed for intergroup comparison. A probability of P < 0.05 was considered to be statistically significant.
Results
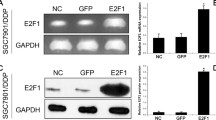
ECRG4 mRNA and protein expression levels were significantly upregulated in SGC-7901/ECRG4 cells induced with tetracycline
ECRG4 mRNA expression levels were significantly upregulated after stimulation with tetracycline in SGC-7901/ ECRG4 cells (Fig. 1). When stimulated with 1 μg/ml tetracycline, the ECRG4 mRNA expression levels significantly increased by about 8.2-fold (P < 0.001). These results were confirmed by Western blot. As shown in Fig. 2, the ECRG4 protein expression levels were elevated markedly when stimulated with tetracycline for the indicated times. This suggests that tetracycline could effectively induce ECRG4 gene expression in SGC-7901/ECRG4 cells.
Overexpression of ECRG4 enhances 5-FU-mediated growth inhibition
To examine whether overexpression of ECRG4 enhances 5-FU-mediated growth inhibition, cell proliferation assays were performed. The growth of SGC-7901/ECRG4 cells was inhibited in a dose-dependent manner when treated with 1, 5, and 10 μmol/l 5-FU for 24 h. The inhibition rates were 12.4, 27.4, and 39.6 %, respectively (Fig. 3). When SGC-7901/ECRG4 cells expressed ECRG4 by induction with tetracycline, the growth inhibition rate was increased to 22.1, 47.8, and 77.9 %, respectively, after treatment with 5-FU for 24 h (P < 0.01; Fig. 3).
Overexpression of ECRG4 increases apoptosis induced by 5-FU
Apoptosis is a major antitumor pathway for chemotherapy. To investigate whether overexpression of ECRG4 increases apoptosis induced by 5-FU, we measured the apoptotic cell death in SGC-7901/ECRG4 cells following ECRG4 overexpression and treatment with 5-FU or treatment with 5-FU alone. As shown in Fig. 4, the apoptotic cell fraction was increased following ECRG4 overexpression and treatment with 5 μmol/l 5-FU, as compared with 5-FU treatment only. Treatment with 5 μmol/l 5-FU resulted in 15.2 % total apoptotic cells. Overexpression of ECRG4 by induction with tetracycline, followed by 5 μmol/l 5-FU resulted in 44.5 % total apoptotic cells (Fig. 5).
Discussion
The ECRG4 gene, officially named C2ORF40, is highly conserved in vertebrates, not in other eukaryotic species, suggesting an important role in vertebrate organisms. Although identified many years ago, the function of the protein encoded by this gene remains unclear, but recent data revealed a potential TSG role in different cancers. The tumor suppressor function of ECRG4 [16] and the cellular consequences of its silencing remain to be investigated in breast cancer. In cell lines of esophageal [11–13] and colorectal cancer [15] and glioma [12], the overexpression of ECRG4 inhibits cell proliferation by blocking the G1/S transition of cell cycle, through increase of p21 and p53 protein expression. The inhibition of proliferation was confirmed in vivo after injection of ECRG4-transfected esophageal cancer cell lines into athymic nude mice, which led to slower tumor growth [11]. Another in vitro effect of ECRG4 overexpression is the inhibition of cell migration and invasion in cell lines from esophageal carcinoma and glioma [12]. To our knowledge, our study is the first one analyzing the effects of ECRG4 expression levels on chemotherapeutic sensitivity of gastric cancer cells.
In our present study, we investigated whether overexpression of ECRG4 could enhance the chemotherapeutic sensitivity of gastric cancer cells. 5-FU is the most frequently used chemotherapy drug for gastric cancer. Inhibition of cell proliferation has been attributed to the antitumor effects of chemotherapy. Our study showed that 5-FU could inhibit SGC-7901 cell growth in a dose-dependent manner, and that the rate of growth inhibition was markedly increased in cells with ECRG4 overexpression after treatment with 5-FU, compared with control cells. Our results suggest that overexpression of ECRG4 enhances the inhibitory effect of 5-FU on SGC-7901 cells.
In conclusion, this study showed that ECRG4 overexpression could increase the sensitivity of SGC-7901 cells to 5-FU by induction of apoptosis. These results will further our understanding of the role of ECRG4 in apoptosis and provide new strategies for improving chemosensitivity in human gastric cancer cells.
References
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al. Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–87. doi:10.1016/S1470-2045(08)70072-X.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi:10.1200/JCO.2005.05.2308.
Macdonald JS. Treatment of localized gastric cancer. Semin Oncol. 2004;31:566–73. doi:10.1053/j.seminoncol.2004.04.022.
Carrato A, Gallego-Plazas J, Guillen-Ponce C. Adjuvant therapy of resected gastric cancer is necessary. Semin Oncol. 2005;32:S105–8. doi:10.1053/j.seminoncol.2005.06.005.
Su T, Liu H, Lu S. Cloning and identification of cDNA fragments related to human esophageal cancer. Zhonghua Zhong Liu Za Zhi. 1998;20:254–7.
Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, et al. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007;17:320–7. doi:10.1101/gr.5755407.
Gonzalez AM, Podvin S, Lin SY, Miller MC, Botfield H, Leadbeater WE, et al. Ecrg4 expression and its product augurin in the choroid plexus: impact on fetal brain development, cerebrospinal fluid homeostasis and neuroprogenitor cell response to CNS injury. Fluids Barriers CNS. 2011;8:6. doi:10.1186/2045-8118-8-6.
Kujuro Y, Suzuki N, Kondo T. Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells. Proc Natl Acad Sci U S A. 2010;107:8259–64. doi:10.1073/pnas.0911446107.
Yue CM, Deng DJ, Bi MX, Guo LP, Lu SH. Expression of ECRG4, a novel esophageal cancer-related gene, downregulated by CpG island hypermethylation in human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9:1174–8.
Mori Y, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Kurehara H, et al. Expression of ECRG4 is an independent prognostic factor for poor survival in patients with esophageal squamous cell carcinoma. Oncol Rep. 2007;18:981–5.
Li LW, Yu XY, Yang Y, Zhang CP, Guo LP, Lu SH. Expression of esophageal cancer related gene 4 (ECRG4), a novel tumor suppressor gene, in esophageal cancer and its inhibitory effect on the tumor growth in vitro and in vivo. Int J Cancer. 2009;125:1505–13. doi:10.1002/ijc.24513.
Li W, Liu X, Zhang B, Qi D, Zhang L, Jin Y, et al. Overexpression of candidate tumor suppressor ECRG4 inhibits glioma proliferation and invasion. J Exp Clin Cancer Res. 2010;29:89. doi:10.1186/1756-9966-29-89.
Li LW, Li YY, Li XY, Zhang CP, Zhou Y, Lu SH. A novel tumor suppressor gene ECRG4 interacts directly with TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal carcinoma. BMC Cancer. 2011;11:52. doi:10.1186/1471-2407-11-52.
Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, Tindall DJ, et al. Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 2009;27:549–60. doi:10.1080/07357900802620794.
Götze S, Feldhaus V, Traska T, Wolter M, Reifenberger G, Tannapfel A, et al. ECRG4 is a candidate tumor suppressor gene frequently hypermethylated in colorectal carcinoma and glioma. BMC Cancer. 2009;9:447. doi:10.1186/1471-2407-9-447.
Ozawa A, Lick AN, Lindberg I. Processing of proaugurin is required to suppress proliferation of tumor cell lines. Mol Endocrinol. 2011;25:776–84. doi:10.1210/me.2010-0389.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, CP., Wu, BH., Wang, BQ. et al. Overexpression of ECRG4 enhances chemosensitivity to 5-fluorouracil in the human gastric cancer SGC-7901 cell line. Tumor Biol. 34, 2269–2273 (2013). https://doi.org/10.1007/s13277-013-0768-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0768-1