Abstract
The S-phase kinase-associated protein 2 (SKP2) oncoprotein is an E3 ubiquitin ligase. Overexpression of SKP2 was found in various human cancers, including colorectal cancers, but its potential role as a prognostic marker after neoadjuvant chemoradiotherapy (CRT) and for therapeutic intervention in rectal cancers is unknown. This study examined the correlation of SKP2 expression in the prognosis of rectal cancer patients and the viability of colorectal cancer cells treated with CRT. SKP2 immunoexpression was retrospectively assessed in pretreatment biopsies of 172 rectal cancer patients treated with neoadjuvant CRT followed by surgery. Results were correlated with clinicopathological features, therapeutic responses, and patient survival. Pharmacologic assays were used to evaluate the therapeutic relevance of Bortezomib in two colorectal cancer cell lines (HT-29 and SW480). High expression of SKP2 was correlated with the advanced Post-Tx nodal status (p = 0.002), Post-Tx International Union for Cancer Control stage (p = 0.002), and a lower-degree tumor regression grade (p < 0.001). Moreover, high expression of SKP2 (p = 0.027, hazard ratio 3.21) was an independent prognostic factor for local recurrence-free survival. In vitro, Bortezomib downregulated SKP2 expression, induced caspase activation, and decreased the viability of colorectal cancer cells with or without a combination with fluorouracil. Bortezomib also promoted caspase activation and gamma-H2AX formation in colorectal cancer cells concurrently treated with CRT. High expression of SKP2 was associated with a poor therapeutic response and adverse outcomes in rectal cancer patients treated with neoadjuvant CRT. In the presence of chemotherapy with or without radiotherapy, the promoted sensitivity of colorectal cancer cells to Bortezomib with an SKP2-repressing effect indicated that it is a potential therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a common and lethal disease globally, and an increased incidence of CRC was recently reported in Taiwan (database from the Bureau of Health Promotion, Department of Health, Taiwan, available at http://www.bhp.doh.gov.tw/BHPNET/Portal/Statistics.aspx). CRC diagnosed distal to the rectosigmoid junction is designated rectal cancer with an estimated 4,900 new cases/year in Taiwan. Currently, neoadjuvant chemoradiotherapy (CRT) followed by surgery is the standard treatment for locally advanced rectal cancer featuring lymph node metastases or perirectal extension (T3, T4, or node metastases) [1–4]. Although the outcomes of this approach are encouraging, 5-year local and distant recurrence rates still persist and respectively range 6∼9.6 and 33∼36 %. This greatly affects the patient survival rate, and research to reduce local recurrence and distant metastases is urgently necessary. Otherwise, another advantage of neoadjuvant CRT might allow some patients to undergo sphincter-preserving low anterior resection rather than abdominoperineal resection. However, variations in the incidence of sphincter preservation ranging 39∼94 % are also a challenge which still needs to be overcome [5–7].
The S-phase kinase-associated protein 2 (SKP2) gene, located at chromosome 5p13, produces a substrate-recognizing E3 ubiquitin ligase and targets p27Kip1 for degradation by the 26S proteasome [8]. SKP2 protein, part of SKP1-CUL1-F-box (SCF) complexes, is considered a negative regulator of the cell-cycle inhibitor, p27Kip1, and positively regulates the G1/S transition. Frequently, SKP2 is overexpressed in various human cancers, including lung cancer, head and neck cancers, myxofibrosarcomas, and CRC [9–12]. Besides accelerating cell growth, SKP2 protein overexpression was also proven to be associated with aggressive phenotypes of promoting cell migration, invasion, and metastases in various human cancers [13, 14]. Recently, correlations of SKP2 with ErbB family-induced Akt ubiquitination, aerobic glycolysis, and tumorigenesis were demonstrated [15]. Targeting glycolysis by an SKP2 deficiency also sensitizes Her2-positive tumors to Herceptin treatment, highlighting the value of SKP2 targeting in clinical cancer therapy. Moreover, overexpression of SKP2 was also demonstrated to increase radioresistance of esophageal squamous cell carcinoma and be associated with poor survival of nasopharyngeal cancer patients treated with radiotherapy [11, 16]. These emerging evidences suggest that SKP2 is involved in resistance to chemotherapy and radiotherapy by human cancers and deserves further evaluation in rectal cancers with neoadjuvant CRT. Nevertheless, thus far, no systematic study has specifically addressed whether SKP2 has effects on CRT for rectal cancers. This is the first study to focus on the expression status, prognostic significance, and in vitro therapeutic effects of chemotherapy and radiotherapy coupled with SKP2 targeting in rectal cancers.
Materials and methods
Patient eligibility and treatment plan
This is a retrospective study composed of 172 patients treated between January 1998 and December 2004 (Table 1). All patients in the study were: (a) histologically confirmed with diagnosis of adenocarcinoma of the rectum, (b) the inferior margin of the tumor being no farther than 16 cm from the anal verge, (c) without distant metastases, (d) previously not having radiotherapy in pelvic region, and (e) treated with neoadjuvant chemoradiotherapy followed by surgery. Preoperatively, radiotherapy with a total dose of 45 Gy in 25 fractions was given over a period of 5 weeks with a concurrent 24-h continuous infusion of 5-fluorouracil (5-FU). All patients were regularly monitored after the diagnosis until death or their last appointment at our hospital. The institutional review board approved procurement of formalin-fixed tissues of locally advanced rectal cancer patients for this study (IRB 10009-L04). Detailed information is provided in the supplementary data.
Histopathologic evaluation and SKP2 immunohistochemistry
After surgery, two pathologists (TJC and HYH) who were blinded to the patient’s clinical information analyzed the tumor specimens. Posttreatment (Post-Tx) T and N stages of all patients were documented according to the seventh American Joint Committee on Cancer TNM staging system [17]. The tumor regression grade (TRG), as described by Dworak et al. [21], was used as the end-point for evaluating the tumor response after CRT. Using a multiheaded microscope to reach a consensus for each case, immunoexpression of SKP2 was scored by two pathologists (TJC and HYH) without prior knowledge of the clinical results. Five groups of various expression levels from 0 to 4+, respectively denoting none, 1∼24 %, 25∼49 %, 50∼74 %, and 75∼100 % of tumor cells with moderate to strong nuclear reactivity, were clarified according to the percentage of tumor cells with SKP2 immunoexpression in each specimen. Detailed experimental protocols are provided in the supplementary data.
Cell culture
HT-29 and SW480 cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). HT-29 cells were maintained in RPMI medium supplemented with 5 % fetal bovine serum (FBS). SW480 cells were maintained in DMEM/F12 medium supplied with 10 % FBS.
Immunoblotting
Cell lysates containing 25 μg protein were separated by 4–12 % gradient NuPAGE gel (Invitrogen), transferred onto polyvinylidene difluoride membranes (Amersham), and probed with antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:3,000; Chemicon) and proteins of interest. The latter included SKP2 (1:200; Zymed), p27kip1 (1:500; Novocastra), cleaved caspase-3 (1:500; Epitomics), and Gamma-H2AX (γ-H2AX; 1:500; Upstate Biotechnology). Proteins were visualized by the Chemiluminescence System (Amersham) after incubation with the secondary antibody.
In vitro 5-FU, Bortezomib, and irradiation treatment of CRC cells
Cancer cells were seeded in 96-well plates at a density of 5 × 103 cells per well the day before treatment with the vehicle control (0.9 % saline), 5-FU, and/or Bortezomib at the indicated concentrations. Irradiation treatment was performed by monolayer irradiation with single-fraction of 5 or 10 Gy.
Cell viability assay
The viability of treated and untreated cells was determined using the XTT Assay Kit (Roche) for 0 to 72 h. In brief, the investigated cells were plated on gelatinized 96-well plates at a density of 5 × 103 cells/well and analyzed on an enzyme-linked immunosorbent assay microplate reader (Promega) at 492 nm.
Apoptosis assay
The evaluation of apoptosis upon treatment was conducted by measuring caspase-3 and caspase-7 activation with a Caspase-Glo 3/7 Assay Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions.
Statistical analysis
SPSS 14 software package was used for statistical analyses. Cases featuring 3+ or 4+ immunoreactivity were defining as high-expression of SKP2 for statistical analysis. The comparisons of SKP2 expression status between the subgroups of various clinicopathological parameters were evaluated by chi-square or Fisher exact test. Local recurrence-free survival (LRFS) metastasis-free survival (MeFS), and disease-specific survival (DSS), calculated from the date of operation to the date of event, were endpoints analyzed in this study. Survival curves were plotted using the Kaplan–Meier method, and log-rank tests were performed to evaluate prognostic differences between groups. The Cox proportional hazards model was used by multivariate analysis. For all analyses, two-sided tests of significance were used, and p < 0.05 is considered significant.
Results
Clinicopathological features and SKP2 expression in association with pre- and posttreatment tumor features
We first examined the expression of SKP2 in clinical specimens of rectal cancer using immunohistochemistry. When detected in cell nuclei, SKP2 immunoexpression was successfully scored in all 172 cases with a wide range of positively stained tumoral nuclei, varying from 5 to 95 % (median, 50 %). Low expression of SKP2, i.e., <50 % of stained tumor cells, was detected in 86 (50 %) cases (Fig. 1a). SKP2 was highly expressed in 86 (50 %) cases that displayed moderate or strong nuclear immunostaining in >50 % of tumor cells (Fig. 1b). As shown in Table 1, high expression of SKP2 was correlated with an advanced Post-Tx nodal status (p = 0.002) and International Union for Cancer Control (UICC) stage (p = 0.002). Of note, the SKP2 expression level was negatively correlated with the TRG degree (p < 0.001, Fig. 1c, d), suggesting its role in resistance to CRT. Otherwise, the SKP2 expression status was not statistically related to other clinicopathological variables. Pretreatment (Pre-Tx) and Post-Tx characteristics of all patients are summarized in Table 1.
Prognostic impact of Skp2 expression in rectal cancer
Next, we examined the correlation between SKP2 expression and the prognosis of CRC patients using a Kaplan–Meier analysis. A number of clinicopathological parameters including the Pre-Tx tumor status, Pre-Tx nodal status, Pre-Tx UICC stage, Post-Tx tumor status, Post-Tx nodal status, Post-Tx UICC stage, vascular invasion, and TRG (Fig. 2a–c) were predictive of at least one of the three endpoints of this study according to a univariate analysis (Table 2). Similarly, high expression of SKP2 was associated with shorter durations of DSS, LRFS, and MeFS (p = 0.0036, 0.0003, and 0.0032, respectively, Fig. 2d–f). More importantly, high expression of SKP2 (p = 0.027, hazard ratio 3.21) remained prognostically significant for LRFS in the multivariate analysis (Table 3). In addition, TRG, Pre-Tx nodal status, and the Post-Tx tumor status were also found to have independent prognostic impacts on survival analysis.
Bortezomib induced downregulation of SKP2 and decreased cell viability with caspase-mediated apoptosis in CRC cells treated with 5-FU
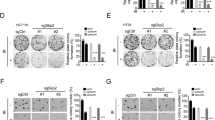
We investigated whether Bortezomib modulated SKP2 expression and affected CRC cell growth and survival. Bortezomib functions as a proteasome inhibitor, and its effect on SKP2 suppression was demonstrated [14, 18]. In our CRC cell models treated with Bortezomib, the suppressed protein level of SKP2 and concomitant increases in p27kip1 expression were confirmed by western blot analyses (Fig. 3c). Furthermore, Bortezomib treatment decreased cell viability in a dose- and time-dependent manner, with substantial inhibition (>50 %) after 72 h at concentrations of as low as 20 nM in HT-29 cells and 40 nM in SW480 cells (Fig. 3a, b). To elucidate whether cell apoptosis might be responsible for the decreased cell viability in Bortezomib-treated CRC cells, activated caspase-3 was analyzed by western blotting. After Bortezomib treatment, activation of caspase-3 with an increasing cleaved form appeared from 24 to 72 h in HT-29 and SW480 cells, as shown in Fig. 3c.
In vitro Bortezomib treatment decreased cell viability and SKP2 expression but induced caspase activation. Using an XTT assay, cell viability was significantly reduced in a dose- and time-dependent manner in both HT-29 (a) and SW480 (b) cell lines treated with the indicated doses and times of Bortezomib. After being treated with 20 nM Bortezomib or the vehicle control for 24∼72 h, protein lysates of both HT-29 and SW480 cells were harvested, and equal amounts of proteins were immunoblotted against SKP2, p27Kip1, and caspase-3 (active form), with GAPDH serving as the loading control. Bortezomib treatment clearly resulted in attenuated SKP2 expression, increased p27 expression, and activation of caspase-3 in both HT-29 and SW480 cells c
Effects of Bortezomib-induced SKP2 suppression in CRC cells treated with chemotherapy or concurrent chemoradiotherapy
5-FU is modern radiosensitizer clinically integrated in neoadjuvant CRT for rectal cancers, and we further checked whether the cytotoxicity of 5-FU against CRC cells was modulated by Bortezomib. As shown in Fig. 4a, b, the viability of CRC cells treated with 5-FU alone was notably higher than that combined with Bortezomib. Moreover, to further verify whether Bortezomib enhanced the cytotoxicity of 5-FU with various radiation doses, a quantitative chemiluminescent caspase-3/7 activity assay was used. When treated with the indicated dose of 5-FU, the addition of Bortezomib (20 nM) increased caspase-3/7 activity in a dose-dependent manner in HT-29 cells (5-fold at 10 μM 5-FU and 12.5-fold at 20 μM 5-FU, Fig. 4c) and SW480 cells (2.5-fold at 10 μM 5-FU and 3.8-fold at 20 μM 5-FU, Fig. 4d), compared to 5-FU alone. This enhanced caspase-3/7 activity modulated by Bortezomib was also observed in CRC cells when CRT with the indicated dose of 5-FU and irradiation were simultaneously added. In the presence of 10 or 20 μM 5-FU combined with 5 or 10 Gy of irradiation, a 3∼5-fold increase in caspase-3/7 activity was induced by Bortezomib in HT-29 cells, compared to the control group (Fig. 4c). Similarly, Bortezomib elicited a 3∼4.5-fold increase in caspase-3/7 activity, compared to the control group, when SW480 cells were concurrently treated with 10 or 20 μM 5-FU and 5 or 10 Gy of irradiation (Fig. 4d).
In vitro Bortezomib treatment enhanced a reduction in cell viability induced by 5-FU and increased caspase-3/7 activity and γ-H2AX formation induced by CRT. According to the XTT assay, cell viability was significantly reduced in a dose-dependent manner in both HT-29 (a) and SW480 (b) cell lines treated with the indicated doses of 5-FU when co-treated with 20 nM Bortezomib for 72 h. The dose-dependent increase in caspase-3/7 activity induced by 5-FU alone or combined with indicated dose or irradiation was confirmed by chemiluminescent assays after HT-29 (c) and SW480 (d) cells were co-treated with 10 and 20 nM Bortezomib for 72 h. In the presence of 5 Gy of irradiation, protein lysates of both HT-29 and SW480 cells were harvested after being treated with 20 μM 5-FU and/or 20 nM Bortezomib or without drug for 24 h, and equal amounts of proteins were immunoblotted against γ-H2AX, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as the loading control. The addition of Bortezomib enhanced γ-H2AX formation induced by concurrent treatment with 5-FU and irradiation in HT-29 (e) and SW480 (f) cells
Because the most damaging lesion introduced by irradiation into cells is the DNA double-strand break (DSB), we would like to determine levels of γ-H2AX, a marker of DSBs, in both cells treated with CRT and Bortezomib. In the presence of 5 Gy of irradiation, the concurrent treatment of 20 nM Bortezomib and 20 μM 5-FU significantly increased γ-H2AX formation in CRC cells (Fig. 4e, f) compared to 5-FU alone, indicating that Bortezomib could enhance DNA damage induced by CRT in our cell models.
Discussion
During stepwise tumorigenesis, deregulation of cell-cycle regulators often promotes progression of and imparts a survival advantage to aggressive tumor subsets, thereby conferring an adverse prognostic effect on patient survival [19]. The SKP2 oncoprotein is known to function as a novel oncogene by modulating the degradation of phosphorylated p27Kip1, an important cell-cycle regulator at the G1–S transition. SKP2 overexpression was also reported in various human cancers, including colon cancers [12, 18, 20], emphasizing its value as a prognostic marker for survival and as a potential therapeutic target to improve CRT outcomes in rectal cancers.
The poor prognostic effect caused by SKP2 overexpression was demonstrated because it represents biological aggressiveness of human cancers [10, 11]. In our clinical specimens, high expression of SKP2 was correlated with the advanced Post-Tx nodal status and UICC stage, implying its aggressive biological behavior with high metastatic potential. Bortezomib functions as a proteasome inhibitor, but its effect on SKP2 suppression was recently clarified [14]. In our cell models, we confirmed that Bortezomib was an SKP2 inhibitor, and its effect reduced CRC cell viability with enhanced apoptosis activation, proving the invasive potential of SKP2 expression. These aggressive biological phenotypes of the high expression of SKP2 may contribute to the poor CRT response and therefore were clinically correlated with the advanced Post-Tx nodal status and UICC stage of rectal cancer patients in this study. A low degree of TRG, indicating a worse response after CRT for rectal cancer, was proven to be closely related to poor survival and high local recurrence [21–23]. In expectation, high-expression of SKP2 was corresponding to lower TRG in our study, also demonstrating the strong correlation between SKP2 expression and poor response after neoadjuvant CRT in rectal cancer patients. Moreover, high expression of SKP2 was shown to be a prognostic factor for poor DSS, LRFS, and MeFS in the univariate analysis and poor LRFS independently in the multivariate analysis. These poor survival results related to the high expression of SKP2 are similar to those reported by Uddin et al. and Shapira et al. in colon cancer patients [18, 20].
Nevertheless, some differences exist between our current study and the two previous studies published by Uddin et al. and Shapira et al. [18, 20]. First, in those two previous studies, the majority of patients enrolled had colon cancers other than rectal cancers, indicating the presence of a selection bias. Moreover, colon cancer patients are often unnecessarily treated with radiotherapy, which differs from the situation with rectal cancers. The majority of patients in these two studies had colon cancers, and most subjects were radiotherapy-naïve. Therefore, we cannot draw conclusions directly from these two published studies as to whether SKP2 expression is correlated to outcomes of rectal cancer patients treated with CRT. Conversely, the studied population in the present study was homogenous for rectal cancer patients who received the same protocol of neoadjuvant CRT. Our results provide powerful evidence that high expression of SKP2 predicts a poor prognostic factor for rectal cancer patients with neoadjuvant CRT.
5-FU, the most commonly used antineoplastic agent applied to CRT in rectal cancers, is an antimetabolite drug that exerts its anticancer effects through inhibiting thymidylate synthase and incorporating its metabolites into RNA and DNA [24]. Resistance to 5-FU also contributes to the treatment response and survival of rectal cancer patients, but its association with SKP2 expression has not yet been clarified. In this study, the reduced cell viability induced by 5-FU in CRC cells was enhanced by the addition of Bortezomib. Furthermore, Bortezomib also enhanced DNA DSBs formation and consequent apoptosis activity in the presence of concurrent chemotherapy and radiotherapy in our cell models. These findings support the possibility of clinically integrating Bortezomib and CRT in rectal cancers. Certainly, the negative regulatory effect of the cell-cycle inhibitor, p27Kip1, may be responsible for CRT resistance modulated by SKP2. Moreover, SKP2 overexpression was demonstrated to be correlated with Akt activation, consequent glycolysis, and breast cancer metastasis and can serve as a marker for a poor prognosis in Her2-positive patients [15]. Distinct biologic functions other than cell-cycle regulation related to CRT resistance may exist in rectal cancers, and further studies are warranted. Otherwise, SKP2 overexpression was also reported to be correlated with increased radioresistance of esophageal squamous cell carcinoma (ESCC) and nasopharyngeal cancer [11, 16]. In ESCC cells, increased SKP2 expression was demonstrated to promote the double-strand break repair ability partly through the homologous recombination pathway, thereby enhancing the radiation resistance of ESCC cells. Although further validation is necessary, these adverse phenotypes of SKP2 to overcome cytotoxicity of radiotherapy are also important in addressing the poor survival of rectal cancer patients in our study.
Bortezomib is modified dipeptidyl boronic acid derived from leucine and phenylalanine that acts as an inhibitor of the 26S proteasome. Its effect on SKP2 suppression regulated through messenger RNA downregulation was demonstrated in sarcoma cells [14]. In clinical trials, clear evidence of the antitumor activity of Bortezomib was found in multiple myelomas and B cell lymphomas with manageable side effects [25]. Therefore, further studies integrating Bortezomib or another SKP2 inhibitor into neoadjuvant CRT protocols for rectal cancers are also warranted.
In conclusion, we found for the first time that high expression of SKP2 was correlated with an advanced node status and a poor response and prognosis of rectal cancer patients who received neoadjuvant CRT. Suppression of SKP2 expression by Bortezomib attenuated the viability of CRC cells and promoted the cytotoxicity of 5-FU alone or in the presence of concurrent irradiation. Our results not only suggest the poor prognosis and resistance of CRT contributed by the high expression of SKP2 but also provide fundamental evidence of SKP2 inhibition applied to CRT protocols in rectal cancers.
Abbreviations
- CRC:
-
Colorectal cancer
- CRT:
-
Chemoradiotherapy
- SKP2:
-
S-phase kinase-associated protein 2
References
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–5.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
National Comprehensive Cancer Network Home Page. http://www.nccn.org/. Accessed 10 Jan 2011.
Hyams DM, Mamounas EP, Petrelli N, Rockette H, Jones J, Wieand HS, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum. Dis Colon Rectum. 1997;40(2):131–9.
Valentini V, Coco C, Cellini N, Picciocchi A, Genovesi D, Mantini G, et al. Preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation. Int J Radiat Oncol Biol Phys. 1998;40(5):1067–75.
Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys. 1998;42(1):51–7.
Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, et al. P45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1(4):207–14.
Yokoi S, Yasui K, Saito-Ohara F, Koshikawa K, Iizasa T, Fujisawa T, et al. A novel target gene, skp2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am J Pathol. 2002;161(1):207–16.
Huang HY, Kang HY, Li CF, Eng HL, Chou SC, Lin CN, et al. Skp2 overexpression is highly representative of intrinsic biological aggressiveness and independently associated with poor prognosis in primary localized myxofibrosarcomas. Clin Cancer Res. 2006;12(2):487–98.
Fang FM, Chien CY, Li CF, Shiu WY, Chen CH, Huang HY. Effect of S-phase kinase-associated protein 2 expression on distant metastasis and survival in nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2009;73(1):202–7.
He QJ, Zeng WF, Sham JST, Xie D, Yang XW, Lin HL, et al. Recurrent genetic alterations in 26 colorectal carcinomas and 21 adenomas from Chinese patients. Cancer Genet Cytogenet. 2003;144(2):112–8.
Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12(5):457–67.
Li CF, Wang JM, Kang HY, Huang CK, Wang JW, Fang FM, et al. Characterization of gene amplification-driven skp2 overexpression in myxofibrosarcoma: potential implications in tumor progression and therapeutics. Clin Cancer Res. 2012;18(6):1598–610.
Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149(5):1098–111.
Wang XC, Tian LL, Tian J, Jiang XY. Overexpression of SKP2 promotes the radiation resistance of esophageal squamous cell carcinoma. Radiat Res. 2011;177(1):52–8.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Uddin S, Ahmed M, Bavi P, El-Sayed R, Al-Sanea N, AbdulJabbar A, et al. Bortezomib (Velcade) induces p27kip1 expression through s-phase kinase protein 2 degradation in colorectal cancer. Cancer Res. 2008;68(9):3379–88.
Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98(9):5043–8.
Shapira M, Ben’Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103(7):1336–46.
Dworak OKL, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Lin CY, Tian YF, Wu LC, Chen LT, Lin LC, Hsing CH, et al. Rsf-1 expression in rectal cancer: with special emphasis on the independent prognostic value after neoadjuvant chemoradiation. J Clin Pathol. 2012;65(8):687–92.
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8.
Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8(8):2505–11.
Acknowledgments
This work was supported in part by grants from the National Science Council, Taiwan (NSC99-2320-B-384-001-MY2 to CF Li) and Department of Health, Taiwan (DOH99-TD-C-111-004). The authors are grateful to the Translational Research Laboratory of Human Cancers of Chi-Mei Medical Center for providing critical technical assistance.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 31 kb)
Rights and permissions
About this article
Cite this article
Tian, YF., Chen, TJ., Lin, CY. et al. SKP2 overexpression is associated with a poor prognosis of rectal cancer treated with chemoradiotherapy and represents a therapeutic target with high potential. Tumor Biol. 34, 1107–1117 (2013). https://doi.org/10.1007/s13277-013-0652-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0652-z