Abstract
Gallbladder cancers (GBC) are associated with high disease-specific mortality rates because of no means of early detection and effective therapies. In this study, we investigated CD146 expression, microvessel densities, and lymph vessel densities in 108 adenocarcinomas, 15 gallbladder polyps, 35 chronic cholecystitis tissues, and 46 peritumoral tissues using immunohistochemistry. We demonstrated that positive CD146 expression, and average microvessel and lymph vessel counts in gallbladder adenocarcinomas were significantly higher than those in peritumoral tissues, polyps, and chronic cholecystitis (ps < 0.01). Positive CD146 expression, and average microvessel and lymph vessel counts were also significantly lower in cases with well-differentiated adenocarcinoma, maximal tumor diameter <2 cm, no metastasis of lymph node, and no invasion of regional tissues than in cases with poorly differentiated adenocarcinoma, maximal tumor diameter ≥2 cm, metastasis in lymph nodes, and invasion of regional tissues (p < 0.05 or p < 0.01). Univariate Kaplan–Meier analysis showed that increased expression of CD146 (p = 0.056), higher average microvessel counts (p < 0.05), and lymph vessel counts (p < 0.05) were associated with decreased overall survival. Multivariate Cox regression analysis showed that average microvessel and lymph vessel counts (ps < 0.05) were independent prognostic predictors in gallbladder adenocarcinoma. Our study suggested that the elevated expression of CD146, angiogenesis, and lymphangiogenesis might be closely related to progression, invasion, metastasis, and prognosis of gallbladder adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder cancers (GBC) are the most common biliary tract malignancy and the fifth most common cancer in the gastrointestinal tract [1, 2]. Most GBCs (over 90%) are adenocarcinomas [3], though mucinous, papillary, squamous, and adenosquamous carcinoma are encountered occasionally [4]. A progressive increase in incidence and mortality for GBC has been reported worldwide [5]. However, effective means of early diagnosis has not been established. More than 90% of cases are diagnosed when tumors are unresectable with local invasion of critical structures or metastasis beyond the primary cancer site [6]. To date, surgery remains the only effective treatment for patients with resectable GBC. For patients with unresectable GBC, palliative chemotherapy and radiotherapy remain the only possible treatments that can be offered, but these therapies provide little benefit to the patient’s survival [7]. Therefore, understanding the tumor biology of GBC may help in developing a more successful strategy for early diagnosis and/or treatment. However, studies currently available in literature do not clearly define the mechanisms involved in the pathogenesis of GBC. Although biomarkers for screening programs and predicting prognosis are under investigation [8], currently none of the proposed markers have reached clinical application.
More and more evidence support the notion that formation of new blood vessels (angiogenesis) significantly contributes to cancer progression and metastasis of solid tumors [9–11]. Recently, microvessel density has been considered an objective marker reflecting progression, metastasis, recurrence, and prognosis of malignant tumors [12, 13]. Analysis of microvessel density in solid tumors is commonly done by staining endothelial cell surface molecules, such as CD31, using immunohistochemistry. CD31 staining has revealed a good relationship between microvessel density in tumor specimen and the prognosis of different tumors including prostate, colon, esophagus, breast cancer, etc. [14]. However, CD31 reacts mildly with fibroblasts and some plasma cells, and generally gives fairly weak expression in tissues. Also, CD31 staining failure is often encountered. For example, failure rate in routinely fixed breast specimens can reach as high as 20%. In contrast, CD34 is a cell surface sialomucin-like glycoprotein expressed by endothelial cells and has the same characteristics as CD31, but with a low rate of staining failure [15, 16]. For instance, a study in non-small cell lung cancer revealed that high microvessel density determined by CD34 is more closely related to poor survival than other neoangiogenetic factors. This may be because CD34 is more closely related to the metastatic process [17]. However, the correlation between CD34 and angiogenesis in GBC progression and prognosis is rarely reported.
The importance of lymphatic metastases in different cancer stages and in treating patients with solid tumors is readily acknowledged in literature. Recent advances in the biology and pathology of lymphangiogenesis have indicated that tumor-induced lymphangiogenesis is a predictive indicator of metastasis to lymph nodes [18, 19]. However, lymphangiogenesis studies are limited by a lack of specific lymphatic endothelial markers. Recently, the commercially available monoclonal antibody D2-40 has demonstrated a selective immunoreactivity for lymphatic endothelium [20]. D2-40 is initially considered to be directed against the M2A antigen, a 40-kDa O-linked sialoglycoprotein found on the cell surface of testicular gonocytes, germ cell tumors, lymphatic endothelium, and mesothelial cells [20]. However, recent studies revealed that D2-40 can also selectively detect lymphatic vessels in other tumor tissues such as breast and tonsillar tissue, etc. [21]. The analysis of lymphangiogenesis with D2-40 in GBC has not yet been reported.
CD146, also known as melanoma cell adhesion molecule, is a thoroughly studied adhesion marker of endothelial cells. CD146 is initially identified as a marker of melanoma progression and metastasis [22]. Recent studies revealed that CD146 is primarily expressed on vascular endothelium, smooth muscle, and other cells in normal tissues and mediates cation-independent adhesion through interactions with an unidentified ligand on the surface of various cells [23]. Moreover, CD146 expression significantly correlates with the progression, angiogenesis, metastasis, and prognosis of some malignant tumors [9, 24, 25]. CD146 is now used as the sole criterion to identify circulating endothelial cells and subsequently isolate these cells from peripheral blood [26]. Therefore, the biological functions and role of CD146 as a diagnostic marker are starting to be recognized. However, the relationship between CD146 and angiogenesis, lymphangiogenesis, as well as the progression and prognosis of GBC have not yet been identified.
In this study, the expressions of CD146, microvessel, and lymph vessel counts in resection specimens, including adenocarcinoma, peritumoral tissue, polyp, and chronic cholecystitis of gallbladder, were examined using immunohistochemistry. The correlations of CD146, microvessel, and lymph vessel counts with the behavior and prognosis of adenocarcinoma as well as its clinical manifestations and survival rate of patients were evaluated.
Materials and methods
Case selection
A total of 204 specimens, including 108 adenocarcinomas, 46 peritumoral tissues, 35 chronic cholecystitis tissues, and 15 gallbladder polyps, were studied ethically with pre-approval from the Ethics Committee of Human Study of Central South University. All of these samples were collected from the First and Second Xiangya Hospitals, Central South University, and the Peoples’ Hospital of Hunan Province. Among the 108 adenocarcinoma, 77 cases were female (71.3%) and 31 cases were male (28.7%) with ages ranging from 37 to 78 years and an average age of 52.6 ± 11.2 years. Diagnosis of adenocarcinomas was based on morphological criteria, immunohistochemical staining, and clinical findings. Histopathologic subtypes of the 108 adenocarcinomas include 36 well-differentiated adenocarcinomas (33.3%), 31 moderately differentiated adenocarcinomas (28.7%), 30 poorly differentiated adenocarcinomas (27.8%), and 11 mucinous adenocarcinomas (10.2%). Invasion and lymph metastases were evaluated according to the standard criteria [27]. Among the 108 adenocarcinomas, 59 cases (54.6%) had invasion of peri-cholecystic tissues and organs, while 59 cases had regional lymph node metastasis (54.6%) and 58 cases had gallstones (53.7%). Surgery included radical resection for 34 adenocarcinomas (31.5%), palliative surgery for 48 adenocarcinomas (44.4%), and no resection for 26 cases (24.1%) with only surgical biopsy. Survival information of 67 cases among the 108 adenocarcinomas was obtained through letters and phone calls. Among them, 20 patients survived over 1 year and 47 cases survived less than 1 year.
Chronic cholecystitis, gallbladder polyp, and peritumoral tissues were diagnosed according to the published standard criteria [28]. Among 35 cases of chronic cholecystitis, only 15 cases had chronic cholecystitis while 20 had chronic cholecystitis accompanied by gallstones. The classification of normal, mild, moderate, and severe dysplasia was determined according to the criteria described by Dowling and Kelly [29]. Among the 35 cases of chronic cholecystitis, 11 cases without cellular atypia were identified as normal mucosa, 12 cases with mild cellular atypia as mild dysplasia, seven cases with moderate cellular atypia as moderate dysplasia, and five cases with severe cellular atypia as severe dysplasia. Among the 15 cases with gallbladder polyp, five cases were males (33.3%) and 10 cases were females (66.7%). The pathological examination confirmed that 10 polyps had epithelial normal to mild dysplasia and five had moderate to severe dysplasia. Among the 46 peritumoral tissues (distance from cancer ≥3 mm), 10 tissues were normal, 10 tissues had mild dysplasia, 12 tissues had moderate dysplasia, and 14 tissues had severe dysplasia.
Immunohistochemistry
Rabbit anti-human CD146 and CD34 antibodies, D2-40 mouse monoclonal antibodies, HRP-conjugated anti-rabbit/mouse secondary antibodies, and streptavidin–peroxidase detection kits were purchased from Gene Tech Company Limited (Switzerland). EnVisionTM Detection kit was purchased from Dako Company (CA, USA). The positive controls of CD146, microvessel, and lymph vessel staining were positive sections provided by Dako Company. The negative controls used 5% fetal bovine serum in place of antibodies. EnVision immunohistochemistry of CD146 expression was performed according to the manufacturer’s protocol. Briefly, 4-μm-thick sections were cut from routinely paraffin-embedded tissues. The sections were deparaffinized and then incubated with peroxidase inhibitor (3% H2O2) in dark for 15 min, followed by EDTA–trypsin digestion (0.125%, pH 9.0) for 15 min. The sections were incubated with rabbit anti-human CD146 primary antibody for 60 min. Solution A (ChemMateTMEnVison +/HRP) was added to the sections for 30 min followed by DAB staining and hematoxylin counterstaining. The slides were dehydrated with different concentrations (70–100%) of alcohol and soaked in xylene for 3 × 5 min and finally mounted with neutral balsam. Fifteen random fields were examined per section. The percent of positively stained cells relative to the total number of cells was determined in random fields at × 200. The staining strength was graded from 1 to 3: 1 score for no positive staining or a weak staining, 2 scores for weak to moderate staining, and 3 scores for moderate to strong staining. The case with positive cells ≥25% and/or scores ≥2 was considered positive [30, 31]. A few sections where percent positive staining was 5% to 10% and staining strength was 3 were also regarded as positive [31].
Microvessel and lymph vessel densities were determined by immunohistochemical staining with anti-CD34 and D2-40 antibody, respectively. Briefly, the sections were treated as described above. The sections were then incubated with rabbit anti-human CD34 primary antibody or mouse D2-40 antibody for 60–120 min at room temperature, followed by HRP-conjugated anti-rabbit/mouse second antibody for 30 min after being soaked with PBS for 3 × 5 min. Solution B was added to the sections for 30 min followed by solution C reagent for 30 min. After sections were washed with PBS for 3 × 5 min, the substrate AEC liquid was added and followed by hematoxylin counterstaining. The slides were then dehydrated with different concentrations of alcohol, soaked in xylene, and mounted permanently with neutral balsam. Microvessels and lymph vessels were counted in 10 random fields at × 200 per section, and average microvessel and lymph vessel count per field was calculated.
Statistical analysis
Data was analyzed using the Statistical Package for the Social Sciences version 13.0 (SPSS 13.0). The inter-relationship of CD146 expression, microvessel, and lymph vessel count with histology or clinical factors was analyzed using χ 2 independence or Fisher’s exact test. Kaplan–Meier and log-rank test were used for univariate survival analysis. Cox proportional hazards model was used for multivariate analysis and for determining the 95% confidence interval. A p <0.05 was considered statistically significant.
Results
CD146 expression and microvessel and lymph vessel counts in adenocarcinoma of gallbladder, peritumoral tissue, polyp, and gallbladder epithelium with chronic cholecystitis
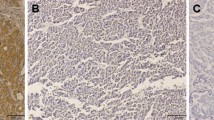
EnVision immunohistochemistry revealed that CD146 positive reaction was mainly localized in the membrane and some in the cytoplasm (Fig. 1b). Significant increases in microvessel and lymph vessel staining were observed in tumor tissues (Figs. 2a and 3a). As shown in Table 1, the rate of CD146 expression was significantly higher in adenocarcinoma of gallbladder (53.7%) than in peritumoral tissues (30.4%), polyps (20.0%), or gallbladder epithelium with chronic cholecystitis (5.7%) (ps < 0.01). Similarly, the average microvessel and lymph vessel counts per field were significantly higher in adenocarcinoma (64.2 ± 11.6 and 11.4 ± 5.2, respectively) than in peritumoral tissues (28.2 ± 12.4 and 6.8 ± 6.2, respectively), polyps (32.4 ± 11.8 and 5.6 ± 2.9, respectively), or gallbladder epithelium with chronic cholecystitis (22.2 ± 11.3 and 5.1 ± 3.9, respectively) (ps < 0.01). As shown in Table 2, the frequencies of the average microvessel counts ≥64.2 or lymph vessel counts ≥11.4 were significantly higher in adenocarcinoma (54.6% and 51.9%, respectively) than in peritumoral tissues (13.0% and 10.9%, respectively), polyps (6.7% and 6.7%, respectively), and gallbladder epithelium with chronic cholecystitis (0.0% and 0.0%, respectively) (ps < 0.01). Of the 58 CD146-positive cases, the average microvessel and lymph vessel counts were 78.6 ± 12.3 and 14.5 ± 5.8, respectively. Of the 50 CD146-negative cases, the average microvessel and lymph vessel counts were 47.4 ± 11.6 and 7.8 ± 5.2, respectively. Average microvessel and lymph vessel counts in CD146-positive cases were significantly higher than those in CD146-negative cases in gallbladder adenocarcinoma (t = 13.51, p < 0.01 and t = 6.32, p < 0.01, respectively). In addition, the high correlation was found between the microvessel and lymph vessel counts in gallbladder adenocarcinoma (r = 0.465, p < 0.01).
Immunohistochemistry of CD146 expression. EnVision immunohistochemistry, original magnification × 200. Positive reaction was mainly localized in the cell membrane and/or cytoplasm. a Negative CD146 expression was observed in benign gallbladder tissue. b Positive CD146 expression in moderately differentiated adenocarcinoma
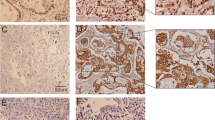
Microvessel staining by immunohistochemistry using anti-CD34 antibody. Immunohistochemistry, original magnification × 200. Significantly more CD34 staining was seen in adenocarcinoma specimen. a Positive CD34 staining in moderately differentiated adenocarcinoma. b Rare CD34 staining was observed in benign gallbladder tissue
Lymph vessel staining by immunohistochemistry using D2-40 antibody. Immunohistochemistry, original magnification × 200. Significantly more D2-40 positive staining was seen in adenocarcinoma specimen. a Positive D2-40 staining in poorly differentiated adenocarcinoma. b Rare D2-40 positive staining was observed in benign gallbladder tissue
CD146 expression, microvessel counts, and lymph vessel counts were associated with clinicopathological characteristics of gallbladder adenocarcinoma
As shown in Table 3, positive expression of CD146, average microvessel counts, and lymph vessel counts were significantly lower in cases with well-differentiated adenocarcinoma, small tumor size (<2 cm), no metastasis in lymph node, and no invasion compared to cases with poorly differentiated adenocarcinoma, larger tumor size (>2 cm), lymph node metastasis, and invasion of the gallbladder surrounding tissues and organs (p < 0.05 or p < 0.01). The expression of CD146 and average counts of microvessel and lymph vessels exhibited no significant association with mucinous adenocarcinoma or other clinicopathological characteristics, such as the sex, age, or history of gallstones (ps > 0.05).
CD146 expression, average microvessel counts, and lymph vessel counts correlated with survival in patients with gallbladder adenocarcinoma
Among the 108 gallbladder adenocarcinoma, survival information of 67 patients was collected. Of the 67 cases, 20 cases survived over 1 year, but 47 cases died within 1 year with a mean survival time of 9.6 ± 5.2 months. Among the 67 cases, 35 cases (52.2%) had CD146-positive expression, while 35 cases (52.2%) showed average microvessel count ≥64.2 and 33 cases (49.3%) showed average lymph vessel count ≥11.4, respectively. Kaplan–Meier survival analysis revealed that the histological type (p < 0.05), tumor size (p < 0.01), lymph node metastasis (p < 0.01), and degree of invasion (p < 0.01) were significantly associated with the average survival time in gallbladder adenocarcinoma (Table 4). Average survival time for CD146-positive patients with average microvessel count (≥64.2) or lymph vessel count (≥11.4) was significantly lower than those with negative CD146 expression, and lower microvessel count and lymph vessel count (p = 0.056, p < 0.05, p < 0.05, respectively) (Fig. 4a–c). Cox multivariate analysis (Table 5) showed that tumor size (≥2 cm), lymph node metastasis, invasion, CD146 expression levels, average microvessel counts, and lymph vessel counts were negatively correlated with survival and positively correlated with mortality, suggesting that CD146, angiogenesis, and lymphangiogenesis are relative risk factors for prognosis.
CD146 expression, microvessel (MV) and lymph vessel (LV) counts, and survival in patients with adenocarcinoma of gallbladder. a Kaplan–Meier plots of overall survival in patients with gallbladder adenocarcinoma and with CD146 positive and negative expression. b Kaplan–Meier plots of overall survival in patients with gallbladder adenocarcinoma and with higher and lower MV counts. c Kaplan–Meier plots of overall survival in patients with gallbladder adenocarcinoma and with higher and lower LV counts
Discussion
Although the overall incidence of gallbladder cancers is much less than that of other cancers, over 90% of cases are diagnosed at an advanced stage with invasion and metastasis. At present, palliative chemotherapy and radiation therapy offer limited benefits to patients’ survival. Few significant improvements were made during the past three decades regarding the treatment of GBC, which might be because of the lack of patients with bile duct neoplasms [32]. Despite the fact that several genetic abnormalities within GBC had been clearly described [33], the molecular mechanisms involved in the pathogenesis of GBC are not fully understood. In this study, we demonstrated that CD146 expression, and average microvessel and lymph vessel counts in gallbladder adenocarcinoma were significantly higher than those in peritumoral tissues, polyp, and chronic cholecystitis. Also, elevated expression of CD146, and higher angiogenic activity and lymphangiogenesis closely related to progression, metastasis, invasion, and prognosis of gallbladder adenocarcinoma.
Angiogenesis plays a very important role in the progression and metastasis of solid tumors. Without angiogenesis, solid tumors can only grow to a critical size of 1–2 mm due to a lack of adequate oxygen and nutrition supply [34]. Highly vascularized primary tumors have revealed a higher incidence of metastasis than poorly vascularized tumors. The role of angiogenesis in GBC progression has been studied [35, 36]. However, microvessel density identified by CD34 has been revealed to be more closely related to metastatic processes than the other neoangiogenetic factors, including CD31 [17]. In this study, we first investigated the correlation of angiogenesis and metastasis in collected GBC samples. Average microvessel counts identified by CD34 expression were significantly higher in cases with metastasis in lymph nodes and invasion of regional tissues. Higher average microvessel counts were also associated with decreased overall survival. Our study suggested that angiogenesis closely relates to progression and prognosis of gallbladder adenocarcinoma. This is consistent with previous findings in other types of solid tumors [14, 37].
Metastasis is a hallmark of poor prognosis for solid cancer [38]. The importance of tumor-induced lymphangiogenesis in solid tumor metastasis has been widely recognized [18, 19]. Lymphangiogenesis is a significant step in the lymphatic metastasis of tumors. Neonatal lymph vessels are susceptible to tumor cells entering lymph vessels and finally cause metastasis to regional lymph nodes [39, 40]. More and more studies have found that lymph vessel density has a close relationship with progression, metastasis, and prognosis of malignant tumors [41, 42]. However, the correlation of lymph vessel density with progression and prognosis of GBC has not been reported. This might be limited by a lack of specific lymphatic endothelial markers as well as a lack of patients with bile duct neoplasms. M2A is a sialoglycoprotein which is heavily expressed in lymphatic endothelial cells. In contrast, matured vascular endothelial cells rarely express M2A [43]. D2-40 monoclonal antibodies are able to recognize a stabile epitope of M2A. Therefore, D2-40 could be used as a specific marker of lymphatic endothelial cells [44, 45]. In this study, we first demonstrated that lymph vessel density identified by D2-40 significantly correlated with progression, metastasis, invasion, and prognosis of gallbladder adenocarcinoma.
To establish metastatic foci, tumor cell arrest must be initialized by local endothelial cells through adhesive interactions with the endothelium [46]. However, key molecules involved in metastatic formation have not yet been fully identified. CD146 is a highly studied adhesion marker of endothelial cells with high expression in neogenetic vessels [23, 24, 47]. With the fact that CD146 has been used as the sole criterion to identify and isolate circulating endothelial cells [26], we proposed that circulating endothelial cells with CD146 expression may play a crucial role in the angiogenesis of metastatic tumors. Recent reports demonstrated that some epithelial malignant tumors such as breast cancer, esophageal cancer, ovarian cancer, melanoma, and prostate cancer have high CD146 expression, whereas their original normal tissues have very low or scarcely any CD146 expression. Furthermore, CD146 expression in these tumor specimens is significantly associated with angiogenesis and metastasis of tumors as well as the progression and prognosis of diseases [9, 23–25]. In this study, we first demonstrated that CD146 level in GBC specimen correlated with tumor progression, metastasis, invasion, and survival of patients. Importantly, average microvessel counts and lymph vessel counts in CD146-positive cases were significantly higher than those in CD146-negative cases with gallbladder adenocarcinoma, which suggests that CD146 is associated with angiogenesis and lymphangiogenesis in GBC.
In conclusion, our study suggests that CD146 is associated with angiogenesis and lymphangiogenesis of gallbladder adenocarcinoma, and positive expression of CD146 is an important biomarker for metastasis, progression, and prognosis of the disease. Microvessel density can be identified by CD34 expression and lymph vessel density can be determined by D2-40 immunohistochemistry. These two indicators could reflect angiogenesis and lymphangiogenesis of gallbladder adenocarcinoma, which significantly correlates with progression, metastasis, and prognosis of the disease. Therefore, the CD146 level, microvessel density, and lymph vessel density could help determine the prognosis of gallbladder adenocarcinoma.
References
Jones RS. Carcinoma of the gallbladder. Surg Clin North Am. 1990;70:1419–28.
Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171–90.
Ootani T, Shirai Y, Tsukada K, Muto T. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647–52.
Jayaraman S, Jarnagin WR. Management of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:331–42.
Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–60.
Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310–5.
Jayaraman S, Jarnagin WR. Management of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:331–42.
Maurya SK, Tewari M, Mishra RR. Genetic aberrations in gallbladder cancer. Surg Oncol. 2010; Sep 28. [Epub ahead of print]
Loges S, Clausen H, Reichelt U, Bubenheim M, Erbersdobler A, Schurr P, et al. Determination of microvessel density by quantitative real-time PCR in esophageal cancer: correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin Cancer Res. 2007;13:76–80.
Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4.
He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta. 2004;1654:3–12.
Delatorre NG, Buley I, Wass JA, et al. Angiogenesis and lymphangiogenesis in thyroid proliferative lesions: relationship to type and tumor behaviors. Endocr Relat Cancer. 2006;13:934–44.
Aldovinni D, Demichelis F, Doglioni C, Di Vizio D, Galligioni E, Brugnara S, et al. MCAM expression as marker of prognosis in epithelial ovarian cancer. Int J Cancer. 2006;119:1920–6.
Hasan J, Byers R, Jayson GC. Intra-tumoural microvessel density in human solid tumours. Br J Cancer. 2002;86:1566–77.
Beenken SW, Bland KI. Biomarkers for breast cancer. Minerva Chir. 2002;57:437–48.
Legan M. New marker of angiogenesis CD105 (endoglin): diagnostic, prognostic and therapeutic role. Radiol Oncol. 2005;39:253–9.
Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, Vincenzi B, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB–IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–7.
Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298–07.
Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–16.
Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s and a subset of angiosarcomas. Mod Pathol. 2002;15:434–40.
Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Modern Pathol. 2005;18:105–10.
Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA. 1989;86:9891–5.
Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795:130–6.
Garcia S, Dales JP, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and C-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–41.
Watson-Hurst K, Becker D. The role of N-cadherin, MCAM and beta3 integrin in melanoma progression, proliferation, migration and invasion. Cancer Biol Ther. 2006;5:1375–82.
Yang H, Wang S, Liu Z, Wu MH, McAlpine B, Ansel J, et al. Isolation and characterization of mouse MUC18 cDNA gene, and correlation of MUC18 expression in mouse melanoma cell lines with metastatic ability. Gene. 2001;265:133–45.
Jayaraman S, Jarnagin WR. Management of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:331–42.
Yamagiwa H, Tomiyama H, Yoshimura H. Histogenesis of carcinoma of gallbladder. Histological study of serial sections of 1000 cases of resected gallbladder. Rinsho Byori. 1986;34:475–80.
Dowling GP, Kelly JK. The histogenesis of adenocarcinoma of the gallbladder. Cancer. 1986;58:1702–8.
Sanada Y, Yoshida K, Ohara M, Tsutani Y. Expression of orotate phosphoribosyltransferase (OPRT) in hepatobiliary and pancreatic carcinoma. Pathol Oncol Res. 2007;13:105–13.
Chang HJ, Yoo BC, Kim SW, Lee BL, Kim WH. Significance of PML and p53 protein as molecular prognostic markers of gallbladder carcinomas. Pathol Oncol Res. 2007;13:326–35.
Tucek S, Tomasek J, Halámkova J. Bile duct malignancies. Klin Onkol. 2010;23:231–41.
Malik IA. Gallbladder cancer: current status. Expert Opin Pharmacother. 2004;5:1271–7.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
Sugawara Y, Makuuchi M, Harihara Y, Noie T, Inoue K, Kubota K, et al. Tumor angiogenesis in gallbladder carcinoma. Hepatogastroenterology. 1999;46:1682–6.
Giatromanolaki A, Sivridis E, Koukourakis MI, Polychronidis A, Simopoulos C. Prognostic role of angiogenesis in operable carcinoma of the gallbladder. Am J Clin Oncol. 2002;25:38–41.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Iiizumi M, Liu W, Pai SK, Furuta E, Watabe K. Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta. 2008;1786:87–104.
Dumoff KL, Chu C, Xu X, Pasha T, Zhang PJ, Acs G. Low D2-40 immunoreactivity correlates with lymphatic invasion and nodal metastasis in early-stage squamous cell carcinoma of the uterine cervix. Mod Pathol. 2005;18:97–104.
Kadota K, Huang CL, Liu D, Ueno M, Kushida Y, Haba R, et al. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44:1057–67.
Hardavella G, Arkoumani E, Dalavanga Y, Galanis P, Agnantis NJ, Constantopoulos S, et al. Prognostic impact of lymphangiogenesis and lymphatic invasion (CD105 expression) in small cell lung carcinoma. Anticancer Res. 2008;28:343–7.
Thelen A, Scholz A, Benckert C, Weichert W, Dietz E, Wiedenmann B, et al. Tumor-associated lymphangiogenesis correlates with lymph node metastases and prognosis in hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:791–9.
Kristiansen G, Yu Y, Schlüns K, Sers C, Dietel M, Petersen I. Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal Cell Pathol. 2003;25:77–81.
Walgenbach-Bruenagel G, Tolba RH, Varnai AD, Bollmann M, Hirner A, Walgenbach KJ. Detection of lymphatic invasion in early stage primary colorectal cancer with the monoclonal antibody D2-40. Eur Surg Res. 2006;38:438–44.
Jackson DG. Lymphatic markers, tumour lymphangiogenesis and lymph node metastasis. Cancer Treat Res. 2007;135:39–53.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72.
Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Yang, Zl., Liu, Jq. et al. Identification of CD146 expression, angiogenesis, and lymphangiogenesis as progression, metastasis, and poor-prognosis related markers for gallbladder adenocarcinoma. Tumor Biol. 33, 173–182 (2012). https://doi.org/10.1007/s13277-011-0260-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0260-8