Abstract
Background
Type Three Secretion Systems (T3SS) are nanomachine complexes, which display the ability to inject effector proteins directly into host cells. This skill allows for gram-negative bacteria to modulate several host cell responses, such as cytoskeleton rearrangement, signal transduction, and cytokine production, which in turn increase the pathogenicity of these bacteria. The Salmonella enterica subsp. enterica serovar Typhimurium (ST) T3SS has been the most characterized so far. Among gram-negative bacterium, ST is one of enterica groups predicted to have two T3SSs activated during different phases of infection.
Objective
To comprise current information about ST T3SS structure and function as well as an overview of its assembly and hierarchical regulation.
Methods
With a brief and straightforward reading, this review summarized aspects of both ST T3SS, such as its structure and function. That was possible due to the development of novel techniques, such as X-ray crystallography, cryoelectron microscopy, and nano-gold labelling, which also elucidated the mechanisms behind T3SS assembly and regulation, which was addressed in this review.
Conclusion
This paper provided fundamental overview of ST T3SS assembly and regulation, besides summarized the structure and function of this complex. Due to T3SS relevance in ST pathogenicity, this complex could become a potential target in therapeutic studies as this nanomachine modulates the infection process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over time and evolution, gram-negative bacteria have developed many resources to increase their entrance and colonization into host cells (Kimbrough and Miller 2002). Many of these resources are modulated by protein secretion, such as DNA replication, motility, transport, antibiotic resistance, and pathogenicity (Portaliou et al. 2016). Thus, the understanding of the molecular mechanisms behind this secretion and the manipulation of host responses through these resources is crucial (Haraga et al. 2008). One of these mechanisms comprises secretion systems, which can transport proteins throughout cell membranes. This is considered a biochemistry accomplishment due to its regulation and complexity (Deng et al. 2017). Gram-negative bacteria currently contain nine of these systems, categorized from I to IX (Desvaux et al. 2009; Vincent et al. 2017). Type III Secretion Systems (T3SS), however, are the most sophisticated and well-studied (Lee 1997; Cornelis 2006; Galkin et al. 2010; Büttner 2012; Fujii et al. 2012; Abby and Rocha 2012). T3SS is present in several gram-negative bacteria, including Shigella, Yersinia, Pseudomonas, Enteropathogenic Escherichia coli (EPEC), and Salmonella spp. (Costa et al. 2015). It is predicted to be an essential and specialized organelle that translocates effector proteins directly into the host cell cytoplasm (Kimbrough and Miller 2002). Once within the cell, these proteins can alter cellular functions, such as cytoskeleton structure, signal transduction, and cytokine production, among others (Haraga et al. 2008). To accomplish this, T3SS covers three cell membranes, namely the inner and outer bacterial membranes and the host eukaryotic cell membrane (Costa et al. 2015). It comprises over 20 proteins related evolutionarily with the flagellar export system (Haraga et al. 2008). T3SS's assembly, like flagella, is complex and is carefully coordinated by regulatory proteins (Portaliou et al. 2016). Thus, the synthesis of proteins that becomes coupled to this machinery follows a strict production and assembly hierarchy, which makes T3SS highly regulated and one of the most complicated known bacteria secretion systems (Portaliou et al. 2016).
Among all T3SS’s, Salmonella enterica T3SS’ is the best characterized so far (Kimbrough and Miller 2000). This species is the only one presenting two T3SS (T3SS-1 and 2) activated during different phases of infection (Hansen-Wester and Hensel 2001). Most studies have been performed in Salmonella enterica subspecies enterica serovar Typhimurium (ST) (Collazo and Galán 1997; Cirillo et al. 1998; Crago and Vassilis 2002; Galkin et al. 2010; Bergeron et al. 2013). In this review, we compile a set of relevant information about T3SS’s ST. With a brief and straightforward reading for quick understanding and knowledge, we report aspects of the T3SS present in this serovar, like structure, assembly, and regulation. As this is a featured article, renowned reviews like Portaliou et al. (2016), Deng et al. (2017), among others, are recommended for more accurate readings. The prefix Secretion and cellular translocation (Sct) was recently adopted to unify the nomenclature of all proteins belonging to T3SS since its structure is present in a diversity of gram-negative bacteria (Hueck 1998). Thus, this review adopts the Sct nomenclature, since it addresses the aspects of the two T3SS present in ST. Table 1 presents the relation between the Sct prefixes and their respective proteins.
ST T3SS functions
T3SS plays a crucial role in gram-negative bacterial infection, since it is required in almost all phases of infection and consequently for bacteria virulence maintenance (van der Heijden and Finlay 2012). In ST, the Salmonella Pathogenicity Island 1 (SPI-1) encodes the first T3SS (T3SS-1) (Haraga et al. 2008), whereas SPI-2 encodes the second, T3SS-2 (Hansen-Wester and Hensel 2001). SPI are chromosomal regions that carry virulence genes, which act as a compact and distinct genetic unit during a specific time of the infection (Marcus et al. 2000). In ST, five main SPIs have been described, with SPI-1 and SPI-2 the best studied, due to their correlation with the invasion phase and persistence inside the cell, respectively (Deiwick et al. 1998; Cirillo et al. 1998; Hensel 2000, 2004; Coburn et al. 2005; Ellermeier and Slauch 2007; Brawn et al. 2007; dos Santos et al. 2018).
T3SS-1 acts during the first moments of invasion, initiating contact between the bacteria and the non-phagocytic host cell membrane (Hueck 1998; Hansen-Wester and Hensel 2001). After contact, a pore in the eukaryotic cell membrane is formed (Cornelis 2006; Galán and Wolf-Watz 2006; Matteï et al. 2011), which allows the passage of the first effector proteins injected by T3SS-1. These effectors are responsible for the conformation change in the cytoskeleton of non-phagocytic cells and consequent formation of so-called ruffles, which allows ST engulfment into the host cell in addition to Salmonella-containing vacuole (SCV) stabilization, formed after bacterium internalization (Francis et al. 1993; Ginocchio et al. 1994). Additionally, these effectors also activate host immune responses through intestinal mucosa inflammation induction (Herrero-Fresno and Olsen 2017). This induction provides a favorable microenvironment for ST since it is less affected by substances released in the inflammation, which gives ST an advantage over resident microbiome (Stecher et al. 2007; Herrero-Fresno and Olsen 2017). Also, some effectors in this phase prevent host cell apoptosis, which increases invasion maintenance (Collazo and Galán 1997).
On the other hand, T3SS-2 is activated inside SCV (Hansen-Wester and Hensel 2001), after ST enter non-phagocytic cells. T3SS-2 initiates contact to deliver effector proteins from intracellular bacteria throughout the SCV membrane into the host cell cytoplasm (Jennings et al. 2017). Even though T3SS-1 and T3SS-2 are activated in different invasion phases, they are not independent of each other since mutations in T3SS-2 were shown to decrease the expression of some T3SS-1 genes significantly and, consequently, the ability of ST to invade cells (Deiwick et al. 1998). Inside the SCV, the effector proteins injected by the T3SS-2 prevent phagolysosome formation and manipulate SCV traffic and maturation, while also promoting ST intracellular survival and replication (Bakowski et al. 2008). SPI-2 encodes most of these effectors (Hensel et al. 1998), despite some being encoded in other chromosome regions and injected by T3SS-2 (Waterman and Holden 2003).
T3SS structure
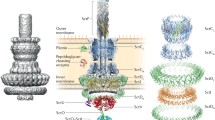
The T3SS-1 structure was first visualized by Kubori et al. (1998) through electronic microscopy. Since then, several advances have occurred, leading to a better understanding of the components that Kubori et al. (1998) first called the Needle-like structure. The development of techniques like X-ray crystallography, nuclear magnetic resonance (NMR) and cryoelectron microscopy (EM) were essential for the visualization and classification of T3SS components (Marlovits et al. 2004; Wang et al. 2010; Lunelli et al. 2011; Fujii et al. 2012; Loquet et al. 2012; Rathinavelan et al. 2014; Worrall et al. 2016; Kuhlen et al. 2018). T3SS is divided into three segments: extracellular components, the basal body, which crosses the two gram-negative cell envelope membranes, and the peripheral inner membrane cytoplasmic components (Büttner 2012) (Fig. 1).
The main structure of T3SS. The illustration is representing the main structure of T3SS using the Sct nomenclature (Hueck 1998) to nominate its proteins. T3SS structure comprises a translocon, formed by the translocators SctB and SctE and the tip complex; the needle, formed by SctF protein and the inner rod, formed by SctI protein; the basal body, formed by the outer and the inner membrane rings; the export apparatus, a Cytoplasmic ring (C-ring) and an ATPase complex. This figure was based on the one illustrated in the article by Portaliou et al. (2016)
The extracellular segment, also known as Needle-like filament, forms a secretion conduit from the top of the inner rod to the interior of the host cell (Kuhlen et al. 2018), which is used for protein passage throughout the type three secretion pathway (Marlovits et al. 2006). This component comprises two structures: a needle and a translocation pore, the latter connected to the eukaryotic host membrane (Portaliou et al. 2016). The extracellular needle is constituted by the SctF protein and is 30–70 nm in length and 10–13 nm in full (Marlovits et al. 2006), connected to the basal body by a different substructure, the inner rod, formed by the SctI protein (Marlovits et al. 2004). On top of the needle, the SctA protein forms a tip complex (Mueller et al. 2005; Espina et al. 2006; Galán et al. 2014). After the translocation pore is formed, translocators SctE and SctB are secreted and anchored to the SctA protein, where they exert their functions (Portaliou et al. 2016; Deng et al. 2017).
The basal body is a series of structures embedded in the inner and outer bacterial membranes (Schraidt et al. 2010; Schraidt and Marlovits 2011; Portaliou et al. 2016). It is formed by two concentric inner rings, connected to two outer rings by a structure known as the neck (Costa et al. 2015). The inner membrane rings are composed by the SctJ and SctD proteins, while the outer membrane rings are formed by the SctC protein, a member of the secretin family (Kubori et al. 1998; Kimbrough and Miller 2002; Costa et al. 2015; Deng et al. 2017). Housed within the base of the basal body is the export apparatus, which is required for the secretion of effector proteins throughout the T3SS (Sukhan et al. 2001). It is assembled from five membrane proteins: SctR, SctS, SctT, SctU, and SctV (Abrusci et al. 2013).
The peripheral inner cytoplasmic component membrane harbors the ATPase complex and the cytoplasmic ring (C-ring) (Portaliou et al. 2016). The C-ring, which is located above the export apparatus, is formed by the SctQ protein (Groisman and Ochman 1993; Morita-Ishihara et al. 2006). The ATPase complex is, in turn, formed by the SctN protein, which shares homology with F0F1 flagellar ATPase family of proteins (Eichelberg et al. 1994). Besides that, the ATPase complex is predicted to be formed as well by the SctO protein, which is required to gain access to epithelial cell (Collazo et al. 1995); the SctL and SctK proteins, which are required to the efficient assembly and stability of StcQ (Lara-Tejero et al. 2011). Both components form a sorting platform for substrate recruitment and secretion (Lara-Tejero et al. 2011; Makino et al. 2016). Besides, other proteins are also related to T3SS regulation. SctP, for example, controls the length of the needle (Marlovits et al. 2006), while SctU and SctW regulate the secretion hierarchy. Moreover, some of the secreted proteins require cognate chaperones to stabilize their assembly (Kimbrough and Miller 2002).
Overview of the assembly of T3SS core components
T3SS assembly occurs following a discrete sequence: first, the basal body and the export apparatus are assembled, followed by the inner rod and the needle, and finally, the tip complex and the translocon (Wagner et al. 2010; Diepold et al. 2011; Diepold and Wagner 2014) (Fig. 2). The assembly of the basal body involves a dependent secretory pathway, the Sec pathway, an essential protein export machinery that transports proteins across the plasma membrane and recognizes their substrates (Economou 1999; Thanassi and Hultgren 2000). This type of secretory pathway, dependent sec-machinery, is used by 95% of the protein exportation that occurs through cell membranes (Portaliou et al. 2016). First, the export apparatus components SctR and SctT initiate the assembly on the inner membrane, recruiting the other apparatus components, namely SctS, SctU, and SctV (Burkinshaw and Strynadka 2014). Then, the inner membrane rings components SctD and SctJ are assembled at the export apparatus site (Diepold and Wagner 2014; Burkinshaw and Strynadka 2014). This mechanism, known as the inside-out mechanism, is similar to the flagellum assembly mechanism, which requires the assembly of inner membrane rings and the export apparatus first, enabling the SctC ring to be correctly located in the outer membrane ring (Minamino and Macnab 1999; Sukhan et al. 2001). The inner rod and the needle, in turn, are assembled by a Sec-independent pathway that depends on ATPase activity (Sukhan et al. 2001). This dependence indicates why it comes before the assembly of the basal body and the export apparatus. In other mechanisms, used by Shigella spp. moreover, Yersinia spp., for example, known as the outside in mechanism, the outer membrane rings are formed first, without the need for inner membrane ring assembly.
Schematic illustration of the inside-out model of T3SS assembly. The T3SS assembly occurs in two different phases. In the Sec-dependent phase, the export apparatus is previously located in the inner membrane (1). The components of the inner membrane rings then assemble around the export apparatus (2). Peptidoglycan-cleaving enzyme locally removes the peptidoglycan layer to enable the assembly of T3SS components present between the inner and the outer membrane (3). The outer membrane rings are localized by secretin (4) and then makes contact with the inner membrane rings (5). In the Sec-independent phase, the ATPase complex and the cytoplasmic ring (C-ring) interact with the export apparatus (6). In this phase, the remain components are assembled by T3SS itself; first, the needle and the inner rod proteins a secreted, followed by the translocators (7); lastly, the effectors (green marbles) are secreted (8) (color figure online). This figure was based on the one illustrated in the article by Portaliou et al. (2016) and Deng et al. (2017)
The basal body
The basal body is composed of the inner and outer membrane rings, which are connected by a structure known as the neck (Kubori et al. 2000; Costa et al. 2015). The inner membrane rings themselves are formed by SctD and SctJ protein oligomers (Kubori et al. 1998, 2000), which are essential to the formation of the others T3SS components, since as described previously they serve as a basis for the linkage of these components (Minamino and Macnab 1999). The complex formed by these two proteins is hollow in the center, corroborating the idea of a central pore that serves as a conduit for the passage of effectors proteins (Galán and Wolf-Watz 2006). Cryo-EM and nano-gold-labeling analyses have demonstrated that SctD, SctJ, and SctC, have a conserved wedge-shaped fold structure with the latter forming the outer membrane ring. This fold is a motif for the inner and outer membrane ring formation and may facilitate ring assembly (Spreter et al. 2009). SctD has the charge distributed in such a way that its amino-terminal domain is located in the cytoplasm, whereas its carboxy-terminal domain in the periplasm. This charge distribution is surrounding a centrally located hydrophobic domain that servers to direct its insertion and retention in the inner membrane (Kimbrough and Miller 2002). This conformation may be necessary for the interaction between basal body components, since the SctC outer membrane ring amino-terminal domain reaches deep into the periplasm and directly contacts the SctD inner membrane ring, creating a bridge that connects the inner and outer membrane rings (Schraidt and Marlovits 2011; Bergeron et al. 2013). SctJ spans the periplasmic space with its sizeable amino-terminal domain and is anchored to the inner membrane through its carboxy-terminal transmembrane domain (Schraidt et al. 2010). This last structure is predicted to function as a stop-transfer signal to anchor SctJ in the inner membrane (Kimbrough and Miller 2002). Recently, near-atomic-resolution and cryo-EM analysis have demonstrated that SctD and SctJ display different 24-fold symmetries and form two nested concentric inner rings, with SctD encompassing SctJ. These rings surround the socket and the cup substructures where the export apparatus will be located (Worrall et al. 2016). The outer membranes ring themselves forms a so-called ring-like oligomeric structure, with approximately 15 nm in diameter and a pore inside the ring with 7 nm in diameter, which allows for the passage of export substrates through the outer membrane (Crago and Vassilis 2002). This structure is composed of SctC protein (Crago and Vassilis 2002), which is an outer membrane secretin family member, present also in Type two secretion and Type four fimbrial morphogenesis in other bacteria (Guilvout et al. 1999). Studies have demonstrated that SctC assembly is InvH-dependent, an SPI-1 encoded protein present only in T3SS-1, known as secretin pilotin (Crago and Vassilis 2002). Even so, the outer membrane ring can be assembled without the presence of this protein, as demonstrated by Sukhan et al. (2001), although its efficiency was significantly reduced.
The export apparatus
The presence of an export apparatus is crucial for the complete assembly of T3SS. The setting up of structures like the inner rod and the needle depending on their protein export through the export apparatus (Sukhan et al. 2001), for example. Five membrane proteins, which are completely conserved among all T3SS, form the export apparatus: SctR, SctS, SctT, SctU and SctV (Cornelis 2006; Galán and Wolf-Watz 2006; Minamino et al. 2008), as well as the soluble ATPase complex (Burkinshaw and Strynadka 2014). Besides, it is estimated that a total of 104 transmembranes domains are involved in the formation of this structure, pointing out its complexity (Zilkenat et al. 2016). Most of the knowledge about the T3SS export apparatus, its associations, and functions, comes from flagella export apparatus system studies (Minamino and Macnab 1999), due to its similarity. It was believed that the export apparatus was housed within the central cavity in the basal body, formed by SctD and SctJ inner membrane ring proteins (Kimbrough and Miller 2002), since FliP and FliR, which are SctR and SctT homologs in the flagellar export apparatus, are also located in a central cavity in MS ring (Fan et al. 1997). Corroborating with this hypothesis, a cryo-EM analysis of Salmonella Typhimurium T3SS with the deletion of export apparatus membrane proteins demonstrated a loss of density in the inner membrane rings of the basal body, in the socket and cup substructures region (Wagner et al. 2010). The same study demonstrated that some of the export apparatus membrane proteins could not be incorporated in a preassembled T3SS, although this may occur in an incompletely assembled T3SS. These results prove that, after complete assembly, the membrane proteins are maintained in a remote site (Wagner et al. 2010), reinforcing the idea of a hierarchy in the recruitment of export apparatus components to the T3SS structure. SctU is an autoprotesase that potentially controls the chronology and specificity of substrate secretion (Edqvist et al. 2003; Zarivach et al. 2008). SctV, in turn, is a so-called export gate, since it oligomerizes to form a cytoplasmic export ring pore, located directly below the secretion pore and above the ATPase complex (Abrusci et al. 2013). The ATPase complex is composed of three domains: an amino-terminal domain; a central ATPase domain, predicted to bind ATP via a conserved Rossmann fold; and a carboxy-domain (Burkinshaw and Strynadka 2014). Another complex, the ATPase sorting platform, is located in the cytoplasm side and has been proposed to include SctQ and SctL proteins, as well as other export apparatus components. This complex may transport substrates in an organized and hierarchical manner, although little is known about this mechanism (Burkinshaw and Strynadka 2014). Thus, this complex, as well as SctR, S, and T, may be the focus of future research.
The inner rod
The inner rod is a compact structure located within the basal body rings, anchored to a socket-like structure on the basal side of the base and extending itself till the needle (Marlovits et al. 2004, 2006). Cryo-EM analysis has demonstrated that this base is hollow, forming an internal chamber filled by the socket-like structure, which may serve as an adaptor that couples the N-fold symmetric base to the inner rod (Marlovits et al. 2004, 2006). Despite this, the inner rod is assembled by the polymerization of SctI protein, characterizing a helical assembly (Kimbrough and Miller 2000). Although little is known about its structure and dimensions, analysis carried out with SctI mutant Salmonella Typhimurium indicates that the SctI protein is essential to the virulence of this pathogen since it slows its invasiveness significantly when compared to a wild-type strain (Klein et al. 2000). In addition, studies have demonstrated an intimate relation between the seven SctI C-terminal residues and the activation of host immune responses (Miao et al. 2010), as it interacts with the NOD-like receptor NLRC4 to activate interleukin-1β maturation (IL-1β), triggering the host inflammatory response against microbial infection (Miao et al. 2010; Miao and Rajan 2011). However, the location of the inner rod structure, inside the basal body rings, requires an environment to fold into its functional form, hindering structural studies concerning both the pure rod and its isolated form (Burkinshaw and Strynadka 2014). Despite this, Zhong et al. (2012) used nuclear magnetic resonance (NMR) spectroscopy and circular dichroism to determine the structural properties of the Salmonella SctI inner rod protein. The results indicate that SctI is a monomeric protein, partially folded and lacking a tertiary structure, with the C-terminal α-helical region presenting a clear structure, which may be responsible for the activation of host immune responses. However, it is unclear if SctI conformation after its assembly into the inner rods would be capable of activating the same responses as those activated by the purified protein (Zhong et al. 2012).
The needle
In contrast to the inner rod, the needle is a polymeric assembly of more than 120 copies of the SctF protein (Kubori et al. 2000; Kimbrough and Miller 2000). Combined with the inner rod, this structure acts as a conduit for T3SS translocator and effector secretion (Galán and Wolf-Watz 2006). Attempting to develop the first model of the Salmonella Typhimurium needle, Loquet et al. (2012) combined solid-state NMR spectroscopy, EM, and Rosetta modeling, using recombinantly produced needles obtained by in vitro polymerization. The results demonstrate that the SctF protein (PrgI in Salmonella Typhimurium T3SS-1), as determined from conformation-dependent chemical shifts, displays a rigid conformation comprising four distinct structural elements: an N-terminal extended domain, an α-helix, a loop and a C-terminal α-helix (Loquet et al. 2012). Each protein is oriented, and thus, consequently, the N-terminal domain is set on the needle surface and the C-terminal lines the central channel of the needle (Loquet et al. 2012). This same arrangement was demonstrated in Shigella needle protein (MxiH), using ssNMR analysis (Fujii et al. 2012), suggesting a conserved architecture. So far, however, it is unclear how the inner rod and the needle are connected.
The tip complex
A structure called the tip complex lies on the top of the needle (Mueller et al. 2005), assembled by tip proteins, mostly SctA (SipD in SPI-1 encoded protein, and SseB in SPI-2 encoded protein) (Chatterjee et al. 2010). Structural characterization by X-ray crystallography indicates that SctA is a highly α-helical protein that can be divided into three structurally different domains: an N-terminal α-helical hairpin, also common in the SctF protein (Wang et al. 2007); a long central coiled-coil domain and, a distal domain located at the end of the coiled-coil domain (Chatterjee et al. 2010; Lunelli et al. 2011). The long central coiled-coil is predicted to give to the tip complex proteins an overall oblong shape (Rathinavelan et al. 2011). Based on the similarity between the α-helical hairpin of SctA in the tip complex and the SctF in the needle, Rathinavelan et al. (2011) hypothesized that the SctA coiled-coil is the primary binding site for the needle protein. The results obtained by NMR paramagnetic relaxation enhancement (PRE) analysis confirmed that SctF binds at the bottom of SctA, and also interacts with two major SctA sites (Rathinavelan et al. 2011). Moreover, several studies have described the interaction between SctA and bile salts of the host duodenum (Chatterjee et al. 2010; Wang et al. 2010; Lunelli et al. 2011), since bile salts can affect Salmonella T3SS activity and, consequently, its invasiveness (Gunn 2000). Thus, bile salts repress T3SS and decrease invasiveness in Salmonella (Prouty and Gunn 2000), while SctA is present on the bacterial surface before host contact could function as a sensor for environmental molecules (Chatterjee et al. 2010).
The translocon
Once the bacteria come into contact with the host cell, translocon components are secreted and predicted to form a translocation pore in the host cell membrane (Collazo and Galán 1997; Nikolaus et al. 2001), through which effector proteins are injected into the host cell cytoplasm (Dickenson et al. 2013). The translocation pore is a hetero-oligomeric complex formed by the translocators SctB and SctE (Portaliou et al. 2016), which are mainly hydrophilic and, therefore, oligomerize with the SctA tip protein on the host cell membrane, where they form the pore (Portaliou et al. 2016). In the absence of some of these components, the effectors are unable to pass to the host cell cytoplasm and, instead, are secreted into the supernatant (Kimbrough and Miller 2002). Nevertheless, translocation pore components possess little high-resolution structural information, and thus, their structures are poorly studied owing to technical challenges, which may be resolved in the future. However, recent studies concerning genome-wide selection indicate that the intermediate filament Vimentin, a cytoskeleton structure that provides structural and mechanical support (Snider et al. 2018), is required for an efficient translocation in Salmonella Typhimurium’s T3SS, since it stabilizes docking, but not for pore formation, indicating that these are two distinct processes (Russo et al. 2016). Moreover, Maserati et al. (2017), based on Salmonella’s survival under extreme desiccation conditions, performed a global transcriptomic analysis comparing Salmonella Typhimurium cells equilibrated to low and high water activity, focusing on the sopB and sctB (sseD) genes, which encode SopB and SctB (SseD) effectors. The results indicate that sopB and sctB mutants exhibited significant viability decreases compared to the wild-types, suggesting that these genes are required for Salmonella survival under desiccation conditions (Maserati et al. 2017).
Overview of T3SS regulation
For correct T3SS assembly and function, the proteins that participate in this event must be secreted in a defined order following a strict hierarchy secretion. To this end, some chaperones play an active role in maintaining this hierarchy (Büttner 2012). In this meaning, they are responsible for keeping substrates in a secreted-competent state, allowing them to travel in an unfolded or partially folded manner (Stebbins and Galán 2001). Chaperones are proteins that interact with one or several partners to prevent premature or incorrect interactions (Parsot et al. 2003). There are though three classes of these molecules that interact with T3SS proteins: the class I chaperones which bind to effectors; the class II chaperones which bind to translocators; and the class III chaperones, which binds to needle proteins (Izoré et al. 2011). Some studies in Yersinia spp. demonstrates that in the absence of their chaperones, some effectors has its amount reduced but not abolished (Wattiau et al. 1994; Cheng et al. 1997). In the same way, the chaperones stabilize an unstable protein and prevent the association of two translocators in the cytoplasm (Tucker and Galán 2000). Thereby, chaperones seem to play an essential role in the stability, translocation, and hierarchy secretion of T3SS proteins.
The secreted proteins are divided into early (needle and inner rod proteins), middle (translocator proteins); and, late (effector proteins) substrates, based on their secretion order (Deng et al. 2017). When the needle reaches a certain length, it signals the accomplishment of a T3SS function and secretion of middle substrates (translocators). Once the translocation pores are formed, T3SS switches to the secretion of late substrates (effectors), which may pass directly into the host cell membrane (Marlovits et al. 2006) (Fig. 3).
Schematic illustration figure for T3SS hierarchy regulation. T3SS secreted proteins follow a strict hierarchy secretion order. They are divided into early (needle and inner rod), middle (translocators), and late (effectors proteins) substrates. First, SctP accessory protein and SctU autoprotease protein regulate the secretion of early substrates (1), which determines the needle lentgh; After th needle assembly, SctW gatekeeper and SctU proteins regulate the switch from early to middle substrates (2), allowing the construction of the translocation pore in the host cell membrane. Last, SctW together with SctN ATPase regulates the switch from the middle to late substrates (3), which enables the translocation of effectors proteins into the host cell. All this process is mediated by chaperones, which play an essential role in maintaining the secretion hierarchy. This figure was based on the one illustrated in the article by Portaliou et al. (2016) and Deng et al. (2017)
In Salmonella, the needle length and the substrate secretion switching depends on the accessory protein SctP (Collazo and Galán 1997; Kubori et al. 2000) and SctU autoprotease protein (Erhardt et al. 2011). The deletion of SctP gene determines the formation of needle-like structures with an indefinite length (Journet 2003), indicating that SctP acts as a molecular ruler to determine needle length (Wee and Hughes 2015). To reinforce that, a study made with Salmonella Typhimurium strains lacking SctP showed the assemble of T3SS with needles much longer than the wide-type strain, disabling them to secrete effector proteins (Marlovits et al. 2006). This inability to secrete effectors proteins could be explained by the fact that in absent of SctP, the main component of the inner rod SctI is also absent although it could be detected in culture supernatants free from the shed needles (Kubori et al. 2000; Sukhan et al. 2003; Lefebre and Galán 2014). This suggests that the inner rod plays a role in needle length (Marlovits et al. 2006) as well as in substrate switching (Lefebre and Galán 2014).
Together with SctP, the self-cleaving SctU protein is also involved in the needle length control and with the switching from early substrates to middle and late substrates. The autocleavage of SctU has been proposed to be the trigger for the substrates switching (Ferris and Minamino 2006). The interaction between the C-terminus of SctP and SctU results in a conformation change in SctU, culminating the initiation of late substrate secretion (Zarivach et al. 2008). However, in Salmonella Typhimurium, the cleavage of the switch protein demonstrated not to be the regulatory signal for the substrate switching (Feria et al. 2015). This because a not-cleavable SctU mutant has a needle length similar to the wild-type strain (Feria et al. 2015), although studies with a homolog protein in Yersinia spp. demonstrates that not-cleavable SctU mutants secrete only early substrates and presents needles much longer than the wild-type (Sorg et al. 2007). Therefore, further studies are required to fully understand the role of SctU in switching substrates in Salmonella Typhimurium.
The switch from the middle to late substrates is regulated by the SctW family of gatekeeper proteins (Büttner 2012). Botteaux et al. (2009) demonstrated that the absence of SctW deregulates the secretion of early and late effectors, affirming the role of SctW in substrates regulation. SctW interacts with inner-rod component SctI, which facilitates switching secretion from translocators to effectors (Cherradi et al. 2013). This interaction is predicted to form a plug on the T3SS entry gate (Cherradi et al. 2013), which may explain the effector hyper-secretion that occurs in SctW mutations (Büttner 2012). In Salmonella Typhimurium, SctW mutations significantly reduce the secretion of translocators SctE; B and A (Kubori and Galán 2002). Besides, SctW interacts with the specific-chaperones of these translocators (Kubori and Galán 2002), which may confirm the ability of SctW to modulate these translocators' secretions. Although much is predicted about T3SS regulation, the exact molecular mechanism of these switches and the primary performance of the secretion hierarchy in Salmonella Typhimurium require further research.
Conclusions
Salmonella enterica subsp. enterica serovar Typhimurium is an intracellular pathogen, and its ability to invade and survive in host cells is strictly related to protein secretion. The delivery of effector proteins into the host cell, in turn, is dependent on the type three secretion system, which makes this complex crucial for ST pathogenicity. Several advances have been observed concerning the study of the structure and function of core T3SS components, as well as its assembly. The development of novel techniques, such as X-ray crystallography, NMR, and cryo-electron microscopy, has been essential to these discoveries. Even so, several challenges still exist concerning the understanding of T3SS. The main structure and assembly of SctR, SctS and SctT membrane proteins of the export apparatus, as well as the translocations, pores in the host cell membrane, for example, are still poorly understood.
In the same way, the so-called ATPase sorting platform also requires further studies. Also, little is known about the real structures and dimensions of the inner rod, since a specific environment is required for folding into its functional form, and recent studies are based on purified proteins. Finally, studies point out that the interaction between the inner rod, needle length, and substrate switching, but the main mechanisms behind this interaction are still unknown. Likewise, the precise molecular mechanisms that coordinate switches of hierarchical protein secretion from early to middle and from the middle to late substrates also remains elusive. Thus, despite many advances, complete knowledge concerning T3SS needs is still lacking. Further studies on T3SS structure and function may allow for therapeutic measures against invasive ST to be developed since knowledge regarding the mechanisms behind this structure can put us one step ahead in the fight against this pathogen.
References
Abby SS, Rocha EPC (2012) The non-flagellar type III secretion system evolved from the bacterial flagellum and diversified into host-cell adapted systems. PLOS Genet 8:e1002983. https://doi.org/10.1371/journal.pgen.1002983
Abrusci P, Vergara-Irigaray M, Johnson S et al (2013) Architecture of the major component of the type III secretion system export apparatus. Nat Struct Mol Biol 20:99–104. https://doi.org/10.1038/nsmb.2452
Bakowski MA, Braun V, Brumell JH (2008) Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic 9:2022–2031. https://doi.org/10.1111/j.1600-0854.2008.00827.x
Bergeron JRC, Worrall LJ, Sgourakis NG et al (2013) a refined model of the prototypical Salmonella SPI-1 T3SS basal body reveals the molecular basis for its assembly. PLOS Pathog 9:e1003307. https://doi.org/10.1371/journal.ppat.1003307
Botteaux A, Sory MP, Biskri L et al (2009) MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol Microbiol 71:449–460. https://doi.org/10.1111/j.1365-2958.2008.06537.x
Brawn LC, Hayward RD, Koronakis V (2007) Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe 1:63–75. https://doi.org/10.1016/j.chom.2007.02.001
Burkinshaw BJ, Strynadka NCJ (2014) Assembly and structure of the T3SS. Biochim Biophys Acta BBA 1843:1649–1663. https://doi.org/10.1016/j.bbamcr.2014.01.035
Büttner D (2012) Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol Rev 76:262–310. https://doi.org/10.1128/MMBR.05017-11
Chatterjee S, Zhong D, Nordhues BA et al (2010) The crystal structures of the Salmonella type III secretion system tip protein SipD in complex with deoxycholate and chenodeoxycholate. Protein Sci 20:75–86. https://doi.org/10.1002/pro.537
Cheng LW, Anderson DM, Schneewind O (1997) Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol 24:757–765. https://doi.org/10.1046/j.1365-2958.1997.3831750.x
Cherradi Y, Schiavolin L, Moussa S et al (2013) Interplay between predicted inner-rod and gatekeeper in controlling substrate specificity of the type III secretion system. Mol Microbiol 87:1183–1199. https://doi.org/10.1111/mmi.12158
Cirillo DM, Valdivia RH, Monack DM, Falkow S (1998) Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol 30:175–188. https://doi.org/10.1046/j.1365-2958.1998.01048.x
Coburn B, Li Y, Owen D et al (2005) Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect Immun 73:3219–3227. https://doi.org/10.1128/IAI.73.6.3219-3227.2005
Collazo CM, Galán JE (1997) The invasion-associated type III system of Salmonella Typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol 24:747–756. https://doi.org/10.1046/j.1365-2958.1997.3781740.x
Collazo CM, Zierler MK, Gatan JE (1995) Functional analysis of the Salmonella Typhimurium invasion genes invl and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol 15:25–38. https://doi.org/10.1111/j.1365-2958.1995.tb02218.x
Cornelis GR (2006) The type III secretion injectisome. Nat Rev Microbiol 4:811–825. https://doi.org/10.1038/nrmicro1526
Costa TRD, Felisberto-Rodrigues C, Meir A et al (2015) Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 13:343–359. https://doi.org/10.1038/nrmicro3456
Crago AM, Vassilis K (2002) Salmonella InvG forms a ring-like multimer that requires the InvH lipoprotein for outer membrane localization. Mol Microbiol 30:47–56. https://doi.org/10.1046/j.1365-2958.1998.01036.x
Deiwick J, Nikolaus T, Shea JE et al (1998) Mutations in Salmonella pathogenicity island 2 (SPI2) genes affecting transcription of SPI1 genes and resistance to antimicrobial agents. J Bacteriol 180:4775–4780
Deng W, Marshall NC, Rowland JL et al (2017) Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol 15:323–337. https://doi.org/10.1038/nrmicro.2017.20
Desvaux M, Hébraud M, Talon R, Henderson IR (2009) Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol 17:139–145. https://doi.org/10.1016/j.tim.2009.01.004
Dickenson NE, Choudhari SP, Adam PR et al (2013) Oligomeric states of the Shigella translocator protein IpaB provide structural insights into formation of the type III secretion translocon. Protein Sci 22:614–627. https://doi.org/10.1002/pro.2245
Diepold A, Wagner S (2014) Assembly of the bacterial type III secretion machinery. FEMS Microbiol Rev 38:802–822. https://doi.org/10.1111/1574-6976.12061
Diepold A, Wiesand U, Cornelis GR (2011) The assembly of the export apparatus (YscR, S, T, U, V) of the Yersinia type III secretion apparatus occurs independently of other structural components and involves the formation of an YscV oligomer. Mol Microbiol 82:502–514. https://doi.org/10.1111/j.1365-2958.2011.07830.x
dos Santos AMP, Ferrari RG, Conte-Junior CA (2018) Virulence factors in SalmonellaTyphimurium: the sagacity of a bacterium. Curr Microbiol. https://doi.org/10.1007/s00284-018-1510-4
Economou A (1999) Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol 7:315–320. https://doi.org/10.1016/S0966-842X(99)01555-3
Edqvist PJ, Olsson J, Lavander M et al (2003) YscP and YscU regulate substrate specificity of the yersinia type iii secretion system. J Bacteriol 185:2259–2266. https://doi.org/10.1128/JB.185.7.2259-2266.2003
Eichelberg K, Ginocchio CC, Galán JE (1994) Molecular and functional characterization of the Salmonella Typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J Bacteriol 176:4501–4510. https://doi.org/10.1128/jb.176.15.4501-4510.1994
Ellermeier JR, Slauch JM (2007) Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10:24–29. https://doi.org/10.1016/j.mib.2006.12.002
Erhardt M, Singer HM, Wee DH et al (2011) An infrequent molecular ruler controls flagellar hook length in Salmonella enterica. EMBO J 30:2948–2961. https://doi.org/10.1038/emboj.2011.185
Espina M, Olive AJ, Kenjale R et al (2006) IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect Immun 74:4391–4400. https://doi.org/10.1128/IAI.00440-06
Fan F, Ohnishi K, Francis NR, Macnab RM (1997) The FliP and FliR proteins of Salmonella Typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol 26:1035–1046. https://doi.org/10.1046/j.1365-2958.1997.6412010.x
Feria JVM, Lefebre MD, Stierhof Y-D et al (2015) Role of autocleavage in the function of a type III secretion specificity switch protein in Salmonella enterica serovar. mBio. https://doi.org/10.1128/mBio.01459-15
Ferris HU, Minamino T (2006) Flipping the switch: bringing order to flagellar assembly. Trends Microbiol 14:519–526. https://doi.org/10.1016/j.tim.2006.10.006
Francis CL, Ryan TA, Jones BD et al (1993) Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639–642. https://doi.org/10.1038/364639a0
Fujii T, Cheung M, Blanco A et al (2012) Structure of a type III secretion needle at 7-Å resolution provides insights into its assembly and signaling mechanisms. Proc Natl Acad Sci 109:4461–4466. https://doi.org/10.1073/pnas.1116126109
Galán JE, Wolf-Watz H (2006) Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573. https://doi.org/10.1038/nature05272
Galán JE, Lara-Tejero M, Marlovits TC, Wagner S (2014) Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 68:415–438. https://doi.org/10.1146/annurev-micro-092412-155725
Galkin VE, Schmied WH, Schraidt O et al (2010) The structure of the Salmonella Typhimurium type III secretion system needle shows divergence from the flagellar system. J Mol Biol 396:1392–1397. https://doi.org/10.1016/j.jmb.2010.01.001
Ginocchio CC, Olmsted SB, Wells CL, Galán JE (1994) Contact with epithelial cells induces the formation of surface appendages on SalmonellaTyphimurium. Cell 76:717–724. https://doi.org/10.1016/0092-8674(94)90510-X
Groisman E, Ochman H (1993) Cognate gene clusters govern invasion of host epithelial cells by Salmonella Typhimurium and Shigella flexneri. EMBO J 12:3779–3787. https://doi.org/10.1002/j.1460-2075.1993.tb06056.x
Guilvout I, Hardie KR, Sauvonnet N, Pugsley AP (1999) Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J Bacteriol 181:7212–7220
Gunn JS (2000) Mechanisms of bacterial resistance and response to bile. Microbes Infect 2:907–913. https://doi.org/10.1016/S1286-4579(00)00392-0
Hansen-Wester I, Hensel M (2001) Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect 3:549–559. https://doi.org/10.1016/S1286-4579(01)01411-3
Haraga A, Ohlson MB, Miller SI (2008) Salmonellae interplay with host cells. Nat Rev Microbiol 6:53–66. https://doi.org/10.1038/nrmicro1788
Hensel M (2000) Salmonella pathogenicity island 2. Mol Microbiol 36:1015–1023. https://doi.org/10.1046/j.1365-2958.2000.01935.x
Hensel M (2004) Evolution of pathogenicity islands of Salmonella enterica. Int J Med Microbiol 294:95–102. https://doi.org/10.1016/j.ijmm.2004.06.025
Hensel M, Shea JE, Waterman SR et al (1998) Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol 30:163–174. https://doi.org/10.1046/j.1365-2958.1998.01047.x
Herrero-Fresno A, Olsen JE (2017) Salmonella Typhimurium metabolism affects virulence in the host : a mini-review. Food Microbiol. https://doi.org/10.1016/j.fm.2017.04.016
Hueck CJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433
Izoré T, Job V, Dessen A (2011) Biogenesis, regulation, and targeting of the type iii secretion system. Structure 19:603–612. https://doi.org/10.1016/j.str.2011.03.015
Jennings E, Thurston TLM, Holden DW (2017) Salmonella SPI-2 type III secretion system effectors: molecular mechanisms and physiological consequences. Cell Host Microbe 22:217–231. https://doi.org/10.1016/j.chom.2017.07.009
Journet L (2003) The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302:1757–1760. https://doi.org/10.1126/science.1091422
Kimbrough TG, Miller SI (2000) Contribution of SalmonellaTyphimurium type III secretion components to needle complex formation. Proc Natl Acad Sci 97:11008–11013. https://doi.org/10.1073/pnas.200209497
Kimbrough TG, Miller SI (2002) Assembly of the type III secretion needle complex of Salmonella Typhimurium. Microbes Infect 4:75–82. https://doi.org/10.1016/S1286-4579(01)01512-X
Klein JR, Fahlen TF, Jones BD (2000) Transcriptional organization and function of invasion genes within Salmonella enterica serovar typhimurium pathogenicity island 1, including the prgH, prgI, prgJ, prgK, orgA, orgB, and orgC Genes. Infect Immun 68:3368–3376. https://doi.org/10.1128/IAI.68.6.3368-3376.2000
Kubori T, Galán JE (2002) Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J Bacteriol 184:4699–4708. https://doi.org/10.1128/JB.184.17.4699-4708.2002
Kubori T, Matsushima Y, Nakamura D et al (1998) Supramolecular structure of the SalmonellaTyphimurium type III protein secretion system. Science 280:602–605. https://doi.org/10.1126/science.280.5363.602
Kubori T, Sukhan A, Aizawa S-I, Galán JE (2000) Molecular characterization and assembly of the needle complex of the SalmonellaTyphimurium type III protein secretion system. Proc Natl Acad Sci 97:10225–10230. https://doi.org/10.1073/pnas.170128997
Kuhlen L, Abrusci P, Johnson S et al (2018) Structure of the core of the type three secretion system export apparatus. bioRxiv. https://doi.org/10.1101/249128
Lara-Tejero M, Kato J, Wagner S et al (2011) A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331:1188–1191. https://doi.org/10.1126/science.1201476
Lee CA (1997) Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol 5:148–156. https://doi.org/10.1016/S0966-842X(97)01029-9
Lefebre MD, Galán JE (2014) The inner rod protein controls substrate switching and needle length in a Salmonella type III secretion system. Proc Natl Acad Sci 111:817–822. https://doi.org/10.1073/pnas.1319698111
Loquet A, Sgourakis NG, Gupta R et al (2012) Atomic model of the type III secretion system needle. Nature 486:276–279. https://doi.org/10.1038/nature11079
Lunelli M, Hurwitz R, Lambers J, Kolbe M (2011) Crystal structure of PrgI-SipD: insight into a secretion competent state of the type three secretion system needle tip and its interaction with host ligands. PLOS Pathog 7:e1002163. https://doi.org/10.1371/journal.ppat.1002163
Makino F, Shen D, Kajimura N et al (2016) The Architecture of the cytoplasmic region of type III secretion systems. Sci Rep 6:33341. https://doi.org/10.1038/srep33341
Marcus SL, Brumell JH, Pfeifer CG, Finlay BB (2000) Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect 2:145–156. https://doi.org/10.1016/S1286-4579(00)00273-2
Marlovits TC, Kubori T, Sukhan A et al (2004) Structural insights into the assembly of the type iii secretion needle complex. Science 306:1040–1042. https://doi.org/10.1126/science.1102610
Marlovits TC, Kubori T, Lara-Tejero M et al (2006) Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441:637–640. https://doi.org/10.1038/nature04822
Maserati A, Fink RC, Lourenco A et al (2017) General response of Salmonella enterica serovar typhimurium to desiccation: a new role for the virulence factors sopD and sseD in survival. PLOS One 12:e0187692. https://doi.org/10.1371/journal.pone.0187692
Matteï P-J, Faudry E, Job V et al (2011) Membrane targeting and pore formation by the type III secretion system translocon. FEBS J 278:414–426. https://doi.org/10.1111/j.1742-4658.2010.07974.x
Miao EA, Rajan JV (2011) Salmonella and Caspase-1: a complex interplay of detection and evasion. Front Microbiol. https://doi.org/10.3389/fmicb.2011.00085
Miao EA, Mao DP, Yudkovsky N et al (2010) Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci 107:3076–3080. https://doi.org/10.1073/pnas.0913087107
Minamino T, Macnab RM (1999) Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol 181:1388–1394
Minamino T, Imada K, Namba K (2008) Mechanisms of type III protein export for bacterial flagellar assembly. Mol Biosyst 4:1105–1115. https://doi.org/10.1039/B808065H
Morita-Ishihara T, Ogawa M, Sagara H et al (2006) Shigella Spa33 is an essential c-ring component of type III secretion machinery. J Biol Chem 281:599–607. https://doi.org/10.1074/jbc.M509644200
Mueller CA, Broz P, Müller SA et al (2005) The V-antigen of yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674–676. https://doi.org/10.1126/science.1118476
Nikolaus T, Deiwick J, Rappl C et al (2001) SseBCD proteins are secreted by the type III secretion system of salmonella pathogenicity island 2 and function as a translocon. J Bacteriol 183:6036–6045. https://doi.org/10.1128/JB.183.20.6036-6045.2001
Parsot C, Hamiaux C, Page A-L (2003) The various and varying roles of specific chaperones in type III secretion systems. Curr Opin Microbiol 6:7–14. https://doi.org/10.1016/S1369-5274(02)00002-4
Portaliou AG, Tsolis KC, Loos MS et al (2016) Type III secretion: building and operating a remarkable nanomachine. Trends Biochem Sci 41:175–189. https://doi.org/10.1016/j.tibs.2015.09.005
Prouty AM, Gunn JS (2000) Salmonella enterica serovar typhimurium invasion is repressed in the presence of bile. Infect Immun 68:6763–6769. https://doi.org/10.1128/IAI.68.12.6763-6769.2000
Rathinavelan T, Tang C, Guzman RND (2011) Characterization of the interaction between the Salmonella type III secretion system tip protein SipD and the needle protein PrgI by paramagnetic relaxation enhancement. J Biol Chem 286:4922–4930. https://doi.org/10.1074/jbc.M110.159434
Rathinavelan T, Lara-Tejero M, Lefebre M et al (2014) NMR model of PrgI–sipd interaction and its implications in the needle-tip assembly of the Salmonella type III secretion system. J Mol Biol 426:2958–2969. https://doi.org/10.1016/j.jmb.2014.06.009
Russo BC, Stamm LM, Raaben M et al (2016) Intermediate filaments enable pathogen docking to trigger type 3 effector translocation. Nat Microbiol 1:16025. https://doi.org/10.1038/nmicrobiol.2016.25
Schraidt O, Marlovits TC (2011) Three-dimensional model of Salmonella’s needle complex at subnanometer resolution. Science 331:1192–1195. https://doi.org/10.1126/science.1199358
Schraidt O, Lefebre MD, Brunner MJ et al (2010) Topology and organization of the SalmonellaTyphimurium type III secretion needle complex components. PLOS Pathog 6:e1000824. https://doi.org/10.1371/journal.ppat.1000824
Snider NT, Ku NO, Omary MB (2018) Intermediate filaments: the sweet side of vimentin. eLife 7:e35336. https://doi.org/10.7554/eLife.35336
Sorg I, Wagner S, Amstutz M et al (2007) YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J 26:3015–3024. https://doi.org/10.1038/sj.emboj.7601731
Spreter T, Yip CK, Sanowar S et al (2009) A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat Struct Mol Biol 16:468–476. https://doi.org/10.1038/nsmb.1603
Stebbins CE, Galán JE (2001) Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 414:77–81. https://doi.org/10.1038/35102073
Stecher B, Robbiani R, Walker AW et al (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLOS Biol 5:e244. https://doi.org/10.1371/journal.pbio.0050244
Sukhan A, Kubori T, Wilson J, Galán JE (2001) Genetic analysis of assembly of the Salmonella enterica serovar typhimurium type III secretion-associated needle complex. J Bacteriol 183:1159–1167. https://doi.org/10.1128/JB.183.4.1159-1167.2001
Sukhan A, Kubori T, Galán JE (2003) Synthesis and Localization of the Salmonella SPI-1 type III secretion needle complex proteins PrgI and PrgJ. J Bacteriol 185:3480–3483. https://doi.org/10.1128/JB.185.11.3480-3483.2003
Thanassi DG, Hultgren SJ (2000) Multiple pathways allow protein secretion across the bacterial outer membrane. Curr Opin Cell Biol 12:420–430. https://doi.org/10.1016/S0955-0674(00)00111-3
Tucker SC, Galán JE (2000) Complex function for SicA, a Salmonella enterica serovar typhimurium type III secretion-associated chaperone. J Bacteriol 182:2262–2268. https://doi.org/10.1128/JB.182.8.2262-2268.2000
van der Heijden J, Finlay BB (2012) Type III effector-mediated processes in Salmonella infection. Future Microbiol 7:685–703. https://doi.org/10.2217/fmb.12.49
Vincent MS, Canestrari MJ, Leone P et al (2017) Characterization of the Porphyromonas gingivalis type IX secretion trans-envelope PorKLMNP core complex. J Biol Chem 292:3252–3261. https://doi.org/10.1074/jbc.M116.765081
Wagner S, Königsmaier L, Lara-Tejero M et al (2010) Organization and coordinated assembly of the type III secretion export apparatus. Proc Natl Acad Sci 107:17745–17750. https://doi.org/10.1073/pnas.1008053107
Wang Y, Ouellette AN, Egan CW et al (2007) Differences in the electrostatic surfaces of the type III secretion needle proteins PrgI, BsaL, and MxiH. J Mol Biol 371:1304–1314. https://doi.org/10.1016/j.jmb.2007.06.034
Wang Y, Nordhues BA, Zhong D, De Guzman RN (2010) NMR Characterization of the interaction of the Salmonella type III secretion system protein SipD and bile salts. Biochemistry 49:4220–4226. https://doi.org/10.1021/bi100335u
Waterman SR, Holden DW (2003) Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 5:501–511. https://doi.org/10.1046/j.1462-5822.2003.00294.x
Wattiau P, Bernier B, Deslée P et al (1994) Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci 91:10493–10497. https://doi.org/10.1073/pnas.91.22.10493
Wee DH, Hughes KT (2015) Molecular ruler determines needle length for the Salmonella Spi-1 injectisome. Proc Natl Acad Sci 112:4098–4103. https://doi.org/10.1073/pnas.1423492112
Worrall LJ, Hong C, Vuckovic M et al (2016) Near-atomic-resolution cryo-EM analysis of the Salmonella T3S injectisome basal body. Nature 540:597–601. https://doi.org/10.1038/nature20576
Zarivach R, Deng W, Vuckovic M et al (2008) Structural analysis of the essential self-cleaving type III secretion proteins EscU and SpaS. Nature 453:124–127. https://doi.org/10.1038/nature06832
Zhong D, Lefebre M, Kaur K et al (2012) The Salmonella type III secretion system inner rod protein PrgJ is partially folded. J Biol Chem 287:25303–25311. https://doi.org/10.1074/jbc.M112.381574
Zilkenat S, Franz-Wachtel M, Stierhof Y-D et al (2016) Determination of the stoichiometry of the complete bacterial type III secretion needle complex using a combined quantitative proteomic approach. Mol Cell Proteom. https://doi.org/10.1074/mcp.M115.056598
Acknowledgements
We thank to Virgínia P. Silveira for the design of the figures.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (process no. 202.225/2017 and E-26/201.577/2018, FAPERJ, Brazil); Conselho Nacional de Desenvolvimento Científico e Tecnológico (process no. 311422/2016-0, CNPq, Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (process no. 125, CAPES/Embrapa 2014, CAPES, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, A.M.P., Ferrari, R.G. & Conte-Junior, C.A. Type three secretion system in Salmonella Typhimurium: the key to infection. Genes Genom 42, 495–506 (2020). https://doi.org/10.1007/s13258-020-00918-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-020-00918-8