Abstract
Planning organ-at-risk volume (PRV) margins can be applied to the bladder and rectum in prostate external beam radiation therapy (EBRT), in order to incorporate the uncertainties resulting from their inter-fraction motion. For each of a total of 16 patients, the bladder and rectum were delineated on CBCT images for five treatment fractions in addition to the planning CT image set. The bladder and rectum boundary displacements across the images were measured and the frequency and size of organ boundary displacements were evaluated. Subsequently, PRV margins were created to cover a specific percentage of organ boundary motion for a specified percentage of the population. In this investigation, two bladder PRV margins were generated to deal with two bladder conditions of low and high-volume variation among fractions. A combined PRV margin was also generated for the rectum by separating the rectum into three parts and deriving independent PRV margins for each segment. Outward coverage and effectiveness metrics allowed evaluation of the margins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common cancer in men worldwide [1] and the third most common cause of cancer death in men in Australia [2]. The number of PCa patients is tending to increase with the ageing of the population [3]. Since the introduction of cobalt teletherapy machines in the 1950 s, an increasing proportion of PCa patients have been treated with prostate external beam radiotherapy (EBRT) [4, 5].
However, the widespread use of prostate radiotherapy is also accompanied by growing concerns about its side effects. Many works have reported the side effects caused by unwanted dose to OARs (organ-at-risk) during EBRT [6, 7]. In addition, the prostate tumour tissue has been found to have significant inter-fraction repair capacity. A significant change in dose-effect can result by reducing the number of treatment fractions [8]. Consequently, radiotherapy with a low number of fractions and large fraction size has become preferable for PCa treatment. For example, stereotactic body radiation therapy (SBRT, or stereotactic ablative body radiotherapy) has shown excellent efficacy for low-risk and intermediate-risk PCa [9,10,11]. With these approaches, careful considerations of OAR motion and deformation are required for PCa EBRT. With a large dose per fraction being used in treatment, the unintended irradiation could impose potentially high toxicity to normal healthy tissue [12, 13].
In order to accommodate the uncertainties caused by OAR motion, ICRU Report 83 recommends adding margins to OARs and defines the volume inclusive of each OAR and its margins as the PRV (planning organ-at-risk volume) [14]. In view of the importance of having suitable PRVs in PCa EBRT, this study focused on generating PRVs for bladder and rectum by evaluating their inter-fraction motion.
Population-derived quantities of OAR motion during radiotherapy are required for creating PRVs appropriate for accommodating inter-fraction motion. Multiple ways to describe and measure organ motion have been suggested in the literature, including: evaluating the shifts of an organ’s center of mass (COM) [15], measuring the amount of motion of an organ’s boundary [16, 17], generating two-dimensional scalar maps of organ motion [18, 19], and deriving geometrical uncertainties and size of PRV margin based on the 3D ellipsoid size of an organ [20].
Subsequent to quantification, OAR motion data can be used in the generation of PRVs by following a margin recipe, the nature of which will depend on the type of measured OAR motion data. For instance, in the research conducted by McKenzie et al. [21], through using the formula \(1.3\times \Sigma -0.5\times \sigma,\) the rectum PRV margin was determined to be 3.7 mm to cover the mean position of the rectum boundary in at least 90 % of cases. In this formula, the random uncertainties \(\left(\sigma \right)\) and the systematic uncertainties \(\left(\Sigma \right)\) for rectum were estimated to be 3 mm and 4 mm, respectively, based on the examination of rectal location on repeated CT scans over a treatment duration of approximately 6 weeks [22].
Given the typical geometrical shape and complexity of the rectum, the rectal boundary displacements show a spatially-variable motion along the rectal length. Several studies have reported the relatively unrestrained superior part of the rectum normally sees a larger boundary displacement than the lower (caudal) part, since the motion of the lower rectum boundary is limited by adjacent muscle tissues such as the puborectalis [17, 20, 23]. To better summarize the rectal boundary displacement pattern and classify different areas of the rectum, Fiorino et al. [17] separated the rectum into a cranial half and a caudal half according to the position of the rectal flexure.
This study aimed to develop a concise and effective PRV margin recipe that could make allowances for bladder and rectum inter-fraction motion in prostate EBRT. The bladder and rectum PRV margins generated in accordance with this recipe aim to cover the outward displacement (the boundary displacement promoted by organ volume expansion) of at least 90 % of the bladder and the rectum boundaries in 90 % of prostate cancer patients.
Materials and methods
Sixteen prostate cancer patients were randomly selected from the GCC (Genesis Cancer Care) database. All patients had EBRT with same prescription of 78 Gy delivered in 39 fractions (2 Gy per fraction). Each patient had implanted prostate fiducial markers and daily cone-beam computed tomography (CBCT) for treatment setup and verification. Patients were between 55 and 79 years old (mean 72.3, median 73.5). Prior to CT and treatment, all patients were counseled to have an evacuated rectum and a comfortably-full bladder.
For each patient, the planning CT and five of the pre-fraction CBCT scans were used (96 scans in total). CBCT scans were randomly sampled from the entire treatment course for each patient, requiring the assumption that the associated organ motion is similar to that observed in the five sampled fractions. The CT scans covered both the abdomen and pelvis from above the bladder to the ischial tuberosity, while CBCT scans only covered the pelvic region from the top of the bladder to the anus. All image sets were acquired with 3 mm thick slices and 3 mm intervals.
Organ delineation
The delineation of organs was undertaken using the MIM software (version 6.8.9 MIM Software Inc., Cleveland OH). The bladder and rectum contours, defined by the external organ walls, were delinated on the image planes which contained planning target volume. Because auto-contouring was not practical with the low quality CBCT images, the entire delineation process was conducted manually by the same person and verified by an experienced senior medical physicist and an experienced radiation therapist. As a result of poor CBCT image quality, the bladder of one patient and the rectum of three patients were not visiable and their delineation was abandoned. Therefore, 15 patients’ bladder contours were selected for the bladder PRV study, and 13 patients’ rectum contours were selected for the rectum PRV study.
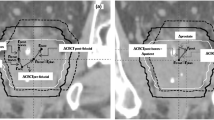
On the basis of the research conducted by Fiorino et al. [17], the whole rectum was also separated about the rectal flexure to form the cranial half and the caudal half (referred to here as “upper” and “lower”, respectively). The upper part of the rectum (cranial half) was then separated further about its midline into the “superior” and “middle” parts as shown in Fig. 1. By following this approach, the rectum could be simply and rapidly separated to generate differential rectum PRV margins for its three parts (superior, middle, and lower rectum); therefore, a combined rectum margin, with an inverted cone-like shape corresponding to the rectum boundary displacement pattern, could be created by forming the union of the three differential margins.
Organ motion measurement
To measure the organ boundary inter-fraction displacement, for each patient the organ contours from five CBCT image sets were transferred to that patient’s CT image set using fiducial marker based rigid image fusion. To simplify the measurement procedure, for each OAR (rectum and bladder) a Boolean union contour was defined which described the union of contours from all five fractions. This way, the largest organ boundary outward displacement, could be evaluated by measuring the distance from the CT contour to the organ union contour.
For each patient, the largest bladder boundary outward displacement was measured in five directions (anterior, posterior, left, right, inferior). The superior direction was not included. The superior bladder boundary displacement has minimal impact on absolute bladder dose metrics, and most CBCT scans did not cover the superior bladder boundary. The largest rectum boundary outward displacement, for each patient, was measured in four directions (anterior, posterior, left, right). The superior and inferior rectal displacements were not considered in this study as they do not reflect the geometry of the rectum of the directions of its boundary motions. These displacements were analysed on transverse slices and mid-sagittal slice (for measuring inferior bladder boundary displacement only) that contained the planning target volume (PTV). This typically corresponded to 15–20 transverse slices and 1 mid-sagittal slice.
Boundary displacement distribution
As the generated PRVs aim to cover 90% of the organ boundary outward displacement, an investigation of the organ boundary displacement distribution has to be conducted to determine the 90 % interval. Hence, in this study three transverse planes were selected for the top, middle, and bottom parts of the bladder and rectum separately. On each plane and each direction (anterior, posterior, left, and right), the distance from CT organ boundary to CBCT organ boundaries were measured in each of the five fractions individually, and the distances were normalized to the largest value. After normalization, a histogram was created to represent the position distribution of organ boundary between the CT and the furthest position on CBCT. The histogram enabled the calculation of the distance range 0–K, representing the range where the interval area occupies 90% of the area of the total distribution. For given mean \({\upmu }\) and standard deviation \({\upsigma },\)the interval can be computed via:
This study only focuses on developing PRV margins to cover organ boundary outward displacement. The margins imposed on CT organ contours will naturally cover all the organ boundary inward motion (the boundary displacement promoted by organ volume contracting). The organ boundary inward displacement was not considered separately in this research. Consequently, Due to the normalization process, “K” should be a positive number less than 1 and the value of “K” stands for the proportion of the furthest distance the CBCT organ boundary can displace The range from 0 to K should encompass the required percentage of organ boundary outward displacements (i.e. 90% in this study).
PRV margin recipe
The PRV margin-generation recipe is designed through the following 4 steps, illustrated in Fig. 2:
-
1.
Calculating the average displacement in every direction by using the largest organ boundary outward displacement values for each patient.
-
2.
Multiplying the average displacement by the K value in each direction and for every patient, to generate a margin that covers the 90 % organ boundary outward motion for that patient.
-
3.
The patients are ranked in each direction, according to their average organ boundary outward displacement values.
-
4.
The 90th-percentile value is selected for all directions as the PRV margin, because one of the aims of generated PRV margin is acceptable for 90 % of patients.
An example flow chart of PRV margin generation process in the posterior direction. The average value of list (a) which contains the largest organ boundary outward displacement for Patient-1 in the posterior direction on all sample slices is calculated to generate list (b), which contains the average of the largest organ boundary outward displacement values in the posterior direction for all patients, ranked from largest to smallest (top to bottom). List (c) which represents the organ PRV margin in the posterior direction for all patients, is created through multiplying the numbers on list (b) by the K value (0.8 in this example). If a PRV is to be created that is acceptable for 90 % of patients, then the 90th -percentile margin value should be selected. In this example, the 90th percentile corresponds with patient-2 in table (c). This process is repeated for all directions to produce list (d) which gives the final width of PRV margins
Bladder PRV margins
Two bladder PRV margins, PRV1_bladder and PRV2_bladder, were generated. PRV1 covered at least 90% of the bladder outward motion for 90% of patients. PRV2_bladder was generated with the same criteria though only considering patients who didn’t experience large bladder volume changes, defined by a bladder volume increase of less than 20% of the baseline bladder volume throughout the treatment course. Twelve patients were included in PRV2_bladder generation.
Rectum PRV margins
Two rectum PRV margins, PRV1_rectum and PRV2_rectum, were generated through the suggested recipe while both margins aim to cover at least 90% of rectum boundary outward motion for 90% of the patients (12/13 patients). However, the PRV2_rectum was produced by forming the union of three PRV margins corresponding to three different rectum parts (superior, middle, and lower parts) as defined using the proposed rectum separation method. The PRV1_rectum was spatially uniform, meaning that the PRV was generated without rectum separation and treated the entire rectum as a whole in the proposed method.
PRV margin evaluation metrics
Three metrics were used in this investigation for evaluating and comparing the PRV margins. These metrics use the following definitions:
-
\({V}_\text{Union}\): the Boolean union volume of the organ on CBCT scans across all five fractions;
-
\({V}_\text{CTorgan}\): the organ volume on original planning CT scan;
-
\({V}_\text{PRV}\): the volume of PRV margin (it doesn’t include the volume of the organ structure).
The metrics are defined as:
-
Original coverage %: the volume of the intersection of \({V}_{CTorgan}\) and \({V}_{Union}\) as a percentage of the union volume:
$$\begin{array}{c}Original\,coverage\,\%=\frac{{V}_{CTorgan}\cap {V}_{Union}}{{V}_{Union}}\times 100\%\end{array}$$(2)
A lower value of Original coverage% indicates the organ boundary experienced a larger outward displacement, while a higher value indicates a smaller outward displacement.
-
Effectiveness%: the volume of the intersection of \({V}_{\text{P}\text{R}\text{V}}\) and \({V}_{Union}\) as a percentage of the union volume:
$$\begin{array}{c}Effectiveness\,\%=\frac{{V}_{PRV}\cap {V}_{Union}}{{V}_{PRV}}\times 100\%\end{array}$$(3)
This represents the percentage of “useful” PRV margin volume, being the PRV margin volume which would cover the organ motion indicated by all CBCT images for each patient. A relatively high Effectiveness% indicates a relatively large portion of the PRV margin volume can cover organ boundary outward motion.
-
Outward coverage%: the volume of the intersection of \({V}_{\text{P}\text{R}\text{V}}\) and \({V}_{Union}\) as a percentage of the outward organ motion volume (which is the relative complement of \({V}_{CTorgan}\) in \({V}_{Union}\)):
$$\begin{array}{c}Outward\,coverage\,\%=\frac{{V}_{PRV}\cap {V}_{Union}}{{V}_{Union}\backslash {V}_{CTorgan}}\times 100\%\end{array}$$(4)
A higher Outward coverage% indicates a larger portion of the organ boundary outward motion is covered and vice versa.
After determining the margins from the PRV margin recipe, they were applied to every patient to obtain the PRV margin evaluation metrics data, which enables evaluation of the PRV margin performance more intuitively.
Results
Organ motion measurement
The statistics of mean organ boundary outward displacement for bladder and rectum are presented in Table 1. In the case of bladder, the anterior boundary displays the largest mean displacement and the posterior boundary shows the largest standard deviation. If 5 % is set as a significant level, the Mann–Whitney–Wilcoxon (MWW) test [24] indicates that the anterior bladder boundary displacement is significantly larger than the right (P-value = 0.02) and inferior (P-value < 0.01) bladder boundaries, while it is not significantly larger than the posterior (P-value = 0.15) and left (P-value = 0.15) bladder boundaries.
The mean rectum boundary outward displacement of all evaluated directions for the different rectal parts is shown in Fig. 3. The MWW test (significance level α = 0.05) indicates there is a significant difference between the upper and lower parts of the rectum (p-value < 0.01), and between the superior and middle parts of the rectum (p-value = 0.02). A decrease in mean boundary displacement is observed when progressing from the superior to inferior rectum, indicating the need for a spatially-varying margin.
Boundary displacement distribution
The normalized bladder boundary position distribution is demonstrated in Fig. 4A. It was approximated to a normal distribution with a mean \({\upmu }\) of 0.5 and standard deviation \({\upsigma }\) of 0.2. The K value for bladder boundary position distribution (K_bladder) for P = 0.9 is found to be approximately 0.77.
The normalized rectum boundary position distribution is shown in Fig. 4B. It too was approximately a normal distribution with a mean of 0.5 and standard deviation of 0.2, and the associated K value for rectum boundary position distribution (K_rectum) was therefore also approximately 0.77. It should be pointed out that both histograms were tested for normality through the Shapiro-Wilk test. Under the null hypothesis that the samples come from a normal distribution, for bladder histogram this gave p-value = 0.022 and for rectum histogram p-value < 0.001.
A Histogram of the normalized bladder boundary position distribution. B Histogram of the normalized rectum boundary position distribution. Values of 0 were not taken into account because this study only considers organ boundary outward displacement (the cases where the organ boundary has not been displaced are not included)
Bladder PRV margins
The values of PRV1_bladder and PRV2_bladder margins are listed in Table 2. Values for evaluation metrics for both margins are provided in Table 3. This table also shows the mean, maximum, minimum, and 10th-percentile values for each evaluation metric.
Rectum PRV margins
The two rectum PRV margins (PRV1_rectum and PRV2_rectum) are listed in Table 4. Table 5 shows the evaluation metrics of both margins. The values of PRV volume and evaluation metrics for the two margins are shown in Fig. 5.
Discussion
Organ motion measurement
From Table 1, the standard deviation of the anterior and posterior bladder boundary displacements (0.53 cm, 0.41 cm) are larger than other directions which suggest the variations in bladder boundary displacement among different fractions are more evident in the anterior and posterior directions. This could be explained by the fact that the left and right bladder boundary motions are restricted by the pelvis and femoral bones while there is no boney tissue to limit the anterior and posterior bladder boundary motions.
The anterior bladder boundary shows the largest mean displacement, but it cannot be concluded that the largest displacement of bladder boundary is always in the anterior direction. The MWW test shows that the anterior bladder boundary displacement is not significantly larger than posterior and left boundaries. This could be the result of an inability to reveal the true bladder motion pattern with the currently limited data set. A limitation was the intensive labour required to delineate multiple pelvic CBCT sets for multiple patients.
Muren et al. [20] previously reported asymmetric left and right bladder boundary motions, the relevant displacements found in this study do not differ statistically.
Although the bladder boundary displacement characteristics vary by direction, for rectum the mean and standard deviation values of all directions are roughly equal to 0.5 and 0.2 cm, indicating that there is minimal variation according to displacement direction. However, displacement does vary with cranio-caudal location along the rectum. As shown in Fig. 3 and as indicated by the MWW test, different parts of the rectum are displaced differently, a large reduction in the displacement of the rectal wall is seen between the superior, middle, and lower parts. This observation supports the concept of applying a PRV margin that varies according to the rectal region. A similar finding can be found in the research conducted by Prabhakar et al. [23], who suggested that using a differential PRV margin for the superior, middle, and lower (referred to as lower rectum in their research) part of rectum is more reasonable. However, they had not reported an unambiguous separation method for generating three parts of the rectum.
Bladder PRV margins
As shown in Table 3, the 10th-percentile Outward coverage for both PRV1_bladder and PRV2_bladder remained at a high level of about 80%, as expected. However, the 10th-percentiles of Effectiveness% for PRV1_bladder and PRV2_bladder were 12.1 and 14.0%, respectively, which means in the worst scenario only about one-tenth of these margins would be sufficient to cover bladder motion.
The low values of Effectiveness% were produced using the current PRV margin creation method. The current creation method is simply adding margins, defined by margin size in the cardinal directions, to the OAR contour on the planning CT to create PRV margins, which means the PRV margin and OAR contour had similar shapes despite the difference in volume. If the motion of OAR in subsequent treatment fractions shows a uniform expansion or contraction (i.e. changing the volume but hardly changing the shape), the PRV margin created by adding margins to OAR contour certainly could maintain a high value of Effectiveness% while achieving the goal of Outward coverage%. However, this study shows that expansion and contraction of bladder and rectum are highly non-uniform between all directions. The change in their volume is usually accompanied with obvious changes in shape. Hence, to meet the requirement of PRV margin Outward coverage%, the size of PRV margin is bound to expand, which results in the low score for margin Effectiveness%. To solve the problem, an anisotropic margin could be applied.
The PRV2_bladder excluded the situation where the bladder volume increased by more than 20% of the baseline bladder volume. Consequently, the size of PRV2_bladder in right, left, and inferior direction is smaller than that of PRV1_bladder. PRV2_bladder was derived for the case where strict bladder volume control measures are applied prior to treatment.
Muren et al. [20] analysed 149 CT scans and 133 sets of EPIs sampled from 20 urinary bladder cancer patients. Applying the 3-D margin tool of the Helax-TMS planning system, the CTV-to-ITV margin was determined as 1.0 cm inferior, 2.0 cm superior, 1.1 cm left, 0.8 cm right, 2.0 cm anterior, and 1.4 cm posterior to encompass all bladder deflections except for the largest outward deflection in all directions in 84 % of patients. Because bony landmark fusion rather than fiducial marker-based rigid image fusion was used in their research, the generated bladder margin is apparently much larger than both PRV1_bladder and PRV2_bladder, which indicates the advantage of applying fiducial markers in reducing bladder geometric uncertainties and the required bladder margin size.
Rectum PRV margins
As a result of the larger margin size of PRV2_rectum in the superior and middle part of the rectum (Table 4), Fig. 5A demonstrate that the volumes of PRV2_rectum are generally larger than that of PRV1_rectum. However, both Table 4 and Fig. 5B illustrate that the effectiveness% of the two margins remains approximately the same, which indicates that the larger expansion in PRV2_rectum relative to PRV1_rectum can correctly cover rectum boundary outward motion. Moreover, based on Fig. 5C, the PRV2_rectum could significantly improve the Outward coverage% score for all 13 patients. For all patients, the PRV2_rectum increased the maximum of Outward coverage% scores by 18.4 % and the average by 10.32% compared to the ordinary margin recipe. Furthermore, when patients were ranked by the Original coverage% (Fig. 5D), an increase was observed in the Outward coverage% using the PRV2_rectum while the patients’ Original coverage% score decreased, which indicates that the PRV2_rectum could greatly increase the Outward coverage% score for patients who experienced a relatively large rectum boundary outward displacement. The derived metrics indicate the benefit of using rectum separation for PRV generation. Specifically, PRV1_rectum achieved a lower Outward coverage% score than PRV2_rectum.
Through examination of 141 CT scans for 19 bladder cancer patients, Muren et al. [20] suggested applying a 1.6 cm anterior and 1.1 cm posterior PRV margin to the whole rectum to account for all rectal motion except for the two most displaced rectum walls in each of these directions in 89% of patients. The size of their margin is similar, though slightly larger, than those derived here for the superior part of the PRV2_rectum. By performing rectum separation as in this current work, a smaller PRV margin size can be used for the middle and lower rectum. By implementing the statistics-based recipe of McKenzie et al. [21], Muren et al. suggested another rectal PRV margin of 0.6 cm anterior and 0.5 cm posterior which can encompass the average wall displacement in 90% of patients. This PRV margin would be an ideal choice when applying a larger margin makes plan optimization too challenging.
PRV margin evaluation metrics
Two evaluation metrics, Effectiveness% and Outward coverage%, were set to examine the performance of PRV margins. The 10th-percentile scores of evaluation metrics were considered sufficient to quantify PRV margin performance, since the PRV margins were generated to suit 90% of patients.
Neither the Effectiveness% nor Outward coverage% should be considered solely, since they cannot fully describe how well the margin accommodates all organ motions. For instance, a high score of Effectiveness% may indicate the margin highly conforms to the organ motion pattern, but an insufficient margin, without sufficient coverage of organ motion, could also achieve a high Effectiveness% score. Thus, evaluation metrics were considered in combination.
The Original coverage% serves for quantifying the degree of boundary displacement. With its use and other evaluation matrices, the inter-patient difference of PRV margin performance can be better observed.
Limitations and considerations
From the 16 patients considered, 624 daily CBCT scans were available. However, due to the intensive nature of the delineation process, only 80 CBCT scans were contoured and investigated. Consequently, the current data set size was limited, and we were required to assume that the associated organ motion is similar to that observed in the five sampled fractions. Similarly, all delineations were completed by the same observer, so this study did not consider the uncertainty of delineation between observers.
By following the proposed margin recipe, a series of PRV margins could be generated to achieve the specified goals. Nevertheless, there are still some concerns about the boundary position distribution, and the authors acknowledge the uncertainties and limitations of the proposed recipe. In this study, using the correct K value in the margin generation process is most important, since the correctness of the K value determines to a large extent whether the generated PRV margin will cover the required percentage of boundary displacement. Considering the K value is deduced from OAR boundary position distribution histograms, it becomes critically important to ensure that OAR boundary displacements can be assessed accurately on a daily basis to generate a histogram that will correctly express the boundary position distribution for each OAR.
Inaccuracies in contouring caused by low quality CBCT images, inadequate number of OAR boundary positions due to insufficient patient data, imprecise OAR boundary position data due to the limitations of manual observer delineation, the histogram generated in this work has limited accuracy. Hence, the question is: Can the K value correctly represent the the required percentage of boundary displacement? Further modifications to the recipe and measurement could be made in future to improve the accuracy of the histogram and K value, such as devoting more resources to preparing a larger patient data set, applying deep learning algorithms to reduce scattering and artefacts, using auto-segmentation for CBCT delineation, and investing more manpower and time to improve the accuracy of contours. Furthermore, as a result of using histograms with limited data, the position distribution of the bladder and rectal boundaries was not normal (as found using the Shapiro-Wilk test), and it had to be assumed rather than proven to be normally distributed in this study.
With the clinician preferences varying, different PRV margin criterion may be required to cover more or less boundary displacement. These criteria can be considered free parameters in this margin derivation process; Therefore, the PRV margin with the required criterion can be generated by adjusting related parameters, such as the K value or the percentile values in the PRV margin recipe.
Another limitation is in the derivation of evaluation metrics from a relatively small number of patients. Only 15 and 13 patients were used for the bladder and rectum studies, respectively. The implicit assumption was that these patients represent the entire patient population. In addition, the organ boundary displacements were measured only on transverse slices that contained the PTV. Moreover, all patient data obtained in this research were from one treatment center. The patient cohort and handling impact could also affect the accuracy of the study. Nevertheless, the recipe was designed to be adaptable and evaluation metrics could be refined as more patient cases are included.
This study has not specifically considered the potential impact of applied PRV margins on dosimetry, in particular the impact of planned and delivered dose to the target volume. Attention should be paid to such effects in any future use of the derived margins.
Finally, owing to the absence of required motion data, the intra-fraction motion was not considered in this study, the potential for revision of PRVs could be provided by taking intrafraction motion information into consideration. Furthermore, there is a paucity of similar studies in the literature. Comparison between the PRV margins generated in this study and other studies is limited.
OAR motion control
While bladder and rectum PRV margins were successfully generated in this study, the use of OAR motion control measures when applying PRV margins in treatment planning could potentially be a better option. Using suitable motion control for OARs, their position in treatment could roughly be the same as OAR positions during simulation; and would enable smaller PRV margins to be used in treatment planning, and as a result, the planning system could have more freedom to optimize dose distributions. In addition, decreased OAR motion may reduce the possibility of inadvertent irradiation, which is particularly important in SBRT (which is characterized by high dose per fraction). Multiple measures are available to control inter-fraction motion of OARs. For instance, variations of the bladder boundary could be minimized through asking patient to drink water to fill the bladder, and the rectum boundary motion can be reduced through keeping the rectum empty for example by using psyllium powder solution or a Fleet enema before simulation and treatment [25], or through the use of rectal fixation such as rectal balloons, studies have pointed out that the use of rectal balloon can not only decrease the exposure to rectal boundary volume [26], but also reduce the prostate intra-fraction motion [27].
Conclusions
In this study, a PRV generating recipe was developed for the bladder and rectum in prostate external beam radiotherapy. A series of PRV margins were generated to meet the bladder and rectum motion coverage objectives. PRV1_bladder, PRV2_bladder, and PRV2_rectum performed acceptably based on the developed evaluation process.
References
Parkin DM et al (2005) Global cancer statistics, 2002. Cancer J Clin 55(2):74–108
Council AC Understanding prostate cancer.. [Web Page]. https://www.cancer.org.au/cancer-information/types-of-cancer/prostate-cancer. Accessed 17 Mar 2021
Ramon J, Denis L (2007) Prostate cancer (recent results in cancer research). Springer, Cham
George FW et al (1965) Cobalt-60 telecurietherapy in the definitive treatment of carcinoma of the prostate: a preliminary report. J Urol 93(1):102–109
Del Regato J (1967) Radiotherapy in the conservative treatment of operable and locally inoperable carcinoma of the prostate. Radiology 88(4):761–766
O’CONNOR KM, Fitzpatrick JM (2006) Side-effects of treatments for locally advanced prostate cancer. BJU Int 97(1):22–28
Sutton E et al (2021) Men’s experiences of radiotherapy treatment for localized prostate cancer and its long-term treatment side effects: a longitudinal qualitative study. Cancer Causes Control 32(3):261–269
Folkert MR, Timmerman RD (2017) Stereotactic ablative body radiosurgery (SABR) or stereotactic body radiation therapy (SBRT). Adv Drug Deliv Rev 109:3–14
Kishan AU, King CR (2017) Stereotactic body radiotherapy for low-and intermediate-risk prostate cancer. Seminars in radiation oncology. Elsevier, Amsterdam
Hannan R et al (2016) Stereotactic body radiation therapy for low and intermediate risk prostate cancer—Results from a multi-institutional clinical trial. Eur J Cancer 59:142–151
Lin Y-W, Lin L-C, Lin K-L (2014) The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Front Oncol 4:278
Janowski E et al (2014) Stereotactic body radiation therapy (SBRT) for prostate cancer in men with large prostates (≥ 50 cm 3). Radiat Oncol 9(1):1–10
Alongi F et al (2019) Phase II study of accelerated Linac-based SBRT in five consecutive fractions for localized prostate cancer. Strahlenther Onkol 195(2):113–120
Hodapp, N., The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft...[et al] (2012) 188(1):97–99
Roeske JC et al (1995) Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys 33(5):1321–1329
Wang X et al (2016) An assessment of interfractional bladder, rectum and vagina motion in postoperative cervical cancer based on daily cone–beam computed tomography. Mol Clin Oncol 4(2):271–277
Fiorino C et al (2005) Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiother Oncol 74(2):187–195
Hoogeman MS et al (2004) Quantification of local rectal wall displacements by virtual rectum unfolding. Radiother Oncol 70(1):21–30
Lotz HT et al (2005) Reproducibility of the bladder shape and bladder shape changes during filling. Med Phys 32(8):2590–2597
Muren LP et al (2004) On the use of margins for geometrical uncertainties around the rectum in radiotherapy planning. Radiother Oncol 70(1):11–19
McKenzie A, van Herk M, Mijnheer B (2002) Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol 62(3):299–307
Remeijer P et al (2000) 3-D portal image analysis in clinical practice: an evaluation of 2-D and 3-D analysis techniques as applied to 30 prostate cancer patients. Int J Radiat Oncol Biol Phys 46(5):1281–1290
Prabhakar R et al (2014) A study on planning organ at risk volume for the rectum using cone beam computed tomography in the treatment of prostate cancer. Med Dosim 39(1):38–43
Mann H, Whitney D (1947) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Ann Math Stat 18(1):50–60
Zelefsky MJ (2019) Stereotactic radiosurgery for prostate cancer. Springer, Cham
Hille A et al (2005) The impact of varying volumes in rectal balloons on rectal dose sparing in conformal radiation therapy of prostate cancer. Strahlenther Onkol 181(11):709–716
Su Z et al (2012) Reduction of prostate intrafraction motion using gas-release rectal balloons. Med Phys 39(10):5869–5873
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No grants were used in this study and there are no conflicts of interest to declare.
Ethical approval
Approval to conduct this study was provided by GenesisCare WA, in accordance with its ethics and approval procedures. This work was assessed to be of negligible risk, with no foreseeable risk of harm, to contributing subjects.
Informed consent
Retrospective data was used and obtained under a waiver of consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Ebert, M.A., Waterhouse, D. et al. Deriving optimal planning organ at risk volume margins in prostate external beam radiotherapy. Phys Eng Sci Med 44, 1071–1080 (2021). https://doi.org/10.1007/s13246-021-01044-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-021-01044-9