Abstract
This prospective study of weekly CT scanning and plan adaption during H&N IMRT reports on the frequency of plan adaptations based on dosimetric differences between original and re-optimised IMRT plans. The volumetric and geometric change occurring in target volumes and salivary glands is also described. Ten H&N cancer patients underwent weekly planning CT imaging and re-optimisation of the IMRT plan if PTV or OAR coverage was unacceptable. Comparisons of PTV and parotid gland dosimetry between the original and adaptive plans were made. Parotid and submandibular gland volume changes and shift were calculated. Eight of ten patients required one or more plan adaptations, with 41% of adaptations occurring by fraction ten. Salivary glands reduced in volume, with a medial shift of the lateral border of the parotid gland and a superior shift of the submandibular gland. Change in PTV coverage did not correlate with weight loss or nutritional score. Inadequate PTV coverage, requiring plan adaptation, occurs early in the course of IMRT. A weekly Adaptive RT (ART) protocol results in significant improvement of PTV coverage. Implementation of a clinical ART protocol should include imaging and dose calculation within the first ten fractions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensity Modulated Radiation Therapy (IMRT) for treatment of Head and Neck (H&N) cancer delivers highly conformal, high dose radiotherapy, utilising an initial planning CT for contouring and dose calculation. This ‘snapshot’ of patient anatomy acquired prior to treatment does not account for morphological changes during treatment. Deliberate placement of steep dose gradients in tissue, where Planning Target Volumes (PTVs) and Organs at Risk (OARs) are in close proximity, results in a dose distribution sensitive to small changes in patient anatomy [1].
Patients undergoing H&N radiotherapy experience weight loss and tumour mass change [2,3,4,5,6], which may affect nearby OARs. Literature reports that parotid glands reduce in volume and shift medially into the high dose region [7]. Other reported volumetric OAR changes affect submandibular glands (reduced volume and superior shift), larynx and constrictor muscles (oedema with increased volume) [6,7,8]. These anatomical changes may result in the delivered dose distribution being significantly different to the one planned, with increased dose to parotid glands, spinal cord and brainstem [7]. Similarly, dose to tumour target volumes can deviate from planned, resulting in decreased dose and increased dose homogeneity in tumour volumes [3,4,5, 9, 10].
Discrepancies between delivered and planned dose distribution would have two consequences. Increased OAR dose results in greater radiation induced organ toxicity [11, 12], impacting on health related quality of life [13, 14]. Inadequate dose delivery to tumour volumes may compromise tumour control.
Patients may benefit from an adaptive radiotherapy (ART) approach, whereby a new treatment plan is created during the course of radiation therapy to account for anatomical changes [15]. Studies investigating the impact of such replanning have demonstrated improvement in tumour target volume coverage [4, 16, 17] as well as a reduction in dose to parotid glands, spinal cord and brainstem [18,19,20,21]. Two prospective case series have demonstrated improved loco-regional control rates in patients undergoing ART [22, 23] .
The clinical implementation of an ART protocol for H&N cancer patients, using multiple repeat CT scans, is a resource intensive process. Furthermore, not all patients may benefit from treatment plan re-optimisation [4, 24]. Therefore, to allocate limited resources appropriately, the patients who would most benefit from the ART process should be identified. Some investigators have attempted to correlate plan dosimetry with factors such as weight loss, initial volume of parotid glands and positional changes; however no statistically robust measures have been clearly identified [7, 18, 25]. Brown et al. [26] proposed a model to predict the need for plan adaptation, based on the tumour site, nodal stage at diagnosis, patient weight and initial nodal size. Equally relevant is the determination of the best timing for ART, with some authors recommending plan adaptations in the first half of treatment [5, 7, 27, 28].
This paper describes an exploratory investigation of a prospective ART protocol, whereby weekly conventional planning CT scans and nutritional (including weight) assessment were performed. The target volumes (TV) and OARs were manually delineated weekly by a radiation oncologist. Patients were subsequently treated with a re-optimised plan, if required. The investigation examined (1) the effect of anatomical changes on dose delivery to the PTV and OARs, (2) the effect of re-optimisation of the IMRT plan, and finally (3) explored the potential utility of weight or nutritional score in selecting patients who may benefit from an ART approach.
Materials and methods
Patient selection
The study protocol attained Ethics Board approval and participants provided informed consent. Ten consecutive patients with histologically proven primary head and neck Squamous Cell Carcinoma (SCC), with measurable disease on CT scan, undergoing primary radiotherapy with curative intent, with or without concurrent systemic therapy, were enrolled onto this prospective study.
Planning CT procedure
Patients were immobilized with a Civco Posifix 5 point fixation thermoplastic head and shoulder mask in supine position. A contrast-enhanced CT scan using a Siemens SOMATOM Sensation 16-slice scanner (SIEMENS, Erlangen, Germany) was acquired with 2 mm axial slices through the head and neck region (from the vertex of the skull to below the clavicular heads), with isocentre placement at the inferior-anterior edge of the C4 vertebral body on mid-line.
Delineation of target volumes and treatment prescription
All delineation was undertaken using the Pinnacle Treatment Planning System Versions 8.0m and 9.0 (Philips Medical Systems, Fitchburg, Wisconsin, USA) by the chief investigator (FA) and checked by a second radiation oncologist (AAM). Treatment volumes were defined using international consensus guidelines [29] and ICRU 62 definitions [30]. Gross Tumour Volume (GTV) was determined by visible tumour (GTVp) and enlarged or suspicious lymph nodes (GTVn), identified clinically and radiographically with CT, MRI or FDG-PET. All the Clinical Target Volumes (CTVs) were defined along anatomical boundaries. The CTV of the primary (CTVp) was defined by an expansion from the GTVp. The CTV of involved lymph nodes (CTVn) was a 3 mm expansion of the GTVn into normal fat. The CTV of uninvolved bilateral nodal regions (CTVn0) was generated as defined by the international consensus guidelines [29], but excluded the CTVp and CTVn volumes. The Planning Target Volume (PTV) was obtained by a 3 mm expansion of the CTV, as per the IGRT protocol at our institution. Two PTVs were defined; PTV60 (tumour, involved lymph nodes and at-risk regions), which was an expansion of the CTVp + CTVn + CTVn0, and PTV70 (tumour and involved lymph nodes), which was an expansion of the CTVp + CTVn. Intraobserver variability, when creating CTVs, was minimized by recording the anatomical boundaries used when creating the TVs, facilitating their reproduction in following weeks.
Organ at risk volumes were identified and contoured, including parotid and submandibular glands, spinal cord, mandible and mucosa (2 mm annulus around the aerodigestive air column). Other tissues (lenses, optic nerves, optic chiasm) were included when considered at risk of exposure.
A total of 70 Gy (2 Gy/fraction) was prescribed to the PTV70 and 60 Gy (1.7 Gy/fraction) to the PTV60 using 6MV photons and a seven-field IMRT with simultaneous integrated boost technique over 35 fractions (10 fractions/week), commencing within a median of 15 days (range 13–27) after planning CT. Daily IGRT using bone matched electronic portal imaging (EPI) correction was performed as per departmental protocol.
Weekly imaging and plan assessment
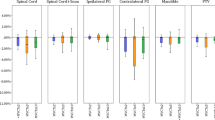
All patients underwent a non-contrast planning CT scan every 5th fraction after commencement of radiation therapy. The final four patients also had an additional planning CT scan on day one of radiation therapy, due to a protocol adjustment to include imaging on day one to assess any anatomical changes between simulation and treatment start. At each CT scan, the fit of the mask was assessed and new masks were provided as required. The newly acquired CT dataset was aligned to the original CT scan using rigid registration. For each CT scan, the tumour volumes (i.e. GTVp, GTVn, CTVs and PTVs), parotid glands and spinal cord were delineated by the chief investigator (FA). Deformable Image Registration (DIR) software was not used, and all contour adjustment was done manually. The original treatment plan was recalculated on the new CT dataset and plan acceptability was assessed via pre-determined DVH parameters for PTV coverage and dose to spinal cord, without dose warping. An unacceptable plan was defined as V95 < 95% for the PTV70 or PTV 60; or a spinal cord Dmax ≥ 45 Gy.The parotid gland dose was noted, but did not affect decision making. Unacceptable plans were re-optimised and this re-optimised plan used for subsequent treatment and weekly comparison. Parotid and submandibular gland movement was calculated by measuring the shift of the Centre of Mass (COM) coordinates relative to the original planning CT scan. The lateral border position of the parotid glands was calculated as half the width of the gland in the lateral direction from the COM point.
Weekly patient assessment
Patients underwent nutritional assessment on the same day as their repeat planning CT scan. Weight was measured without shoes and before chemotherapy. A Patient Generated Subjective Global Assessment (PG-SGA) was performed, in order to document the patient nutritional status. The PG-SGA is a nutritional tool, validated in the oncology population, which provides a global assessment of the nutritional status (categories A,B,C), as well as a numerical score, which increases with worsening nutritional status [31].
Statistical analysis
Sample t-tests were used to assess volumetric, geometric and dosimetric changes in the unadapted IMRT plans. Comparisons between the original and adapted plans were performed using two-sided paired t-tests. A probability value < 0.05 was considered significant. Correlation between patient nutritional score and weight versus PTV70 coverage over the treatment was investigated using Pearson’s correlation; since the Shapiro–Wilk test demonstrated that the data exhibited a normal distribution.
Results
Patients
Ten eligible patients were enrolled (Table 1); nine were male. The mean age of the patients was 63.7 years (range 49–82). Eight patients received concurrent systemic therapy, with either weekly Cisplatin or Cetuximab. One patient switched from Cisplatin to Cetuximab in the third week due to ototoxicity.
Mean weight loss during treatment for all patients was 5.6 kg (range 0.3–10.0 kg). Two patients required new immobilisation masks; both at fraction 20. Their weight loss at fraction 20 (6.2 kg and 6.8 kg) was double the mean weight loss of 3.1 kg (range + 0.8 kg to − 6.8 kg) at this time. The mean PG-SGA score increased during the treatment course, from a score of 5 (range 1–11) prior to treatment to 17 (range 11–28) at fraction 35, indicating worsening nutritional status (Supplementary Fig. 1).
Target volume volumetric changes
TVs changed over the treatment period (Table 2), with significant reduction by fraction 35; 25% for the GTVp, 44% GTVn, 18% CTVp, 28% CTVn and 11% PTV70. However CTVn0 and PTV60 did not demonstrate significant volume change.
Adaptive replanning
A total of 84 CT scans were analysed. A total of 22 plan adaptations were undertaken (Fig. 1), with a mean of 2 (range 0–5) for the patient cohort. Two of four patients, who underwent scanning at fraction 1, required immediate plan adaptation due to significant underdosing of the PTV. Two patients with early stage node-negative (T1N0) oropharyngeal SCC required no treatment plan adaptation. All 22 plan adaptations were due to inadequate PTV coverage; with 9 (41%) occurring by fraction 10 and 12 (55%) by fraction 15. All first plan adaptations and half of the second plan adaptations had occurred by fraction 15.
Target volume coverage with and without adaptive replanning
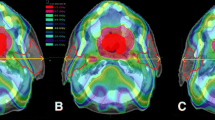
Figure 2 and Table 3 demonstrate the weekly PTV70 and PTV60 coverage for the original treatment plan on the weekly anatomy (as if no plan optimisation was performed) and for the adaptive planning strategy (≥ 1 optimisation events) for all 10 patients. Adaptive replanning significantly improved both PTV70 coverage (P = 0.0004) and PTV 60 coverage (P = 0.0012).
Salivary gland volume change, shift and dose change
The parotid gland volume significantly reduced by 28.0% during treatment (P < 0.01). The lateral borders of the parotid glands demonstrated a significant medial shift of 1.2 mm (range − 0.1 to 3.4 mm; P = 0.02), however the centres of mass did not shift medially (P = 0.51). The submandibular glands reduced significantly in volume by 26% (P < 0.001). A significant superior shift of the submandibular glands was observed at all fractions except for fractions 20 and 35. At fraction 30, a 2.4 mm superior shift (range − 2.3 to 10.3 mm, P = 0.02) was observed. Although the adapted plans met the parotid gland dose constraints (V26 < 50%), the adapted plan delivered a higher parotid gland dose compared to the original plan (38% vs. 44%, P = 0.001). However, further retrospective replanning (ART2), with optimisation parameters placing more importance on the parotid gland dose, was able to obtain both improved TV coverage and reduced parotid gland dose (Fig. 3).
Correlations with inadequate tumour coverage
There was no correlation between the change in patient PG-SGA score and change in PTV70 coverage (correlation coefficient − 0.046; P = 0.703). Similarly, no correlation was found between patient weight changes and the change in PTV70 coverage (correlation coefficient − 0.135; P = 0.256). However, initial nodal volume correlated with the number of plan adaptations (correlation coefficient 0.708; P = 0.022).
Discussion
The strength of our study lies in the weekly dosimetric evaluation of oncologist-defined structures on planning CT scans, which allowed for regular assessment of the “actual” dose delivery as anatomical changes occurred. This permits a truly adaptive approach to be implemented as patients were treated with a re-optimised plan as required, within 24 h of the new planning CT scan. Our frequency of dosimetric evaluation is similar to the study published by Schwartz et al. [19], where 24 patients underwent daily in-room CT scanning with weekly dose calculation on contours created by deformable-image registration. They report that all patients required at least one replan due to “CTV and normal tissues changes”. However, underdosing of the CTV was not observed with the IGRT or ART techniques, suggesting that replanning was required due to excessive OAR dose. By contrast, our investigation demonstrated inadequate PTV coverage in 80% of the patient cohort, requiring IMRT plan adaptation during the course of treatment. Plan adaptation was required due to inadequate PTV coverage in all cases, rather than excessive OAR dose; and resulted in significant improvement in TV coverage. Other investigators [4, 5, 18] have reported lower rates of plan adaptations; however the frequency of CT scanning and dosimetric analysis in these reports is less than weekly. Ahn et al. [4], who imaged at three time points during the treatment course, demonstrated that 65% of their patients (15 of 23) required replanning, predominantly due to inadequate PTV coverage. Wang et al. [24], using a single re-imaging, reported that 50% of unadapted plans were noncompliant for OAR dose criteria.
A limitation of our study is that the patient cohort was small (n = 10), however the number of scans undertaken (84 scans) is comparable to that seen in other prospective studies which have reported on the effect of an ART approach on TV and OAR coverage (range 30–91 scans) [4, 16,17,18,19, 21, 24, 25]. Larger studies tend to image and calculate the dose less frequently [4, 16, 18, 21, 24]. Other studies, employing weekly imaging and dose calculation have similarly smaller patient cohorts (n = 11–15) [17, 20, 25, 32]. Schwartz et al. [19], however performed weekly imaging on a larger patient cohort (n = 24). It was not possible in increase the number of patients in this study due to departmental resource constraints.
Whilst dose calculation on repeat imaging studies is performed and reported widely, interpretation of the data needs to consider the variability in results occurring due to factors aside from volumetric and geometric changes within the patient. The image registration process and interfraction positional variation of the patient may introduce random variability, which would impact on results of contour shift and dosimetric comparison. Delana et al. [33] quantified the impact of set-up variations on parotid gland dose. A 3% increase in mean parotid dose was observed with each millimetre displacement in the craniocaudal and lateral dimensions. Additionally, the movement of interstitial fluid, resulting in tissue deformation, may vary during treatment [34]; this was not accounted for in this study.
We have demonstrated that the GTVs (GTVp, GTVn) and their associated CTVs (CTVp, CTVn) reduced in volume over the treatment period. In a recent study by Hamming-Vrieze et al. [35], it was demonstrated that the tissue surrounding the GTV (containing microscopic disease) did not shrink as rapidly as the visible tumour. Therefore, contouring a shrinking tumour volume during the course of treatment risks underdosing microscopic disease. However, this issue is mitigated if CTVs strictly adhere to anatomical boundaries, as we have done. We demonstrated that the mean CTVn0 (representing the high risk nodal area) did not show significant volume reduction, nor did the mean PTV60 volume, whose construct is dominated by the CTVn0. Therefore we feel that our protocol minimised the risk of undertreating microscopic disease.
Whilst the optimal timing to engage an ART technique is unknown, some authors recommend undertaking plan adaptation early in the treatment course [7, 17, 27, 28]. Zhang et al. [17] recommend plan adaptation at weeks one, two and five. Schwartz et al. [19] report the median timing of first replan for their cohort was at fraction 16. Significant changes in anatomy occurring in the first half of treatment have previously been reported by others [7]. 41% of our replanning events occurred at or before fraction 10. All first IMRT plan adaptations and half of the second plan adaptations had occurred by fraction 15. This supports the view that inadequate IMRT plan dosimetry can occur early and we would recommend initiating repeat imaging and plan assessment early in the treatment course, as early as fraction 5 or 10. However, plan assessment should also be undertaken later in the course of RT, e.g., at fraction 25, as suboptimal dose delivery can still occur at this stage.
Although the parotid gland dose remained within acceptable IMRT planning dose constraints (i.e., V26 < 50%), our patients received a higher parotid gland dose with the adaptive plans, compared to the original treatment plan. This occurred because, in order to begin treatment using the adaptive plan within 24 h, the first acceptable adaptive treatment plan meeting the requirements for PTV coverage and the parotid gland dose constraints (V26 < 50%) was utilised. Subsequent retrospective replanning demonstrated that it was possible to create an adaptive plan with improved coverage of both the PTV and the parotids, with little additional planning time. A limitation of our study is that prospective parotid gland sparing was not prioritised sufficiently. This could have been avoided, without slowing the replanning process, by modifying the optimisation criteria for the parotid glands at the time of adaptive replanning.
Significant resources are required to maintain a comprehensive ART strategy for all H&N cancer patients. Manual delineation of the required anatomical structures takes approximately 2 h [36], but can vary widely depending on the amount of structures contoured and physician experience. Further time and resources are required for treatment plan generation, dosimetry calculation and the Quality Assurance process before an adapted plan can be delivered [36]. We were able to achieve a turnaround time of 24 h to delivery of an adapted plan, with existing staff resources, but this required good communication and coordination between the team. Due to resource limitations, the challenge of identifying those patients who would most benefit from ART remains. We found a correlation between initial nodal size and frequency of plan adaptations. This is consistent with the investigation by Brown et al. [26], where initial nodal size correlated with the need for plan adaptation; and was subsequently a predictive factor included in their model to identify patients who may need ART. Selection criteria for ART suggested by other authors include initial planning doses achieved in the TV and parotid glands [4, 7, 37]. Whilst weight change could be a convenient surrogate measure of IMRT plan suitability, previous studies have found it to be an unreliable indicator [18, 25, 34]. In our investigation, neither weight loss nor PG-SGA score correlated with inadequate tumour coverage; however we are limited by a small sample size. Nevertheless, we feel that the PG-SGA is a potentially useful tool to examine further in prospectively designed larger scale investigations, since the score is derived from a group of measures, only one of which is weight.
Implementing an ART strategy is challenging in large numbers outside of a trial protocol, due to the significant resources required. Larger scale prospective investigations would be useful to identify reliable surrogate measures that would suggest a need to adapt an IMRT plan; hence directing the resources where they would be most beneficial. Cone Beam CT, deformable registration and autosegmentation tools were not in clinical use in the centre when this study was developed. Whilst this study required significant staffing resources, we have shown that it is feasible to implement an ART strategy on a small scale, even in the absence of these potentially time-saving tools. Streamlining the ART process, with the use of autosegmentation and planning and Cone Beam CT Imaging may also result in a more practical ART strategy, and should be investigated further. However, there are limitations of using these tools. Compared the planning CT images, Cone Beam CT images have reduced soft tissue contrast, greater image noise and the longer acquisition time may result in blurring due to internal structure motion [38, 39]. The image quality is inferior to diagnostic quality CT images, which may result in difficulty with the accurate delineation of the GTV and at- risk nodal regions [39, 40]. Whilst there is good concordance between oncologist defined contours and autosegmented contours for OARs, the GTV is also less well replicated by autosegmentation [41].We consider oncologist defined contours to still be the gold-standard. Additionally, the maximum field length of Cone Beam CT images is limited to 26–27 cm [38], therefore the full head and neck field cannot be imaged in a single acquisition; thus preventing complete assessment of patient anatomy and dose delivery.
Ultimately, an ART strategy which improves plan dosimetry would only be worthwhile if it results in an improvement in patient clinical outcomes. Good tumour control was reported in one prospective ART study; with 100% local and 95% regional disease control at 2 years reported (19). Castelli et al. [32] demonstrated a reduction in xerostomia risk with weekly replanning using radiobiological modelling. Radiobiological analysis of clinical outcome measures should be incorporated into future prospective investigations, in addition to the consideration of dosimetric outcome measures.
Conclusion
80% of the patient cohort benefitted from ART during the course of IMRT in our investigation. Our results suggest that inadequate target volume coverage can occur early, as all first plan adaptations and half of the second plan adaptations had occurred by fraction 15. A weekly ART protocol resulted in significant improvement of tumour coverage. During the implementation of a clinical ART protocol, imaging and dose calculation within the first ten fractions could be considered. Patients with large initial volume of nodal disease may benefit from more frequent imaging and plan assessment.
References
Ezzell GA et al (2003) Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT subcommittee of the AAPM radiation therapy committee. Med Phys 30(8):2089
Barker JL Jr et al (2004) Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 59(4):960–970
Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P (2006) Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol 64(2):355–362
Ahn PH et al (2011) Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol 80(3):677–685
Cheng HCY et al (2012) A prospective study on volumetric and dosimetric changes during intensity-modulated radiotherapy for nasopharyngeal carcinoma patients. Radiother Oncol 104(3):317–323
Bando R et al (2013) Changes of tumor and normal structures of the neck during radiation therapy for head and neck cancer requires adaptive strategy. J Med Invest 60(1.2):46–51
Brouwer CL, Steenbakkers RJHM, Langendijk JA, Sijtsema NM (2015) Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol 115(3):285–294
Ricchetti F et al (2011) Volumetric change of selected organs at risk during IMRT for oropharyngeal cancer. Int J Radiat Oncol 80(1):161–168
Bhide SA et al (2010) Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys 76(5):1360–1368
Bhandari V, Patel P, Gurjar O, Gupta K (2014) Impact of repeat computerized tomography replans in the radiation therapy of head and neck cancers. J Med Phys 39(3):164
Deasy JO, Moiseenko V, Marks L, Chao KC, Nam J, Eisbruch A (2010) Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 76(3):S58–S63
Rancati T et al (2010) Radiation dose–volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys 76(3):S64–S69
Dornfeld K et al (2007) Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol 68(3):750–757
Lin A, Kim HM, Terrell JE, Dawson LA, Ship JA, Eisbruch A (2003) Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol 57(1):61–70
Castadot P, Lee JA, Geets X, Grégoire V (2010) Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol 20:84–93
Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S (2011) The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol 98(1):23–27
Zhang P et al (2016) Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol 120(1):41–47
Wang X et al (2010) Anatomic and dosimetric changes during the treatment course of intensity-modulated radiotherapy for locally advanced nasopharyngeal carcinoma. Med Dosim 35(2):151–157
Schwartz DL et al (2012) Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol 83(3):986–993
Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A (2009) Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol 75(3):924–932
Nishi T, Nishimura Y, Shibata T, Tamura M, Nishigaito N, Okumura M (2013) Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother Oncol 106(1):85–89
Yang H, Hu W, Wang W, Chen P, Ding W, Luo W (Jan. 2013) Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol 85(1):e47–e54
Chen AM, Daly ME, Cui J, Mathai M, Benedict S, Purdy JA (2014) Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning: adaptive replanning for head and neck cancer. Head Neck 36(11):1541–1546
Wang W et al (2010) Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol 77(2):617–621
Zhang X et al (2012) Dosimetric variations of target volumes and organs at risk in nasopharyngeal carcinoma intensity-modulated radiotherapy. Br J Radiol 85(1016):e506–e513
Brown E et al (2015) Predicting the need for adaptive radiotherapy in head and neck cancer. Radiother Oncol 116(1):57–63
Fiorentino A et al (2012) Parotid gland volumetric changes during intensity-modulated radiotherapy in head and neck cancer. Br J Radiol 85(1018):1415–1419
Huang H et al (2015) Determining appropriate timing of adaptive radiation therapy for nasopharyngeal carcinoma during intensity-modulated radiation therapy. Radiat Oncol 10(1):192
Grégoire V et al (2003) CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol 69(3):227–236
International Commission on Radiation Units and Measurements (1999) ICRU Report 62: prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50). ICRU, Bethesda
Bauer JD et al (2006) Evidence based practice guidelines for the nutritional management of cancer cachexia. Nutr Diet 63(s2):S3–S32
Castelli J et al (2015) Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol 10(1):6
Delana A et al (2009) Impact of residual setup error on parotid gland dose in intensity-modulated radiation therapy with or without planning organ-at-risk margin. Strahlenther Onkol 185(7):453–459
Lee C et al. (2008) Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol 71(5):1563–1571
Hamming-Vrieze O et al (2017) Analysis of GTV reduction during radiotherapy for oropharyngeal cancer: implications for adaptive radiotherapy. Radiother Oncol 122(2):224–228
Budach W et al (2011) Evaluation of time, attendance of medical staff, and resources during radiotherapy for head and neck cancer patients: the DEGRO-QUIRO trial. Strahlenther Onkol 187(8):449–460
Castelli J et al (2016) A Nomogram to predict parotid gland overdose in head and neck IMRT. Radiat Oncol 11(1):79
Bissonnette J-P et al (2012) Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179: QA for image-guided radiation therapy utilizing CT-based technologies. Med Phys 39(4):1946–1963
Boda-Heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M (2011) kV Cone-Beam CT-Based IGRT: a clinical review. Strahlenther Onkol 187(5):284–291
Ho KF et al (2012) Monitoring dosimetric impact of weight loss with kilovoltage (KV) cone beam CT (CBCT) during parotid-sparing IMRT and concurrent chemotherapy. Int J Radiat Oncol 82(3):e375–e382
Peroni M et al (2012) Automatic segmentation and Online virtualCT in head-and-neck adaptive radiation therapy. Int J Radiat Oncol 84(3):e427–e433
Acknowledgements
Anthony Arnold, Justin Dixon and Stephen Jones for planning assistance throughout the study, Michael Bailey for data management assistance and Lois Holloway for strategic guidance. This research has been conducted with the support of the Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig 1
Patient weight loss and PGSGA score during treatment (TIF 55 KB)
Supplementary Fig 2
Coverage of the PTV70 on original plan (left) and adaptive plan (right). CTVp orange, CTVn light orange, PTV70 purple, 95% isodose line green (TIF 1245 KB)
Supplementary Fig 3
Coverage of the PTV60 on original plan (left) and adaptive plan (right). CTVp orange, CTVn light orange, CTVn0 yellow, PTV60 purple, 95% isodose line green (TIF 1341 KB)
Rights and permissions
About this article
Cite this article
Aly, F., Miller, A.A., Jameson, M.G. et al. A prospective study of weekly intensity modulated radiation therapy plan adaptation for head and neck cancer: improved target coverage and organ at risk sparing. Australas Phys Eng Sci Med 42, 43–51 (2019). https://doi.org/10.1007/s13246-018-0707-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-018-0707-y