Abstract
Background
Current design strategies for small diameter vascular grafts (< 6 mm internal diameter; ID) are focused on mimicking native vascular tissue because the commercially available grafts still fail at small diameters, notably due to development of intimal hyperplasia and thrombosis. To overcome these challenges, various design approaches, material selection, and surface modification strategies have been employed to improve the patency of small-diameter grafts.
Review
The purpose of this review is to outline various considerations in the development of small-diameter vascular grafts, including material choice, surface modifications to enhance biocompatibility/endothelialization, and mechanical properties of the graft, that are currently being implanted. Additionally, we have taken into account the general vascular physiology, tissue engineering approaches, and collective achievements of the authors in this area. We reviewed both commercially available synthetic grafts (e-PTFE and PET), elastic polymers such as polyurethane and biodegradable and bioresorbable materials. We included naturally occurring materials by focusing on their potential application in the development of future vascular alternatives.
Conclusion
Until now, there are few comprehensive reviews regarding considerations in the design of small-diameter vascular grafts in the literature. Here-in, we have discussed in-depth the various strategies employed to generate engineered vascular graft due to their high demand for vascular surgeries. While some TEVG design strategies have shown greater potential in contrast to autologous or synthetic ePTFE conduits, many are still hindered by high production cost which prevents their widespread adoption. Nonetheless, as tissue engineers continue to develop on their strategies and procedures for improved TEVGs, soon, a reliable engineered graft will be available in the market. Hence, we anticipate a viable TEVG with resorbable property, fabricated via electrospinning approach to hold a greater potential that can overcome the challenges observed in both autologous and allogenic grafts. This is because they can be mechanically tuned, incorporated/surface-functionalized with bioactive molecules and mass-manufactured in a reproducible manner. It is also found that most of the success in engineered vascular graft approaching commercialization is for large vessels rather than small-diameter grafts used as cardiovascular bypass grafts. Consequently, the field of vascular engineering is still available for future innovators that can take up the challenge to create a functional arterial substitute.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Cardiovascular disease is among the leading causes of death worldwide. An approximately 3 million procedures performed on blood vessels and the heart each year in the US according to the American Heart Association have prompted a challenge to develop a functional small-diameter vascular graft.169 Surgical intervention with bypass graft is a procedure often employed on a defective blood vessel.26,177 Bypass surgery with a patient’s vein or artery remains the preferred treatment of both peripheral and coronary artery disease.32 However, these vasculatures are usually limited by availability because of the patient’s condition or they have been used previously. Hence, a patient with recurrent occlusive lesions would be rendered inoperable resulting in loss of a limb in cases of the peripheral disease.
An alternative to an autograft (patient’s vessel) is the use of engineered grafts. These grafts have the future potential over autografts given their limitless availability, range of material selection, and the ability to optimize their properties to match the mechanical properties of the native vessel.
The use of well-known, commercially available, non-degradable synthetic grafts such as expanded polytetrafluoroethylene (e-PTFE*) and polyethylene terephthalate (PET*) for large arterial reconstructions such as that of the aorta, arch vessels, and common femoral artery, where there are high flow and low resistance, has shown success in clinical trials.52,80,122,170 Although graft infection (< 3%),146 dilation (35%),72 and occlusion (2%)72 remain challenging problems for a small number of patients who have undergone procedures using these grafts, the majority of patients can expect permanent patency and minimal need for repeated procedures.114,138 A recent study showed that the use of e-PTFE* in the bypass procedure had patency of 39% at 5 years.170 However, small diameter bypass grafts tend to fail often in short- and long-term observations. This observation has been linked to poor endothelial regeneration and compliance mismatch between the graft and native vessels.103 Hence, resolving these issues would lead to lower morbidity and mortality especially in children born with congenital heart disease.
An ideal small-caliber graft should have [1] good mechanical strength and compliance to withstand hemodynamic stress; [2] suturability; [3] “off-the-shelf” availability in various sizes in case of emergency; [4] ease of use in handling to minimize duration of surgery, cost, and risk; [5] resistance to thrombus and infection; [6] biocompatibility to completely integrate with the body and yield neo-vessels similar to native arteries in property and function; [7] reasonable manufacturing cost; [8] long-term patency;[9] rapid endothelialization and [10] good porosity > 50 µm for easy cell infiltration. A functional small-caliber vascular graft that would meet all the above requirements needs a biomimetic design coupled with improved cellular and molecular understanding of the biology of the vessel wall.32 Finally, incorporating signaling cues on the surface of the graft would aid in faster endothelialization and tissue remodeling.166
Rapid endothelialization is an important aspect of consideration for the success of any given small-diameter vascular graft to prevent thrombus formation. In this process, porosity and pore size distribution plays an important role in the structure and remodeling of the graft. Over the past five decades, countless studies have shown the importance of porosity in graft design.24,31,33,61 Recent findings show that the porous luminal surface of the graft plays an important role in stabilizing the intima and aids in the infiltration of cells, whereas the porous abluminal surface enhances penetration of peri-graft tissue, thus, acting as anchorage and inhibiting graft kinking.225 However, porous grafts with good compliance such as those made with polyurethane (PU) materials79 often lose their radial mechanical strength over time as a result of internal pressure and, hence, may lead to aneurysm formation. Therefore, one alternative way to eliminate this obstacle is to apply external reinforcement to the graft.
In the past two decades, several studies have used polyester tubular knitted mesh,46 weft-knitted tubular fabric 213 and polyester-spandex fabric216 to reinforce small-diameter vascular grafts (≤ 6 mm). However, these studies only focused on radial tensile properties and compliance in vitro without any in vivo data.
Recently, Xie et al.211 published an in vivo study on reinforced vascular grafts using polyester as the reinforcement material. In their study, three different PU grafts were fabricated into five structural designs and chemical components. The grafts (ID, 6.0 mm) were implanted in a canine abdominal aorta model and compared in terms of material stability, healing characteristics, and device function for 1–6 months. The reinforcement material used ranged from external polyester mesh to internal polyester monofilament including a design with an impenetrable PU layer. Although their experiments showed unsatisfactory results due to thrombus formation in all grafts, however, a notable difference was observed in grafts with different structural designs. The graft with a filamentous, porous, interconnected structure and externally reinforced polyester mesh were superior to grafts with low porosity or impenetrable walls.
Understanding the Structure and Function of Native Blood Vessels
The ultimate design goal of vascular grafts is to mimic the native vessel both in structure and function. Hence, prior knowledge of the native vessel is paramount to construct an appropriate matching graft. Arteries, capillaries, and veins are the major components of the cardiovascular system responsible for the transport of waste, nutrients, oxygen, and biological factors to other parts of the body.76 A typical arterial wall is composed of various distinct layers which are divided into three main groups (adventitia, media, and intima). Understanding the structure and functions of these layers, that vary in thickness depending on their anatomical location, will be necessary when designing a suitable functional graft substitute. The intima, located on the luminal side of the vessel, consists of a monolayer of endothelial cells (ECs) anchored to a thin basal lamina composed of collagen type IV, fibronectin, and proteoglycans.165 The function of the endothelial monolayer is to maintain the vessel by providing a non-thrombogenic surface and to control molecular diffusion through the wall. The internal elastic lamina, composed of mostly elastin, separates the intima from the media.150 The media layer consists of circumferentially organized laminae of smooth muscle cells (SMCs) dominant with elastin and collagen sheets. Their variable thickness is proportional to wall shear stress (WSS) of the vessel location, with the abdominal aorta having the highest value.105 The external elastic lamina that demarcates the media from the outer layer of the artery is called the adventitia. The adventitia helps to attach the vessel to surrounding tissue and provides structural support by allowing the vessel to stretch and recoil. This layer is rich in collagen and is composed of mainly fibroblast cells.160,162
Apart from the various cells that make up blood vessels, the extracellular matrix (ECM), which is composed of macromolecules that support the cells and hold the vessel wall together, is an important component that determines the tissue’s physical property.4 Other functions performed by the ECM include providing structural support, bearing mechanical stresses, regulating cell proliferation and differentiation, modulating growth factors, and contributing to the flexibility of the vessel.47
Tissue Engineering
Vascular grafts can be categorized into four groups depending on their source: engineered grafts, xenografts, allografts, and autografts. The use of a patient’s tissue or tissues from someone of the same genome such as a monozygotic twin is usually referred to as autologous graft. The source of these grafts is mostly from the internal mammary artery (ITA) or saphenous vein (SV) due to their lower immune response.149 Arterial autografts are usually preferred owing to their infection resistance, little to no inflammation, and easy attachment to the native vessel wall.197 However, the non-viability of autografts in elderly patients coupled with long preparation and processing time ex vivo before surgery renders this approach less attractive.55 Both allografts and xenografts are tissues harvested from other patients or species, respectively. Several concerns over the use of allografts include infection, cost, availability, graft failure, and immune rejection.126 While a loss of mechanical and biological integrity has been observed in the processing of a sterile allograft, use of xenograft has faced multiple ethical objections related to the patient’s perspective regarding the humane treatment of animals.81
Another alternative to autologous grafts is bioengineered grafts. The preference of these grafts to their autologous counterparts is often because of their limitless availability, a broad range of material options, and the possibility of optimizing the mechanical property to match that of the biological replacement. Currently, there are two major commercially available synthetic grafts: PTFE (Teflon) and PET (Dacron). Both graft types have several drawbacks, as listed in the introductory section of this manuscript, necessitating active research for an alternative biomaterial with superior qualities.
Design Considerations in TEVG
Tissue engineering of small diameter vessels involves innovative design and fabrication of a material capable of replacing a particular diseased tissue in both property and function without eliciting adverse immune responses.
The purpose of engineered grafts is to provide support to the native vessel and promote rapid endothelialization and vessel remodeling.111 Hence some properties of a functional scaffolds should be appropriate architecture, biocompatibility, antithrombotic, bioactivity, and favorable mechanical properties.28 Apart from these properties, it also necessary that the material adjusts to the prevailing hemodynamic conditions both instantaneously and in long-term. Good porosity with appropriate architecture, not only enhances cell proliferation, communication, and integration but also sustains mechanical stability during remodeling.28 Another important property of biodegradable scaffolds is that degradation should balance with tissue regeneration that mimics the function of the ECM in normal tissue.165 Taking into account that different materials interact with cells in ways that might trigger various responses, surface bioactive materials (cell-stimulating signals) could alter cellular responses in ways that facilitate positive growth. Therefore, these qualities would allow for modulating cellular function and behavior and may also promote tissue growth.199
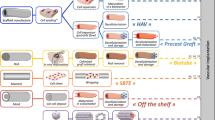
The important methodologies/considerations in designing a functional tissue-engineered vascular graft (TEVG) discussed in this review are illustrated in Fig. 1.
Material Selection and Properties
The most common commercially available non-degradable synthetic grafts for peripheral vascular reconstruction are e-PTFE (Teflon) and PET- widely known as Dacron. These biostable grafts are predominantly used clinically because of their excellent resistance to degradation, good mechanical strength, and affordability in larger diameter applications.
e-PTFE
PTFE is a fluorocarbon polymer that usually undergoes architectural transformation to form e-PTFE (Gore-Tex) via extrusion and sintering to introduce a microporous structure of circumferentially aligned nodes in their matrix. This material possesses electronegative luminal surface that offer its anti-thrombogenic property which are suitable for lower-limb bypass grafting (7–9 mm). Most surgeons prefer an impervious and impregnated knitted-e-PTFE graft to avoid the need for pre-clotting.157 The poor compliance of this graft to the native vessel due to it’s rigidity has led to non- endothelized surface, which results in poor patency after surgery.172 Recently, vigorous research efforts have been focused on surface modification and external reinforcement of this product to mitigate these challenges in small diameter grafts.
Dacron
PET is a polyester material with multiple filaments that usually comes knitted or woven into a vascular graft. These type of grafts are used as alternatives to e-PTFE; however, their poor patency in long-term in vivo small diameter vessels still limits their use clinically.184 Sauvage et al. reported a successful result of up to 16 months for an adult implanted with a knitted Dacron vascular graft (4 cm long and 3.5 mm ID) between the aorta and right coronary artery.175 This was followed by two other successful studies using Dacron as an aorto-coronary prosthesis in pediatric patients with coronary anomalies.34,73 In all these successful studies, the short grafts were used in high flow positions as interposition grafts between the ascending aorta and the proximal end of the coronary artery. However, several recent studies with knitted Dacron show dilation over time and required pre-clotting with gelatin, collagen, or albumin to seal the large pores and prevent sweating or plasma leakage after surgery.195 Another limitation of this prosthesis is the surface hydrophobicity, which prevents EC adhesion and proliferation,42 thus, leading to platelet activation and thrombosis.202 Additional intensive research is needed to increase the surface hydrophilicity, enhance EC attachment, and suppress thrombosis.173 According to a recent study, plasma surface treatment was employed to functionalize the Dacron surface with atmospheric gases, thus, making it more hydrophilic.42 The findings showed that cell interaction was enhanced without adverse inflammatory tissue reaction.161 Another alternative is surface immobilization of antithrombotic molecules such as heparin or bioactive proteins that enhance faster endothelialization.214 More recently, numerous researchers have used electrospinning to combine Dacron with hydrophilic polymers-like gelatin to improve its biocompatibility.117
PUs
This polymer was introduced as a vascular graft in the 1960s211 to combat challenges such as intima hyperplasia due to compliance mismatch observed in vascular conduits being used at that time. The preference of this graft over e-PTFE was due to better compliance and mechanical properties close to that of the native vessel.22 PUs are usually composed of soft amorphous and hard crystalline segments held together by hydrogen bonding and Van der Waal forces, resulting in it’s inherent stiffness and flexibility.174 In contrast to e-PTFE, the low thrombogenic surface of PU grafts can self-heal instantaneously after being punctured with a needle, thus, resulting in minimal plasma leakage after anastomosis.48 The compliance properties can be optimized to inhibit intima hyperplasia by controlling the concentration of hard and soft segments.91, 177 Despite their improved mechanical compliance, early in vivo trials failed because of poor biostability and autonomous degradation. Two categories of PUs- polyester, and polyether degrade via hydrolysis and oxidation respectively with release of toxic byproducts, as early as 8 weeks after implantation.69,176 Small diameter PU grafts (5 and 6 mm ID) meant for below-knee vessel replacement were withdrawn from the market after the first year as a result of multiple cases of thrombosis, where eight out of 15 grafts were occluded with little EC coverage.224 In the quest to improve the biostability of PU grafts, one particular study replaced the macrodiols with either siloxane or carbonates resulting in the generation of a new graft with a polycarbonate soft segment showing enhanced resistance to biodegradation, and thus, could remain at the site for a prolonged period.214 In a comparative in vivo study of 1.5 mm polycarbonate and e-PFTE, grafts implanted in a rat aorta were observed to be stable for up to 6 months. The findings showed that polycarbonate grafts outperformed e-PTFE in terms of faster endothelialization and suppression of intima hyperplasia; however, there was no obvious difference in patency (~ 80%).83 The author emphasized that small diameter high flow in the abdominal aorta of rat does not reflect the low flow condition for patency studies; hence, a small diameter artery (such as the carotid) in a large animal should be used instead.41 While some results from PU grafts appeared promising, an alternative approach to surface modification needs to be explored for applicability of this material.
Bioresorbable and Biodegradable Polymers
Due to complications such as late intima hyperplasia observed on biostable materials in vivo, researchers have recently begun concentrating their effort on developing biodegradable and bioresorbable materials in the form of a scaffold. These materials usually act as support and matrix for cell infiltration and deposition of the ECM, and they ultimately dissolve leaving behind a functional tissue thereby minimizing long-term consequences. An important difference between biodegradable and bioresorbable polymers is that bioresorbable polymers form fragments and are metabolized in the body while biodegradable polymers degrade and remain at the site of implantation.50 Electrospinning is one of the strategies employed on these polymers to fabricate tubular porous grafts composed of micro- and nanosize fibers suitable for cell infiltration, differentiation, and new capillary formation.154In vivo metabolism of these grafts, post-implantation reduces the possibility of adverse tissue interactions that usually cause graft failure. Surgeons are often skeptical about the implantation of biodegradable grafts because of the fear that rapid degradation may not counter-balance natural tissue regeneration, thus, leading to unintended consequences such as aneurysm.41 Therefore, recent research on biodegradable polymers, such as polydioxanone (PDO), poly(ɛ-caprolactone) (PCL), polyglycolic acid (PGA), polylactic acid (PLA), and polyglycerol-sebacate (PGS), has focused on tailoring the degradation profile to meet appropriate tissue regeneration. The degradation profile of a graft can also be optimized by designing composite polymers with different degradation rates. Another strategy is to combine degradable and non-degradable polymers to enhance mechanical strength.
PGS
This elastomeric material was first synthesized in 2002 and since then, its properties such as flexibility, biocompatibility, and biodegradability have attracted numerous researchers. Polycondensation of glycerol and sebacic acid monomers remains the main process to synthesize this material. The interesting elastomeric property of this material is a result of covalent crosslinking coupled with multiple hydrogen bonds among the hydroxyl groups on the backbone.201 This material can biodegrade and be reabsorbed in the body via normal pathways. In a previous study, PGS was confirmed to biodegrade roughly 60 days after subcutaneous implantation in rats leaving no scar tissue around the implant.201
Although both in vitro and in vivo studies on this material are still in the preliminary stage, the results so far are interesting. Vascular scaffolds constructed with PGS have macro and micropores ranging from 75 to 150 and 5 to 20 µm, respectively, encouraging good cellular activity such as the proliferation of ECs and infiltration of vascular SMCs (VSMCs) capable of enhancing vessel remodeling.64,164 In an In vitro comparative hemocompatibility testing of PGS, e-PTFE, and poly(lactide-co-glycolide) (PLGA) showed that decreased platelets and inflammatory potential were observed on PGS in comparison to e-PTFE and PLGA.132 Subcutaneous implantation of both PLGA and PGS in rats showed a similar inflammatory response. However, a thick fibrous capsule was formed on PLGA while a thinner collagenous layer was deposited on PGS, which were both highly vascularized.201 The PGS scaffold was found to enhance elastin expression, thus, increasing the mechanical property five-fold toward matching that of the native vessel within 3 weeks of implantation.63 Therefore, future design and fabrication of a composite graft that composed of PGS is expected to show very interesting results.
PGA and PLA
PGA and PLA polymers are bioresorbable and composed of saturated poly- -hydroxyester, which forms the amorphous composite of PLGA when combined. The extra methyl group on PLA makes it more hydrophobic than crystalline/hydrophilic PGA with a faster degradation profile.221 These polymers degrade via hydrolysis of the ester bond on their backbone resulting in the formation of glycolic and lactic acid monomers, which are metabolized normally. To avoid local pH decrease around the implantation site during graft degradation, it is recommended that these materials be applied to dynamic tissues such as vessels.12 A recent in vivo study of PGA, PLA, and PLGA nanofibers in the vascular application was challenging due to rapid resorption within 2 months.220 In another study, a composite graft made of PLA and PGA (vicryl*) showed loss of mechanical strength and aneurysm formation as a result of rapid polymer degradation that could not withstand the arterial pressure.
-hydroxyester, which forms the amorphous composite of PLGA when combined. The extra methyl group on PLA makes it more hydrophobic than crystalline/hydrophilic PGA with a faster degradation profile.221 These polymers degrade via hydrolysis of the ester bond on their backbone resulting in the formation of glycolic and lactic acid monomers, which are metabolized normally. To avoid local pH decrease around the implantation site during graft degradation, it is recommended that these materials be applied to dynamic tissues such as vessels.12 A recent in vivo study of PGA, PLA, and PLGA nanofibers in the vascular application was challenging due to rapid resorption within 2 months.220 In another study, a composite graft made of PLA and PGA (vicryl*) showed loss of mechanical strength and aneurysm formation as a result of rapid polymer degradation that could not withstand the arterial pressure.
PDO
PDO is a colorless, crystalline, biodegradable synthetic polymer. The chemical structure contains a multiple repeating ether ester unit. The glass transition temperature of PDO ranges from − 10 to 0 °C with a crystallinity of approximately 55%. This polymer can be easily extruded into a flexible fiber.
PDO has been previously applied in pins, suture clips, and plastic surgery material, as well as in drug delivery systems, cardiovascular devices, and tissue engineering, which slowly degrade and are resorbed by the body while the remainder is excreted in the urine and exhaled as CO2. However, the recent application of PDO in vascular graft design has shown good luminal endothelialization. Burst pressure testing on an explanted specimen of PDO could withstand up to 6000 mmHg without fatigue. Multiple studies have examined the use of PDO stents in both vascular and non-vascular organs such as the esophagus, trachea, and intestine.106,110,198
According to Boland et al., the most interesting unique property of PDO in contrast to other polyesters is the mechanical similarity to the native vascular ECM- specifically, collagen and elastin.20 This coupled with its shape memory behavior that provides rebound and kink resistance.20
PCL
PCL is a biocompatible and bioresorbable polymer that is applicable in many biomedical areas owing to its favorable mechanical properties and simple synthesis.208 It is a hydrophobic polymer with a semi-crystalline structure having a low melting point of ~ 60 °C.137 The degradation profile of PCL follows a two-step process which depends on the molecular weight and synthesis method. After implantation of PCL in a physiologic environment, the poly(α-OH) ester linkage undergoes non-enzymatic hydrolysis releasing 30,000 MW non-toxic by-products. Next, the surrounding cells, including macrophages and phagosomes, degrade the low MW fragments through intracellular processes.156,181 The degradation timeline of PCL material was observed to show changes in their mechanical property (stiffer) due to reduced amorphous ratio and enhanced crystallinity leading to a longer presence in the body (up to 2 years) in contrast with other biodegradable polymers.41 This, thus, provides enough time for cell infiltration and tissue regeneration.144 The approval of PCL and other PCL products by the FDA has led numerous researchers to focus on constructing viable vascular grafts with this material.208
In long-term studies, cast molded PCL vascular grafts have met numerous challenges such as plastic deformation due to cyclic stress in dynamic vascular tissues. Structural stability over a long period holds the key for successful vascular remodeling as infiltrating cells receive proliferative cues from surrounding cells.205 Hence, PCL grafts are often fabricated using the electrospinning method, which mimics the elastic ECM unlike the cast molding method.101
The morphology of electrospun PCL (porosity and fiber diameter) can be tuned by varying the spinning parameters such as polymer concentration, and solvent used. In vivo examination of 2 mm, PCL and e-PTFE grafts in a rat aorta showed successful endothelialization of PCL at 12 weeks with better patency than e-PTFE.154 Long-term observation up to 18 months showed a similar trend with stable endothelialization and no aneurysmal dilation or thrombosis for the PCL graft.41 Observation up to 12 months showed progressive cellular infiltration from the adventitia layer of the native artery, which was induced by macrophages that secrete angiogenic factors, thus, enabling a capillary formation and graft colonization by myofibroblasts. However, after 12 months, regressive tissue remodeling was observed as fewer macrophages caused a reduced influx of cells leading to myofibroblast starvation and graft calcification at 18 months.41 A similar observation was made in a long-term study on collagen-, PLA-, and PGA-based grafts at 6 months; thus, a new vascular graft design that make use of these materials requires further long-term investigation. To overcome the challenges observed in small-diameter vascular grafts (< 6 mm), we proposed a new design strategy by combining the last two interesting biodegradable polymers due to their property which resembles that of the ECM. We employed an electrospinning approach to incorporate a thin layer of P1 nanofiber at the luminal surface, whereas the abluminal surface was composed of co-electrospun PCL nanofibers doped with an antithrombotic drug and P1 nanofibers. In between stages 1 and 3 of the spinning process (Fig. 2), a 3D printed P2 thread was interposed as reinforcement to prevent vessel collapse or kinking during tissue remodeling.
Schematic illustration of our proposed reinforced small diameter graft fabrication process. Stage 1-from left involves electrospinning of the luminal surface with faster degradable polymer after which the construct is reinforced with a 3D printer. The final step (stage 3) involves the spinning of two polymer solutions doped with an antithrombotic drug (P 1-polymer 1; P 2- polymer 2).
The fabricated bilayered tubular graft utilizing electrospinning approach Showed that both layers are elastic and, hence, will improve the flexibility and compliance of the graft in the physiological environment. The graft has both appropriate stress and elongation at break to maintain the elasticity under pulsatile conditions. More so, the graft has a desirable tensile property that mimics the natural artery, while the stress and elongation values are comparable to that of native vasculature.
Natural Polymers
These polymers are natural proteins used as scaffolds for tissue regeneration since they possess a high degree of biocompatibility and degradability, thus, facilitating cellular growth. More importantly, their ability to interact and bind easily with cells and enhance cellular activity makes them preferable over synthetic polymers such as PET.186 Some of the natural proteins explored for vascular graft development so far, which are present in the ECM, are collagen and elastin, coupled with other with biocompatible compounds such as silk fibroin. One of the challenges using natural polymers in vascular graft fabrication is their inherent weak mechanical property, which can fail due to constant pulsatile stress on the vascular wall, leading to aneurysm and vessel rupture.14
Elastin
Elastin, a dominant elastic protein in blood vessels, seems to be an attractive choice for vascular graft fabrication. Roughly 50% dry weight of the internal and external elastic lamellae layer of arterial walls is made up of elastin.150 Elastin is synthesized by polymerization of the tropoelastin monomer via a multi-stage process.219 The two important properties of elastin that promote its recognition in TEVGs are its excellent elasticity and signaling capabilities. A graft matrix made of collagen and incorporated with elastin was found to have good elastic recoil, thus, withstanding the stress during pulsatile flow.25,186 Moreover, elastin was observed to be responsible for modulating VSMC migration and proliferation, thus, inhibiting intima hyperplasia in vivo.150
A previous study utilized a range of enzymatic crosslinkers to crosslink the collagen/elastin matrix to maintain good mechanical strength. This allows the design of a multi-layered vascular scaffold, with each layer having a unique microenvironment for cell infiltration.102,219 MacClure et al. employed this idea to fabricate a tri-layered vascular graft composed of an endothelial stimulating inner layer, a middle elastic layer, and a strong outer layer to prevent aneurysm dilation.125 Incorporating elastin in the vascular construct is promising, however its derivation from animal tissues could limit its production and consistency due to possible remnants of pathogens or toxic chemicals during the long processing period.219 To enhance the mechanical property of elastin, recent studies evaluated the co-electrospinning of tropoelastin with other synthetic biodegradable polymers such as PCL and PLGA.207 In contrast to collagen, the non-thrombogenic property of tropoelastin remains an added advantage especially when co-spun. In another study, an elastin blend with PDO graft showed enhanced burst pressure matching that of the native vessel.183 The interesting data above regarding elastin show that it can enhance both the mechanical and biological properties of tissue-engineered small diameter grafts.
Collagen
Collagen is produced by SMCs and fibroblast cells in the media and adventitia of native blood vessels which is also a major component of the vascular ECM.21 Collagen acts as a stabilizer to these cells by maintaining their structural integrity and limiting their elongation.19 Boccafoschi et al. designed a vascular scaffold with collagen, which showed a good proliferation of endothelial and SMCs.19 However, due to the inherent thrombogenicity of collagen, that can trigger platelet adhesion and activation, the use in blood-contacting applications presents a serious challenge.207 One strategy to avoid this obstacle is to pre-seed ECs on the luminal surface via a bioreactor to mask the effect of platelet adhesion.192 In a previous study, acid-soluble type 1 collagen film was tested for thrombogenicity in comparison to e-PTFE, which showed surprisingly higher clot resistance due to the absence of platelet-activating collagen fibrils.19
Collagen can be obtained via extraction from animal tissues or through recombinant processes. This material is readily soluble and can form a gel capable of cell encapsulation similar to the 3D microenvironment observed in the native vascular wall.186 However, it is very difficult to construct vascular grafts with gels alone due to their weak mechanical strength. Weinberg and Bell, in 1986, were the first to construct tissue-engineered vessels via in vitro culturing of bovine ECs, SMCs, and fibroblasts, mimicking the intima, media, and adventitia, respectively, onto a collagen type 1 gel.204 However, the weak mechanical strength of these gels presents a very low burst pressure similar to the results observed by other researchers when reinforced with synthetic non-degradable PET mesh.186
Kakisis et al. suggested that low density and poor orientation of the SMCs seeded on collagen gels might be linked to the weak mechanical properties.87 SMCs seeded on collagen gel typically have longitudinal orientation rather than circumferential orientation confirming the poor mechanical strength of collagen-based vessels.87,212 To resolve this challenge, numerous researchers used bioreactors to apply pulsatile flow to seeded cells, hence, creating an environment similar to that occurring physiologically.89,174 Cells seeded in this manner were circumferentially oriented with improved mechanical strength. Other studies used cyclic strain and platelet growth factors to not only enhance the mechanical strength of tubular collagen constructs but also increase the SMC compaction.178,186
Collagen type 1 nanofibers mat fabricated into 2 mm diameter tubular grafts and then crosslinked with glutaraldehyde were stable up to 12 weeks without aneurysmal dilation after implantation in the rat inferior vena cava.210 Collagen alone can also be electrospun for tissue engineering of blood vessels; however, co-electrospinning with other biodegradable materials such as elastin and PCL improved the mechanical strength and bioactivity in a study.21,214 These findings suggest that collagen combined with other synthetic materials could offer a better engineered vascular substitute in the future; however, collagen was found to degrade to gelatin after electrospinning, thus, eliminating its unique structural properties.222
Silk
Silk fibers extracted from cocoons of the Bombyx mori silkworm have been used in various biomedical processes including suture threads.62 The two active components of silk are fibroin and sericin that provide elasticity and glue-like properties, respectively.53 This material is biocompatible, non-thrombogenic, and degrades slowly in vivo, hence, standing out as a viable alternative for constructing small diameter vascular conduits.6,53,116 In a previous study, electrospun silk fibroin was found to possess sufficient burst strength capable of withstanding physiological pressure and good mechanical strength that resisted aneurysm dilation and rupture.120 The compliance of silk fibroin is far greater in comparison to synthetic grafts such as e-PTFE and PET,215 though this value is still below that of the SV.120
Vascular grafts constructed with silk fibroin showed enhanced bioactivity observed by migration and proliferation of both SMCs and ECs in vivo.116 A silk fibroin (1.5 mm) graft implanted in a rat abdominal aorta showed higher patency (85%) for up to 1 year than that observed for e-PTFE (30%).53 The construct showed formation of a confluent endothelium which is free from thrombus at 12 weeks and an enhanced deposition of ECM proteins as the fibroin degraded, coupled with increased the mechanical properties that inhibited aneurysmal dilation. Furthermore, a long-term large animal study on a low flow artery such as the carotid is needed to confirm the clinical relevance of silk fibroin vascular constructs.
Hyaluronan
Hyaluronic acid (HA) is a non-sulfated glycosaminoglycan (GAG) that was first isolated from the vitreous humor in 1934 by Meyer and Palmer,190 which is composed of glucuronic acid and N-acetylglucosamine. This material is transparent and can be synthesized in large quantities via microbial fermentation. HA is a rapidly biodegradable, hydrophilic, non-adhesive, and biocompatible natural polymer.148 It can modulate cell differentiation, migration, and angiogenesis without eliciting any foreign-body response, making it an intriguing material in the field of tissue engineering. In a previous study, HA was found to promote adhesion and proliferation of ECs via CD44, ICAM-1, and RHAMM cell receptors to enhance tissue growth and repair.54,182 The mechanical property could be tuned by the degree of modification and crosslinking.189
Chenhui et al. combined HA and human-like collagen to produce a vascular scaffold with enhanced biophysical and mechanical properties that were close to those of the natural ECM.227
Fibrin
Fibrin is an insoluble protein that often participates in tissue repair and wound healing process. It is formed as a result of fibrinogen polymerization activated by thrombin, thus resulting in a fibrillar network gel.44 Fibrin scaffolds can aid the adhesion, proliferation, and migration of cells involved in tissue remodeling.35,148 During post tissue remodeling, the gel network is broken down through fibrinolytic processing of the fibrils, which can be finally be resorbed via fibrinolysis.35,148 One of the challenges working with fibrin gel is due to it’s poor mechanical properties similar to that of the collagen scaffolds used in vascular tissue engineering. Tubular fibrin gel seeded with cells over 24 h and implanted in the external jugular vein of an ovine model was monitored for 12 weeks.188 The graft showed substantial vascular remodeling with significant deposition of ECM and enhanced mechanical properties. There was minimal aneurysmal dilation which did not affect the patency of the vessel until the time of explantation.
In another study, fibrin-based vascular grafts entrapped with SMCs were implanted as vein interposition grafts in a lamb and was observed for up to 15 weeks.188 The grafts were patent (25%) with ECM deposition that enhanced the mechanical properties comparative to the native aorta. Instead of SMCs, a varied concentration of fibrinogen grafts were seeded with bone marrow-derived SMC progenitor cells, which produced vessels with better mechanical properties; however, implantation in artrial vessels is still under investigation.112,217 Another technique to improve the strength of fibrin-based tissue-engineered vessels is hypoxia and insulin supplementation which enhances collagen deposition of the entrapped neonatal human dermal fibroblasts.18 However, the drawback of this approach was the inability to produce a vessel with appropriate clinical length due to the loss of their length during culture in a bioreactor.
Hybrid Scaffolds
A combination of natural and synthetic material in graft fabrication could offer improved graft properties that are superior to the properties of either material alone. Within the last decade, several in vivo experiments have been performed on TEVGs to demonstrate the feasibility of a hybrid graft design approach. Recently, many researchers focused their efforts on this approach because of the positive results it offers. To mimic the structurally, morphologically, and mechanically property of a blood vessel, Haghjooy Javanmard et al. fabricated a three-layer vascular graft that sandwiched collagen in PCL and poly-l-lactic acid (PLLA).71 In another study, PCL was combined with gelatin,84 collagen,15 and elastin207 to enhance the cell adhesion and mechanical property of the graft. A hybrid graft composed of PCL blended with silk and chitosan maintained patency for up to 8 weeks in a rat model.226 After electrospun HA was first developed, Ekaputra et al. fabricated a hybrid mesh formed by electrospinning HA with PCL and collagen; which supported capillary networks from co-culture of EC and lung fibroblast.51
In an ovine model, a fibrin scaffold reinforced by poly(l/d) lactide (P(l/d)LA) mesh and seeded with autologous arterial-derived cells was observed up to 6 months as an interposed carotid artery graft.99 At 6 months, there was no evidence of calcification, thrombus, or aneurysm dilation on the luminal surface of the explant. Young Min Ju et al. used a PCL/collagen scaffold seeded with autologous ECs and VSMCs in their study on ovine carotid interposition for 6 months. Serial computed tomography (CT) and ultrasound (US) images showed no observable stenosis and the vessel showed stable structural integrity. Although this hybrid graft approach presents some interesting results, long-term conditioning with outstanding processing skills are required, which could be a serious challenge for large scale manufacturing.86
Decellularized Natural Matrices
In the quest to obtain a TEVG that mimics the native vasculature in both structural and biomechanical performance while avoiding adverse immunologic responses, a decellularized scaffold was developed.206 Decellularization is the process (Fig. 3) of stripping off antigenic cellular material from tissue with the aid of detergents and enzymes, leaving behind a well-organized acellular matrix as a scaffold for autologous cell infiltration and tissue remodeling.30
Tissue engineering manufacturing process. Briefly, the tissue is explanted from the source (human or animal) and decellularized using detergent and chemicals under agitation. 2D tissue can be explanted from amniotic membranes or small intestine submucosa while vascular tissue is obtained from blood vessels. The decellularized 2D tissue, which is composed of extracellular matrix (ECM) only, can be rolled into vascular constructs and seeded with a patient’s explanted cells.
Rosenberg et al. were the first to develop a decellularized vascular prosthesis using bovine carotid in the 1960s.171 From then until now, numerous clinical products based on decellularized tissue from both autografts, allografts, and xenografts have become available for a wide range of applications such as cardiac, ophthalmic, dentistry, dermal and soft tissues.36 The commercial names for these grafts are ArtegraftTM, SolcograftTM, and ProColTM, which were derived from bovine blood vessels. The SynerGraftTM model 100 was derived from decellularized bovine ureter.29,100 Lack of large-scale adoption of these grafts might be due to the results of several prospective randomized trials, which concluded that there is no clear advantage of decellularized xenografts in comparison to alternative synthetic conduits.45,107 Apart from the high processing cost of decellularized grafts, both grafts (synthetic and xenografts) have a similar patency rate and also the chances of graft recovery after infection or pseudoaneurysm was lower.
Products based on decellularized human donor vein such as those used for arteriovenous fistulas (AVFs) (SynerGraftTM, derived from human cadaver vein allograft) are commercially available but not yet widely adopted in the clinical field. In a previous study, these grafts appeared to be more resistant to infection though with aneurysmal dilation in contrast to synthetic conduits, however no improvement in patency was observed.118 The limited supply and complex ethical/regulatory issues associated with the commercialization of allogenic decellularized matrix remain another challenge.
Although decellularized scaffolds present interesting results147 as alternative TEVGs, challenges such as inadequate decellularization have led to adverse immune reactions and implant failure, while hash decellularization has led to the loss of mechanical properties caused by degenerative structural graft failure. Other important obstacles in this approach that could limit quality control are variations in geometry, mechanical properties, and chemistry due to age and health of the donor. Hence, no viable prediction can be made on the prospect of this approach.
Complete Biologic TEVGs
This approach utilizes various cells to fabricate a vascular construct with the aim of avoiding problems observed with synthetic materials such as inflammation, stenosis, and infection.148,153 Currently, there are three main developed approaches: the cell-sheet based, in vivo bioreactor, and cell-ring based methods.
The cell-sheet approach involves expanding the cell population in the presence of ascorbic acid as a medium.153 The cells are monitored to a certain period (maturation time) after which the cell-sheets are carefully removed and placed onto a round tubular construct,153 followed by 8 weeks of dynamic culture to unify the layers into a vascular structure (Ref. Fig. 4). Researchers have utilized different cell sources to construct cell-sheet tissue TEVGs with burst pressures approaching roughly ~ 2600 mmHg, which is well above that of the SV. In a previous study, grafts obtain via this approach could withstand physiological pressure after implantation in a canine model as a femoral arterial interposition.104 However, the long period of in vitro processing (between 6 and 9 months) required to generate TEVGs still limits this approach.124
The bioreactor approach was another innovative approach developed to generate TEVGs. Here, the body acts as a bioreactor by initiating an inflammatory response after implantation of foreign material at the peritoneal cavity, thus triggering the formation of a fibrous capsule mostly composed of myofibroblasts and collagen matrix layers encapsulated by a layer of mesothelial cells77 (Ref. Fig. 4). In a previous study, implantation of silastic tubing in a rat’s peritoneal cavity led to encapsulation by myofibroblasts, a formation of a collagen matrix, and a single layer mesothelium after 2 weeks.148 After the tubing was explanted, the vessel-like structure was turned inside out: the mesothelial outside layer became the intima; the collagen and elastin layers became the media; the outside collagen layer became the adventitia. Subsequently, the graft was implanted in the carotid position of the same rat which remained patent up to 4 months with properties similar to that of the elastic lamella. Among the implant tubing evaluated so far, polyethylene tubing was found to produce an outstanding graftable vessel in 50% of cases. Though this model seems to be a viable option to develop TEVGs within a short period without fear of antigenicity, the dual invasive surgeries coupled with the possibility of tissue adhesion to the peritoneal wall during maturation may limit this approach.39,153
The cell-ring based method was developed to overcome the challenges of possible damage to the cell sheet through peeling or manipulation during the post culturing process by utilizing a mold composed of agarose wells. In this approach, high-density cells are suspended in the agarose matrix for ECM secretion, thus serving as building blocks for vascular conduit formation121 (Ref. Fig. 4). Previously, human arteries derived from fibroblasts and ECs were deposited and conditioned up to 14 days under pulsatile flow that included mechanical stimulation in a 3 mm ID mold. This generated a vessel-like tissue composed of ECM components.94 Similarly, this approach has been tried in a small animal model (rat) after immortalized rat aortic SMCs were cultured in agarose well (2–6 mm in ID) for up to 2 weeks to generate fused tissue rings.70 This ring was further developed into a fused vascular construct when placed in a silicon tube mandrel and cultured for 7 days. One limitation of this approach was the limited shape of the fabricated graft which led to the development of bioprinting. With this new approach, patient-specific vasculature could be developed. Due to the flexibility, several studies have employed this approach to develop in vitro micro vascularized constructs.37 Norotte et al. developed simple and branched vasculatures via precise deposition and merging of multicellular spheroids and cylinders143 (Ref. Fig. 4). The construct demonstrated a burst pressure of around 773 mmHg after 21 days in a bioreactor. The ability to engineer vessels of distinct shapes and hierarchical trees via this approach seems compelling; however, the processing time, which usually takes up to 7 days, coupled with sterility issues could be obstacles in generating stable structures.
Fabrication Method of TEVG
Nowadays, electrospinning is among the versatile approach used by tissue engineers to fabricate degradable scaffolds since it is compatible with many polymers and is customizable enough to fabricate vascular conduits with optimized properties.
The process transform polymers dissolved in a volatile solvent into nano and microfibers on application electric field. However, to create a tubular structure such as blood vessels, a fast-rotating mandrel with a diameter matching the targeted vessel is often used. By controlling various parameters such voltage, flow rate, polymer concentration, nozzle size, distance, rotation speed and solvent, the thickness, density and physical properties of the resultant scaffold can be changed.191 Montini-Ballarin et al. demonstrated the use of this approach to generate a vascular construct that has properties akin to a physiologic vessel.131
Freeze-drying or lyophilization209 is another method that uses principles of sublimation to remove solvent present in a polymer solution. In this method, the polymer solution is poured in a mold and transferred into a freeze-dryer where the solvent is allowed to sublime. The pore size of the construct can be optimized by varying the freezing rate and solute concentration.
Porogen or particulates leaching is another method, where porogen (sugar, salt, wax) or particulates are used to generate pores before being leached via evaporation.203
Some other approaches used to generate vascular construct includes—solvent casting, foaming, phase separation, fiber bonding, self-assembly, rapid prototyping, melt molding, and membrane lamination. Bioreactors are utilized in tissue engineering to translate cells and tissue-based constructs into large-scale biological products that are clinically effective and safe. A study employed bioreactor to seed human induced pluripotent stem cells onto a PGA scaffold up to 9 weeks, which produced vessels composed of cells largely positive for alpha-smooth muscle actin.68
Most of the fabrication approach employed in TEBV to date were illustrated in Fig. 5 below.
From the above descriptions of tissue-engineered vascular graft strategies irrespective of their cell source, they can simply be divided into two categories: scaffold guided (synthetic, natural or decellularized matrix) and self-assembly (complete biologic). Hence, the selection of a vascular graft with optimum property should be a priority and a summary can be seen in Table 1 below.
Mechanical Properties of TEVG
The mechanical behavior of TEVG is an integral part of graft design consideration due to the load-bearing nature of blood vessels. This means that grafts should have sufficient mechanical strength to withstand pressurized blood flow and resist deformation. TEVGs require sufficient mechanical strength to retain vessel integrity and resist permanent deformation under physiological conditions. Thus, designing a TEVG that mimics the mechanical properties of native vessels could be a challenging task owing to their complex viscoelastic property. Tensile strength, burst pressure, suture retention, and compliance are the most important factors to consider for mechanical strength when choosing material for TEVGs. However, among researchers developing TEVGs, there is currently no agreement over the target values for these particular graft properties- whether to focus on mimicking the current gold standard graft property of the SV or ITA. Developing TEVGs matching the mechanical properties of the SV may be preferable for easy clinical adoption; however, the patency may still be limited because they are not designed to handle high-pressure vessels. Samand Pashneh-Tala et al. presented data on the mechanical properties of some reported TEVGs and compared them to that of both the ITA and SV.149 According to the study, no TEVG has matched either the ITA or SV in terms of compliance, burst pressure, or suture retention strength; however, a few of them were approaching these targets. The author concluded that the best option to fabricate future TEVGs is to tailor them to patient-specific implantation sites.
Tensile Strength
Tensile strength is the ability of graft material to resist a static or applied force while pulling at both secured ends. Since graft material encounters forces like longitudinal and axial stresses during the systolic and diastolic cycles, longitudinal tensile strength is a crucial measure of a graft’s resistance to internal stresses after implantation. Blood vessels are well known to behave like viscoelastic material as observed by their j-shaped stress-strain response. It has been observed that low strain produces only small changes in strain induced by the compliant and elastic response of the elastin fibers. At maximum systolic pressure, crimped collagen fibers are open and engaged in a tensile manner thus leading to increased stress.43 Several studies have utilized natural soft tissue such as small intestine submucosa (SIS) to engineered grafts, which showed mechanical responses similar to that of the native ovine carotid artery.155 In another study, layered PCL and PGA composite sheets seeded with bovine fibroblasts, SMCs, and ECs for up to 2 weeks showed a similar stress-strain response to that of bovine arteries.135 The unpredictable transition of mechanical behavior of synthetic TEVGs over time as the scaffold degrades requires a long-term in vivo study with appropriate assays at each time point to observe the graft mechanics regarding degradation.
Compliance Mismatch
As we mentioned earlier, the blood vessel is a complex organ with a viscoelastic property; the motion of the arterial wall is pressure-dependent due to elasticity and time-dependent owing to viscosity. Hence, to fabricate an ideal graft that would replicate these complexities is usually a challenging task. Therefore, compliance is a term used to simplify the clinical approach to a very complex physiological phenomenon. This property is essentially expressed as a dimensional change concerning luminal pressure change as represented below; where D is the internal diameter in diastole, ∆D is the ID change and Pp is the pulse pressure.
Compliance mismatch between the host artery and the graft was suggested as part of physical forces associated with vascular graft intima hyperplasia (IH) as a result of differential mechanical strain and other hemodynamic WSS parameters.167
Dacron and e-PTFE are the most widely used synthetic graft materials, which happen to be comparatively rigid in comparison to native tissue, thus resulting in a compliance mismatch at the anastomosis site.67 This difference in the mechanical property of materials at the anastomotic site could alter the blood flow profile and trigger a flow separation zone.5 Figure 6 illustrates the possible three different hemodynamic flow patterns observed at the distal end after anastomosis of the graft to native vessels because of variation in graft diameter or change in artery diameter as a result of pulsatile blood flow. According to previous studies, this convergent and divergent flow pattern was found to cause intimal thickening at the distal end.1,17,123 This indicates that divergent flow geometry reduces mean WSS and could trigger flow separation while convergent flow geometry increases shear stress and may result in EC damage and platelet activation.96,98
Effect of compliance (graft diameter) on hemodynamic flow at the distal anastomosis. From the left: convergent flow as a result of large diameter graft (mismatch) to small vessel anastomosis; divergent flow observed because of small diameter graft to large vessel anastomosis; laminar compliance flow owing to highly compliance graft and vessel anastomosis.
Since arteries can adjust their diameter and maintain a relatively uniform WSS, but a rigid synthetic graft cannot. Hence, optimal sizing of grafting is needed to maintain uniform WSS. In Fig. 6, a graft whose diameter is larger than that of the native vessel can create a state of low-velocity blood flow and shear stress that promotes failure. Conversely, when a graft is smaller than the native vessel, especially when it is smaller in diameter, the deposition of a fibrin layer over the synthetic surface post-implantation may trigger thrombotic obstruction.
As a result of these intrinsic differences between most synthetic grafts and native vessels in terms of moduli, coupled with anastomotic complications, complete compliance matching in a clinical setting is almost impossible.9,193,194 Hence, it has been confirmed experimentally that non-compliant (rigid) graft failures were a result of their lack of distensibility in contrast to the native vessels.1 Compliance mismatch can be categorized into two aspects: tubular and anastomotic compliance mismatch. Whereas tubular compliance mismatch results from lack of seamless mechanotransduction (change in impedance) from the native vessel through the synthetic graft, anastomotic compliance originates from the anastomosis which leads to a decrease in diameter and a drastic reduction in compliance.187 Additionally, the stiffness and suture technique used-continuous and interrupted may contribute immensely to compliance mismatch.
Burst Pressure
The burst pressure test was designed to measure the capability of a graft to withstand blood pressure similar to that found under physiological vascular conditions, including both systolic and diastolic level. Here, we can obtain the maximum pressure limits the graft can withstand before diameter expansion and ultimately graft failure. The ability of a graft to withstand its initial dimension after implantation in an in vivo microenvironment is termed burst strength.136 In small diameter grafts, it is required that a single pulse pressure between 80 and 120 mmHg should be maintained. In a previous study, synthetic engineered grafts with a burst pressure up to 1200 ± 500 mmHg, similar to that of the standard autologous SV have been obtained.115
One way to perform this test is usually with the aid of a balloon catheter whereby the balloon portion is inserted into the graft and pumped with air thus expanding it while an attached pressure gauge monitors the rise in pressure until graft failure.
Surface Modification of Vascular Grafts
Considering the repeated failures of commercially available grafts (e.g. e-PTFE and PET), especially at small diameters, researchers in this field have focused great effort toward surface modifications to optimize their antithrombotic property. However, understanding how the tissue reacts to biomaterial implants is essential for designing new cardiovascular devices or surface modification strategies.129 There are two methods usually employed to modify the surface of blood-contacting implants: (1) a physicochemical approach which is mostly applied for a permanent vascular replacement that has a non-adhesive, inert, non-biofouling surface involving electrochemical polishing, surface roughening, ordered patterning, plasma treatment,185 etching and passive or covalent surface coating; (2) a surface modification to enhance (or activate) a biological activity which will eventually speed up tissue remodeling. This approach is more attractive to recent researchers whereby they employ biological tools to design a biomimetic surface via incorporation of bioactive or inactive molecules that modulate cellular responses.88,90,141 These approaches aim to enhance patency and thereby inhibit thrombogenic events at the blood-implant interphase. It is widely known in vascular tissue engineering that graft endothelialization remains the ultimate solution to address thrombogenicity and other graft associated complications.
Physicochemical or Hydrophilic Modification
Surface modifications have been widely adopted in the field of biomedical engineering because of the significant role they play in changing the surface behavior of a material to the desired property without altering its bulk property. They are two classes of physicochemical surface modifications: physical and chemical. Physical surface modifications range from flame treatment, corona discharge treatment, plasma treatment, etching, ultraviolet (UV) radiation exposure, laser ablation treatment, gamma irradiation to X-ray treatments. In a chemical surface modification, chemical agents and functional groups such as surface oxidation, hydrolysis, chemical grafting, and surface coatings are usually employed to change the surface behavior of the biomaterial. Most of these coating approaches have been reviewed in depth by Alves et al.56 and Meyers et al.129 Among the important physical properties that modulate cell behavior, such as protein adsorption, platelet adhesion, and thrombogenicity, the addition of pores, grooves and pits on their surface could also be alternative modifications.113 Slight changes at the micron and nanometer level could amount to drastic alterations in surface properties as both physical modifications and chemical coating work synergistically. In a recent study, it was observed that plasma proteins such as fibrinogen tend to adhere to rough surfaces and bind to platelet receptors more tightly than on a smooth surface.218 As porosity plays a crucial role in vascular graft topography, a comparative study of EC growth on 5–90 µm porous surfaces shows that ECs proliferate to a greater extent on smaller pores (5–20 µm). Hence, nanocomposite material has shown a favorable outcome in most cardiovascular implants as they influence desirable protein, cellular and tissue interaction at the blood-biomaterial interface thus, enhancing their patency and reducing thrombogenicity.145 Most amphiphilic nanocomposite polymer coatings are hemodynamically favorable and thermodynamically stable in contrast to conventional materials when used as vascular grafts.92 The favorable viscoelastic and antithrombotic properties of polyhedral-oligomeric-silsesquioxane-poly(carbonate-urea) urethane (POSS-PCU) coating observed in various cardiovascular implants such as stents, heart valves, and bypass grafts was due to its comparable properties with that of the native artery.65
Cardiovascular implants passivated with non-biofouling and non-adhesive material such as polyethylene glycol (PEG), dextran hydrogels and polyethylene oxide (PEO) were observed to prevent protein adsorption and platelet adhesion in several studies.8,40 Karrer et al.93 coated e-PTFE with polypropylene sulfide PEG (PPS-PEG) and evaluated its performance in a model as an extracorporeal arteriovenous shunt. Here, the study was grouped into three categories: heparinized and non-heparinized, cell adhesion testing, and thrombus deposition. The outcome of the study showed that there was no difference in terms of cell adhesion in comparison with that of the control samples; however, the heparin-coated samples showed marked suppression of thrombus formation. Hydrophilic polymers such as dextran, PEG, and PEO can be chemically modified on their backbone with cell-selective peptides to enhance specific cell adhesion. Ryan et al. employed the spin shearing method to attach poly(1,8-octanediol-co-citrate) (POC) on an e-PTFE graft after which heparin was immobilized and the experiment was monitored in vitro.78 The results showed that after 1 month, the POC-heparin coated e-PTFE grafts maintained bioactivity with higher hemocompatibility than that by bare e-PTFE grafts.
Antithrombotic Surface
The primary function of the endothelium is cardio-protection which involves regulating the coagulation of plasma proteins via the release of vasoactive factors such as nitric oxide (NO) and prostacyclin (PGI2), plasminogen activators, thrombomodulin, and heparin sulfate.49 These molecules control coagulation and promote fibrinolysis. Deep in the vessel wall, thrombotic elements such as collagen, von Willebrand factor (vWF) and tissue factor (TF) induce the blood coagulation cascade.139 Hence, there is great interest among vascular tissue engineers to mimic the function of ECs via surface passivation on blood-contacting implants. Various strategies have been adopted such as the incorporation of antithrombotic drugs including heparin, warfarin, hirudin, dipyridamole, clopidogrel, aspirin, cilostazol, and glycoprotein IIb/IIIa inhibitor (abciximab, eptifibatide, tirofiban) in a synthetic graft matrix with the aim of slow release, thus inhibiting platelet adhesion.7,95,163
Another strategy that is of interest among vascular tissue engineers is the modification of graft material to produce NO, thus suppressing IH.3 To read more about NO release system, please refer to other review articles.60,130
Surface Modification of Vascular Implants to Enhance Endothelialization
Commercially available vascular implants such as e-PTFE and PET fail because of their inability to induce faster in situ graft endothelialization. Therefore, surface modification strategies have been employed to enhance the endothelialization of these implants to maintain their normal vessel function.180
Peptide Coating
Another strategy employed to enhance EC attachment to vascular constructs involves the immobilization of specific peptide sequences responsible for specific binding with ECs. To date, RGD remains the most common integrin-binding site which is composed of an arginine, aspartic acid and glycine sequence found on many proteins such as fibronectin (RGDS), vWF (RGDS), vitronectin (RGDV), collagen I (RGDN), collagen IV (RGDX, where X is a variant), thrombospondin (RGDA) and laminin (RGDN). Another integrin has been identified by several researchers which includes REDV and LDV on fibronectin133 and YIGSR on laminin.66 Trudel et al. covalently bonded RGD and YIGSR peptides on Dacron and e-PTFE and observed the behavior of ECs seeded on them. The results showed that the coated sample promoted significantly higher EC attachment and spreading than the un-coated samples.57 In another study, e-PTFE coated with a cell adhesion-promoting RGD-containing synthetic peptide and fibronectin showed enhanced EC attachment even under shear stress in contrast to a fibronectin-coated surface.200
Antibody Coating
An antibody as a marker coated on artificial grafts is another alternative to induce self-endothelialization. The main objective of this approach is to promote attachment and proliferation of ECs and EPCs on the luminal portion of the graft after implantation. Monoclonal antibody adsorbed against EC membrane proteins, fibronectin, or vWF improved cell adhesion and proliferation. Bhatia et al. created an avidin-biotin system that showed enhanced EC attachment and spreading.16
Plasma Surface Treatment
Plasma surface treatment is another approach to modify a biomaterial surface to enhance its surface properties such as its ability to recruit, adhere, and retain functional ECs. Here, samples are placed inside a high vacuum chamber, and gas is introduced, which is ionized by microwaves or radio waves, to create a plasma that reacts with samples. This creates surface functional groups that are dependent on the gas and material used. Some of the groups generated through this process are hydroxyl (–OH), carboxyl (–COOH), carbonyl (–CO), ketone (=O), carbonate (–CO32−), and amine (–NH2). The benefits of plasma surface treatment are numerous, ranging from secondary immobilization procedures to increased biocompatibility or increased cell attachment and material sterilization. One of the challenges observed in this process is material degradation due to the generation of free radicals; however, because of the rapid treatment process (within seconds), damages are mostly observed in extremely susceptible materials. Pu et al. successfully modified the surface of the clinically available vascular implant-Dacron and e-PTFE with ammonia plasma treatment and observed the behavior of ECs seeded on them.161 The results showed that the plasma-treated surface-enhanced EC attachment in comparison to untreated samples. Furthermore, in another study, plasma treatment was employed to functionalize the e-PTFE surface before secondary biomolecule immobilization, which showed better EC adhesion and proliferation.10
Protein Coating
Biomaterial protein-coated surfaces offer an alternative strategy to enhance additional binding sites for cell adhesion receptors, which are widely used to improve the attachment and spreading of ECs on a vascular construct. Some of the proteins currently used to enhance EC attachment include laminin, poly-l-lysine, gelatin, and fibrin (Table 2). The combination of these proteins which form the basement membrane tends to enhance EC attachment in contrast to single protein coating. Among these proteins, collagen and fibronectin are used often given that they are also a component of the ECM. A conformational change is often observed when proteins are adsorbed on a biomaterial surface.142 A hydrophobic core and the hydrophilic surface of a protein aid in its solubilization in aqueous media. When a protein is exposed to a hydrophobic surface, a conformational change occurs, resulting in exposure of the hydrophobic core and strong adsorption onto the material. However, in hydrophilic materials, no conformational changes occur, but they bind weakly with the hydrophobic groups on the protein surface.42,196 The conformational change on protein adsorption could influence the exposure of their binding sites, thus, altering their activity.
An in vivo carotid sheep interposition model of 4 mm e-PTFE and PU graft coated with fibronectin and subsequently seeded with ECs in a bioreactor showed ECs coverage of 80–90% after 3 weeks with no thrombus formation.159 In another study, a 6 mm Dacron graft coated with gelatin and seeded with autologous ECs were implanted into the vena cava of a canine model which similarly showed no thrombus formation.179
Human trials of endothelialized vascular implants according to the procedures stated above have been investigated. A randomized clinical study was conducted up to 7 years using fibronectin-coated e-PTFE grafts, which was in-vitro lined with autologous ECs, showed higher performance (75.8%) compared to control (0%) and grafts lined with ECs only (66%).128 The same procedures have been replicated by other investigators, and the results confirmed that endothelialized grafts with autologous cells improved the patency and enhanced healing of vascular conduits.127
VEGF is another important signaling protein that is involved in both vasculogenesis and angiogenesis processes by mediating the migration and mitosis of ECs including methane mono-oxygenase and αyβ3 activities.140 The important biological function of VEGF is due to its specific membrane receptor and vascular endothelial growth factor receptor (VEGFR), which is categorized into three main receptors, VEGFR1 (Flt1), VEGFR2 (KDR), and VEGFR3 (Flt4). Among these VEGFRs and VEGF2 were found to express mesodermal cells responsible for the enhanced proliferation of EPCs.58,168
Several investigators have utilized nanoparticles and microgels for the controlled release of encapsulated VEGF and VEGF genes to treat diseases such as coronary artery disease.13,59,97,134 In another study, gelatin microparticles (GMP) loaded with VEGF were investigated for prolonged-release activity in an in vivo study, which led to a significant increase in vessel formation as observed in the histology results.158
A core-shell electrospun fibrous membrane with double-layer structure has been developed for dual-delivery of VEGF and platelet-derived growth factor-bb (PDGF) aimed at modulating proliferation of ECs and VSMCs. The function of the PDGF was to stimulate SMC proliferation, which plays a vital role in vascular remodeling.223 Yuan et al. utilized poly(ethylene glycol)-b-poly(L-lactide-co-ε-caprolactone) (PELCL), poly(L-lactide-co-glycolide) (PLGA), poly(ε-caprolactone) (PCL) and gelatin to developed a multilayer vascular graft (1.5 mm in diameter) loaded with VEGF and PDGF, which showed good mechanical properties by employing electrospinning method.74
Hence, to reduce cases of implant rejection, we propose a new surface modification of the TEVG with VEGF via the polydopamine coating method. The process involved incubation of fabricated vascular samples in a VEGF solution according to the protocol after polydopamine coating. Figure 7 illustrates the immobilization process.
Manufacturing Cost as an Evaluation for Graft Selection
In the surgical field, it is common to see widespread adoption of new devices or more expensive procedures, before their proven superiority over older devices or practices.
However, when these new devices are interesting to surgical personnel, a conflict may arise if the scarcity of resources limits their widespread adoption. Hence, the economic implications followed by the medical advantages of the new device should be critically evaluated and analyzed via an appropriate controlled clinical trial. There are numerous quantitative tools11,82,151 that can be used to access this information and their results can be beneficial in faster adoption of the new graft or devices.
The adoption of a new graft -which in most cases is likely to be more expensive can only be justified economically in the absence of a saphenous vein, considering the initial cumulative patency figures of the new graft and the known performance of the older grafts.
A good example of cost analysis methodology was employed by Rapheal adar et al, in femoropopliteal grafting of a male patient for limb-threatening ischemia.2 The study confirmed that the degree of improved performance (the break-even patency) of the new graft over dacron would be economically preferred, despite their higher price due to relatively small advantage, in terms of cumulative patency rates. The successful utilization of decision theory, in this case, was because of the availability of reliable data that can be applied to different scenarios. However, a difficulty may arise due to non-standardized method of reporting clinical results.
The pros associated with the use of decision theory: (1) It points out different factors affecting the decision process and the elements which render them more expensive. (2) It identifies those areas that require a more detailed database. (3) It is used as a sensitivity analysis for testing how a variation in the basic data or different sets of assumptions can alter the decision-making process.2
The manufacturing cost of different vascular grafts can be summarized as thus: synthetic grafts < natural polymer < decellularized matrix < cell-sheet approach.
Conclusions
The quest for a synthetic biomaterial with good mechanical properties capable of integrating with the host tissue and that can also remodel and grow with the patient, remains the driving force behind designing a new generation of clinically viable small-diameter vascular replacements. Hence, to realize this goal, the material choice for the graft must be optimized to have good mechanical properties with bioactive surface signaling cues of native tissue. Additionally, the material should have properties that closely match the elasticity and burst pressure of the native vessel to overcome the risk of flow variation and mechanical failure.
Nowadays, the outcomes of experimental and clinical trials using synthetic commercially available small diameter TEVGs show that these grafts seem to perform poorly after surgery. Hence, it is likely that in the future, a reinforced bioresorbable synthetic material with antithrombotic property and outstanding biocompatibility coupled with tailored degradability will become a desirable option for small vascular surgeries. Though, most of the successes in engineered vascular grafts approaching commercialization are for large vessels rather than small-diameter grafts used as cardiovascular bypass grafts. Consequently, the field of vascular engineering is still available for future innovators that can take up the challenge to create a functional arterial substitute.
Review Criteria
Publications up to 2019 were identified by searches performed on PubMed, MEDLINE, and Google scholar using the following keywords, alone and in combination: “small diameter vascular tissue engineering”, scaffold-based vascular tissue engineering”, “self-assembled”, “decellularized matrix”, “review”,” books”. materials for vascular tissue engineering”, mechanical properties of engineered graft”, “surface modification of vascular grafts”. In this review, we consider mostly full-text, original articles that were written in English and published between 1965 and 2019, and also searched the reference list of these papers for further leads.
Abbreviations
- e-PTFE:
-
Expanded polytetrafluoroethylene
- PET:
-
Polyethylenetelephtalate
- VEGF:
-
Vascular endothelial growth factor
- SMCs:
-
Smooth muscle cells
- HUVECs:
-
Human umbilical vein endothelial cells
- ECM:
-
Extracellular matrix
- PU:
-
Polyurethane
- PGS:
-
Polyglycerol sebacate
- PCL:
-
Polycaprolactone
- PDO:
-
Polydioxanone
- PLA:
-
Polylactic acid
- PGA:
-
Polyglycolic acid
- TEVG:
-
Tissue engineered vascular graft
- IH:
-
Intima hyperplasia
- ITA:
-
Internal mammary artery
- SV:
-
Saphenous vein
- WSS:
-
Wall shear stress
- *:
-
Commercialized products
References
Abbott, W. M., J. Megerman, J. E. Hasson, G. L’Italien, and D. F. Warnock. Effect of compliance mismatch on vascular graft patency. J. Vasc. Surg. 5(2):376–382, 1987.
Adar, R., and N. Pliskin. Cost analysis of the utilization of new vascular grafts. Metamedicine 1(2):213–223, 1980.
Ahanchi, S. S., N. D. Tsihlis, and M. R. Kibbe. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J. Vasc. Surg. 45 Suppl A:A64–A73, 2007.
Alberts, B., A. Johnson, J. Lewis, M. Rafi, K. Roberts, and P. Walter. Molecular Biology of the Cell (5th ed.). LLC, New York: Garland Science Garland Science, Taylor & Francis Group, 2008.
Asakura, T., and T. Karino. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 66(4):1045–1066, 1990.
Aytemiz, D., W. Sakiyama, Y. Suzuki, N. Nakaizumi, R. Tanaka, Y. Ogawa, et al. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv. Healthc. Mater. 2(2):361–368, 2013.
Bae, S., M. J. DiBalsi, N. Meilinger, C. Zhang, E. Beal, G. Korneva, et al. Heparin-eluting electrospun nanofiber yarns for antithrombotic vascular sutures. ACS Appl. Mater. Interfaces 10(10):8426–8435, 2018.
Bajpai, A. K., J. Bajpai, R. K. Saini, P. Agrawal, and A. Tiwari. Smart Biomaterial Devices: Polymers in Biomedical Sciences. Boca Raton: CRC Press, 2016.
Ballyk, P. D., C. Walsh, J. Butany, and M. Ojha. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J. Biomech. 31(3):229–237, 1998.
Baquey, C., F. Palumbo, M. C. Porte-Durrieu, G. Legeay, A. Tressaud, and R. d’Agostino. Plasma treatment of expanded PTFE offers a way to a biofunctionalization of its surface. Nucl. Instrum. Methods Phys. Res. Sect. B 151(1):255–262, 1999.
Barnes, B. A., and A. B. Barnes. Evaluation of surgical therapy by cost-benefit analysis. Surgery 82(1):21–33, 1977.
Barnes, C. P., S. A. Sell, E. D. Boland, D. G. Simpson, and G. L. Bowlin. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 59(14):1413–1433, 2007.
Belair, D. G., N. N. Le, and W. L. Murphy. Design of growth factor sequestering biomaterials. Chem. Commun. 50(99):15651–15668, 2014.
Berglund, J. D., R. M. Nerem, and A. Sambanis. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Eng. 10(9–10):1526–1535, 2004.
Bertram, U., D. Steiner, B. Poppitz, D. Dippold, K. Kohn, J. P. Beier, et al. Vascular tissue engineering: effects of integrating collagen into a PCL based nanofiber material. Biomed. Res. Int. 2017:9616939, 2017.
Bhat, V. D., G. A. Truskey, and W. M. Reichert. Using avidin-mediated binding to enhance initial endothelial cell attachment and spreading. J. Biomed. Mater. Res. 40(1):57–65, 1998.
Binns, R. L., D. N. Ku, M. T. Stewart, J. P. Ansley, and K. A. Coyle. Optimal graft diameter: effect of wall shear stress on vascular healing. J. Vasc. Surg. 10(3):326–337, 1989.
Bjork, J. W., L. A. Meier, S. L. Johnson, Z. H. Syedain, and R. T. Tranquillo. Hypoxic culture and insulin yield improvements to fibrin-based engineered tissue. Tissue Eng. Part A 18(7–8):785–795, 2012.
Boccafoschi, F., J. Habermehl, S. Vesentini, and D. Mantovani. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials 26(35):7410–7417, 2005.
Boland, E. D., B. D. Coleman, C. P. Barnes, D. G. Simpson, G. E. Wnek, and G. L. Bowlin. Electrospinning polydioxanone for biomedical applications. Acta Biomater. 1(1):115–123, 2005.
Boland, E. D., J. A. Matthews, K. J. Pawlowski, D. G. Simpson, G. E. Wnek, and G. L. Bowlin. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front. Biosci. 9:1422–1432, 2004.
Bos, G. W., A. A. Poot, T. Beugeling, W. G. van Aken, and J. Feijen. Small-diameter vascular graft prostheses: current status. Arch. Physiol. Biochem. 106(2):100–115, 1998.
Bourget, J. M., R. Gauvin, D. Larouche, A. Lavoie, R. Labbe, F. A. Auger, et al. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials 33(36):9205–9213, 2012.
Bradham, R. R. The importance of porosity in vascular prostheses. Am. J. Surg. 100(4):557–560, 1960.
Buttafoco, L., N. G. Kolkman, P. Engbers-Buijtenhuijs, A. A. Poot, P. J. Dijkstra, I. Vermes, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 27(5):724–734, 2006.
Catto, V., S. Farè, G. Freddi, and M. C. Tanzi. Vascular tissue engineering: recent advances in small diameter blood vessel regeneration. ISRN Vasc. Med. 2014:27, 2014.
Chan, A. H. P., E. C. Filipe, R. P. Tan, M. Santos, N. Yang, J. Hung, et al. Altered processing enhances the efficacy of small-diameter silk fibroin vascular grafts. Sci. Rep. 9(1):17461, 2019.
Chan, B. P., and K. W. Leong. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 17(Suppl 4):467–479, 2008.
Chemla, E. S., and M. Morsy. Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access. Br. J. Surg. 96(1):34–39, 2009.
Cho, S. W., S. H. Lim, I. K. Kim, Y. S. Hong, S. S. Kim, K. J. Yoo, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann. Surg. 241(3):506–515, 2005.
Clowes, A. W., R. K. Zacharias, and T. R. Kirkman. Early endothelial coverage of synthetic arterial grafts: porosity revisited. Am. J. Surg. 153(5):501–504, 1987.
Conte, M. S. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 12(1):43–45, 1998.
Contreras, M. A., W. C. Quist, and F. W. Logerfo. Effect of porosity on small-diameter vascular graft healing. Microsurgery 20(1):15–21, 2000.
Cooley, D. A., G. L. Hallman, and R. D. Bloodwell. Definitive surgical treatment of anomalous origin of left coronary artery from pulmonary artery: indications and results. J. Thorac. Cardiovasc. Surg. 52(6):798–808, 1966.
Couet, F., N. Rajan, and D. Mantovani. Macromolecular biomaterials for scaffold-based vascular tissue engineering. Macromol. Biosci. 7(5):701–718, 2007.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32(12):3233–3243, 2011.
Cui, X., and T. Boland. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30(31):6221–6227, 2009.
Dahl, S. L. M., A. P. Kypson, J. H. Lawson, J. L. Blum, J. T. Strader, Y. Li, et al. Readily available tissue-engineered vascular grafts. Sci. Transl. Med. 3(68):68ra9, 2011.
de Mel, A., C. Bolvin, M. Edirisinghe, G. Hamilton, and A. M. Seifalian. Development of cardiovascular bypass grafts: endothelialization and applications of nanotechnology. Exp. Rev. Cardiovasc. Ther. 6(9):1259–1277, 2008.
de Mel, A., B. G. Cousins, and A. M. Seifalian. Surface modification of biomaterials: a quest for blood compatibility. Int. J. Biomater. 2012:707863, 2012.
de Valence, S., J. C. Tille, D. Mugnai, W. Mrowczynski, R. Gurny, M. Moller, et al. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 33(1):38–47, 2012.
Dekker, A., K. Reitsma, T. Beugeling, A. Bantjes, J. Feijen, and W. G. van Aken. Adhesion of endothelial cells and adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials 12(2):130–138, 1991.
Diamant, J., A. Keller, E. Baer, M. Litt, R. G. C. Arridge, and F. C. Frank. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc. R. Soc. Lond. B 180(1060):293–315, 1972.
Dietrich, M., J. Heselhaus, J. Wozniak, S. Weinandy, P. Mela, B. Tschoeke, et al. Fibrin-based tissue engineering: comparison of different methods of autologous fibrinogen isolation. Tissue Eng. Part C Methods 19(3):216–226, 2013.
Dukkipati, R., M. Peck, R. Dhamija, D. M. Hentschel, T. Reynolds, G. Tammewar, et al. Biological grafts for hemodialysis access: historical lessons, state-of-the-art and future directions. Semin. Dial. 26(2):233–239, 2013.
Eberhart, A., Z. Zhang, R. Guidoin, G. Laroche, L. Guay, D. De La Faye, et al. A new generation of polyurethane vascular prostheses: rara avis or ignis fatuus? J. Biomed. Mater. Res. 48(4):546–558, 1999.
Eble, J. A., and S. Niland. The extracellular matrix of blood vessels. Curr. Pharm. Des. 15(12):1385–1400, 2009.
Edwards, A., R. J. Carson, S. Bowald, and W. C. Quist. Development of a microporous compliant small bore vascular graft. J. Biomater. Appl. 10(2):171–187, 1995.
Egashira, K. Clinical importance of endothelial function in arteriosclerosis and ischemic heart disease. Circ. J. 66(6):529–533, 2002.
Eglin, D., and M. Alini. Degradable polymeric materials for osteosynthesis: tutorial. Eur. Cells Mater. 16:80–91, 2008.
Ekaputra, A. K., G. D. Prestwich, S. M. Cool, and D. W. Hutmacher. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (epsilon-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 32(32):8108–8117, 2011.
El-Massry, S., E. Saad, L. R. Sauvage, M. Zammit, C. C. Davis, J. C. Smith, et al. Axillofemoral bypass with externally supported, knitted Dacron grafts: a follow-up through twelve years. J. Vasc. Surg. 17(1):107–114, 1993.
Enomoto, S., M. Sumi, K. Kajimoto, Y. Nakazawa, R. Takahashi, C. Takabayashi, et al. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 51(1):155–164, 2010.
Entwistle, J., C. L. Hall, and E. A. Turley. HA receptors: regulators of signalling to the cytoskeleton. J. Cell. Biochem. 61(4):569–577, 1996.
Fauza, B. D. A. D. O. Autologous Approaches to Tissue Engineering. Cambridge, MA: Harvard Stem Cell Institute, 2012.
Ferreira, P., P. Alves, P. Coimbra, and M. H. Gil. Improving polymeric surfaces for biomedical applications: a review. J. Coat. Technol. Res. 12(3):463–475, 2015.
Fittkau, M., P. Zilla, D. Bezuidenhout, M. Lutolf, P. Human, J. A. Hubbell, et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials 26:167–174, 2005.
Flamme, I., and W. Risau. Induction of vasculogenesis and hematopoiesis in vitro. Development 116(2):435–439, 1992.
Fontana, G., A. Srivastava, D. Thomas, P. Lalor, P. Dockery, and A. Pandit. Three-dimensional microgel platform for the production of cell factories tailored for the nucleus pulposus. Bioconjug. Chem. 26(7):1297–1306, 2015.
Frost, M. C., M. M. Reynolds, and M. E. Meyerhoff. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contactincy medical devices. Biomaterials 26(14):1685–1693, 2005.
Fry, W. J., M. S. DeWeese, R. O. Kraft, and C. B. Ernst. Importance of porosity in arterial prostheses. Arch. Surg. 88(5):836–842, 1964.
Fukayama, T., K. Takagi, R. Tanaka, Y. Hatakeyama, D. Aytemiz, Y. Suzuki, et al. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann. Vasc. Surg. 29(2):341–352, 2015.
Gao, J., P. Crapo, R. Nerem, and Y. Wang. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. J. Biomed. Mater. Res. Part A 85(4):1120–1128, 2008.
Gao, J., A. E. Ensley, R. M. Nerem, and Y. Wang. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J. Biomed. Mater. Res. Part A 83(4):1070–1075, 2007.
Ghanbari, H., A. de Mel, and A. M. Seifalian. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizons. Int. J. Nanomed. 6:775–786, 2011.
Graf, J., R. C. Ogle, F. A. Robey, M. Sasaki, G. R. Martin, Y. Yamada, et al. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds to 67000 laminin receptor. Biochemistry 26(22):6896–6900, 1987.
Greisler, H. P., K. A. Joyce, D. U. Kim, S. M. Pham, S. A. Berceli, and H. S. Borovetz. Spatial and temporal changes in compliance following implantation of bioresorbable vascular grafts. J. Biomed. Mater. Res. 26(11):1449–1461, 1992.
Gui, L., B. C. Dash, J. Luo, L. Qin, L. Zhao, K. Yamamoto, et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102:120–129, 2016.
Gunatillake, T., D. J. Martin, G. F. Meijs, S. McCarthy, and R. Adhikari. Designing biostable polyurethane elastomers for biomedical implants. Aust. J. Chem. 56:545–557, 2003.
Gwyther, T. A., J. Z. Hu, A. G. Christakis, J. K. Skorinko, S. M. Shaw, K. L. Billiar, et al. Engineered vascular tissue fabricated from aggregated smooth muscle cells. Cells Tissues Org. 194(1):13–24, 2011.
Haghjooy Javanmard, S., J. Anari, A. Zargar Kharazi, and E. Vatankhah. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 31(3):438–449, 2016.
Hallett, J. W., D. M. Marshall, T. M. Petterson, D. T. Gray, T. C. Bower, K. J. Cherry, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J. Vasc. Surg. 25(2):277–286, 1997.
Hallman, G. L., D. A. Cooley, D. G. McNamara, and J. R. Latson. Single left coronary artery with fistula to right ventricle: reconstruction of two-coronary system with dacron graft. Circulation 32:293–297, 1965.
Han, F., X. Jia, D. Dai, X. Yang, J. Zhao, Y. Zhao, et al. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 34(30):7302–7313, 2013.
Hibino, N., E. McGillicuddy, G. Matsumura, Y. Ichihara, Y. Naito, C. Breuer, et al. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139(2):431-6, 6.e1-2, 2010.
Hillis, D. M., D. E. Sadava, R. W. Hill, and M. V. Price. Principles of Life (2nd ed.). New York: W.H.Freeman & Co Ltd., 2014.
Hoenig, M. R., G. R. Campbell, B. E. Rolfe, and J. H. Campbell. Tissue-engineered blood vessels: alternative to autologous grafts? Arterioscler. Thromb. Vasc. Biol. 25(6):1128–1134, 2005.
Hoshi, R. A., R. Van Lith, M. C. Jen, J. B. Allen, K. A. Lapidos, and G. Ameer. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials 34(1):30–41, 2013.
Hu, Z.-J., Z.-L. Li, L.-Y. Hu, W. He, R.-M. Liu, Y.-S. Qin, et al. The in vivo performance of small-caliber nanofibrous polyurethane vascular grafts. BMC Cardiovasc. Disord. 12(1):115, 2012.
Ingle, H., G. Fishwick, A. Garnham, M. M. Thompson, and P. R. Bell. Long-term results of endovascular AAA repair using a homemade aortomonoiliac PTFE device. J. Endovasc. Ther. 9(4):481–487, 2002.
Institute of Medicine Committee on Xenograft Transplantation: Ethical I, Public P. The National Academies Collection: Reports funded by National Institutes of Health. Xenotransplantation: Science, Ethics, and Public Policy. Washington, DC: National Academies Press (US), National Academy of Sciences, 1996.
Jenicek, M. Foundations of evidence-based medicine: clinical epidemiology and beyond (2nd ed.). Boca Raton: CRC Press, 2019.
Jeschke, M. G., V. Hermanutz, S. E. Wolf, and G. B. Köveker. Polyurethane vascular prostheses decreases neointimal formation compared with expanded polytetrafluoroethylene. J. Vasc. Surg. 29(1):168–176, 1999.
Jiang, Y. C., L. Jiang, A. Huang, X. F. Wang, Q. Li, and L. S. Turng. Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 71:901–908, 2017.
Johnson, J. Development of novel, bioresorbable, small-diameter electrospun vascular grafts. J. Tissue Sci. Eng. 06:1, 2015.
Ju, Y. M., H. Ahn, J. Arenas-Herrera, C. Kim, M. Abolbashari, A. Atala, et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: a preclinical large animal study. Acta Biomater. 59:58–67, 2017.
Kakisis, J. D., C. D. Liapis, C. Breuer, and B. E. Sumpio. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J. Vasc. Surg. 41(2):349–354, 2005.
Kammerer, P. W., M. Heller, J. Brieger, M. O. Klein, B. Al-Nawas, and M. Gabriel. Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur. Cells Mater. 21:364–372, 2011.
Kanda, K., and T. Matsuda. Mechanical stress-induced orientation and ultrastructural change of smooth muscle cells cultured in three-dimensional collagen lattices. Cell Transpl. 3(6):481–492, 1994.
Kanie, K., R. Kato, Y. Zhao, Y. Narita, M. Okochi, and H. Honda. Amino acid sequence preferences to control cell-specific organization of endothelial cells, smooth muscle cells, and fibroblasts. J. Pept. Sci. 17(6):479–486, 2011.
Kannan, R. Y., H. J. Salacinski, P. E. Butler, G. Hamilton, and A. M. Seifalian. Current status of prosthetic bypass grafts: a review. J. Biomed. Mater. Res. B Appl. Biomater. 74(1):570–581, 2005.
Kannan, R. Y., H. J. Salacinski, J. De Groot, I. Clatworthy, L. Bozec, M. Horton, et al. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules 7(1):215–223, 2006.
Karrer, L., J. Duwe, A. H. Zisch, E. Khabiri, M. Cikirikcioglu, A. Napoli, et al. PPS-PEG surface coating to reduce thrombogenicity of small diameter ePTFE vascular grafts. Int. J. Artif. Org. 28:993–1002, 2005.
Kelm, J. M., V. Lorber, J. G. Snedeker, D. Schmidt, A. Broggini-Tenzer, M. Weisstanner, et al. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J. Biotechnol. 148(1):46–55, 2010.
Kidane, A. G., H. Salacinski, A. Tiwari, K. R. Bruckdorfer, and A. M. Seifalian. Anticoagulant and antiplatelet agents: their clinical and device application(s) together with usages to engineer surfaces. Biomacromolecules 5(3):798–813, 2004.
Kim, Y. H., K. B. Chandran, T. J. Bower, and J. D. Corson. Flow dynamics across end-to-end vascular bypass graft anastomoses. Ann. Biomed. Eng. 21(4):311–320, 1993.
Kim, P. H., H. G. Yim, Y. J. Choi, B. J. Kang, J. Kim, S. M. Kwon, et al. Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 187:1–13, 2014.
Kinley, C. E., and A. E. Marble. Compliance: a continuing problem with vascular grafts. J. Cardiovasc. Surg. 21(2):163–170, 1980.
Koch, S., T. C. Flanagan, J. S. Sachweh, F. Tanios, H. Schnoering, T. Deichmann, et al. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 31(17):4731–4739, 2010.
Kovalic, A. J., D. K. Beattie, and A. H. Davies. Outcome of ProCol, a bovine mesenteric vein graft, in infrainguinal reconstruction. Eur. J. Vasc. Endovasc. Surg. 24(6):533–534, 2002.
Lee, K. H., H. Y. Kim, M. S. Khil, Y. M. Ra, and D. R. Lee. Characterization of nano-structured poly(ε-caprolactone) nonwoven mats via electrospinning. Polymer 44(4):1287–1294, 2003.
Lee, S. J., J. J. Yoo, G. J. Lim, A. Atala, and J. Stitzel. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. Part A 83(4):999–1008, 2007.
L’Heureux, N., N. Dusserre, A. Marini, S. Garrido, L. de la Fuente, and T. McAllister. Technology insight: the evolution of tissue-engineered vascular grafts–from research to clinical practice. Nat. Clin. Pract. Cardiovasc. Med. 4(7):389–395, 2007.
L’Heureux, N., S. Paquet, R. Labbe, L. Germain, and F. A. Auger. A completely biological tissue-engineered human blood vessel. FASEB J. 12(1):47–56, 1998.
Li, D. Y., G. Faury, D. G. Taylor, E. C. Davis, W. A. Boyle, R. P. Mecham, et al. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Investig. 102(10):1783–1787, 1998.
Li, G., Y. Li, P. Lan, J. Li, Z. Zhao, X. He, et al. Biodegradable weft-knitted intestinal stents: fabrication and physical changes investigation in vitro degradation. J. Biomed. Mater. Res. Part A 102(4):982–990, 2014.
Li, L., C. M. Terry, Y.-T. E. Shiu, and A. K. Cheung. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int. 74(10):1247–1261, 2008.
Li, R. K., and R. D. Weisel. Cardiac Regeneration and Repair: Biomaterials and Tissue Engineering. Amsterdam: Elsevier, 2014.
Li, X., J. Xu, C. T. Nicolescu, J. T. Marinelli, and J. Tien. Generation, endothelialization, and microsurgical suture anastomosis of strong 1-mm-diameter collagen tubes. Tissue Eng. Part A 23(7–8):335–344, 2017.
Lischke, R., J. Pozniak, D. Vondrys, and M. J. Elliott. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur. J. Cardio-thorac. Surg. 40(3):619–624, 2011.
Liu, S., C. Dong, G. Lu, Q. Lu, Z. Li, D. L. Kaplan, et al. Bilayered vascular grafts based on silk proteins. Acta Biomater. 9(11):8991–9003, 2013.
Liu, J. Y., D. D. Swartz, H. F. Peng, S. F. Gugino, J. A. Russell, and S. T. Andreadis. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc. Res. 75(3):618–628, 2007.
Lord, M. S., B. Cheng, S. J. McCarthy, M. Jung, and J. M. Whitelock. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 32(28):6655–6662, 2011.
Lorentzen, J. E., O. M. Nielsen, H. Arendrup, H. H. Kimose, S. Bille, J. Andersen, et al. Vascular graft infection: an analysis of sixty-two graft infections in 2411 consecutively implanted synthetic vascular grafts. Surgery 98(1):81–86, 1985.
Lovett, M., C. Cannizzaro, L. Daheron, B. Messmer, G. Vunjak-Novakovic, and D. L. Kaplan. Silk fibroin microtubes for blood vessel engineering. Biomaterials 28(35):5271–5279, 2007.
Lovett, M., G. Eng, J. A. Kluge, C. Cannizzaro, G. Vunjak-Novakovic, and D. L. Kaplan. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 6(4):217–224, 2010.
Ma, Z., M. Kotaki, T. Yong, W. He, and S. Ramakrishna. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials 26(15):2527–2536, 2005.
Madden, R. L., G. S. Lipkowitz, B. J. Browne, and A. Kurbanov. Experience with cryopreserved cadaveric femoral vein allografts used for hemodialysis access. Ann. Vasc. Surg. 18(4):453–458, 2004.
Marelli, B., M. Achilli, A. Alessandrino, G. Freddi, M. C. Tanzi, S. Fare, et al. Collagen-reinforced electrospun silk fibroin tubular construct as small calibre vascular graft. Macromol. Biosci. 12(11):1566–1574, 2012.
Marelli, B., A. Alessandrino, S. Fare, G. Freddi, D. Mantovani, and M. C. Tanzi. Compliant electrospun silk fibroin tubes for small vessel bypass grafting. Acta Biomater. 6(10):4019–4026, 2010.
Marga, F., K. Jakab, C. Khatiwala, B. Shepherd, S. Dorfman, B. Hubbard, et al. Toward engineering functional organ modules by additive manufacturing. Biofabrication 4(2):022001, 2012.
Martins, M. S. D. S., M. P. L. D. Sá, L. Abad, E. S. Bastos, N. Franklin Junior, A. L. X. D. B. M. Baptista, et al. Tratamento cirúrgico da aorta ascendente e arco com perfusão cerebral anterógrada e hipotermia moderada. Braz. J. Cardiovasc. Surg. 21:461–467, 2006.
Matsumoto, T., T. Naiki, and K. Hayashi. Flow visualization analysis in a model of artery-graft anastomosis. Bio-Med. Mater. Eng. 2(4):171–183, 1992.
McAllister, T. N., M. Maruszewski, S. A. Garrido, W. Wystrychowski, N. Dusserre, A. Marini, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373(9673):1440–1446, 2009.
McClure, M. J., S. A. Sell, D. G. Simpson, B. H. Walpoth, and G. L. Bowlin. A three-layered electrospun matrix to mimic native arterial architecture using polycaprolactone, elastin, and collagen: a preliminary study. Acta Biomater. 6(7):2422–2433, 2010.
McKee, J. Autograft or Allograft for ACL Reconstruction?. Rosemont: AAOS, 2012.
Meinhart, J. G., M. Deutsch, T. Fischlein, N. Howanietz, A. Froschl, and P. Zilla. Clinical autologous in vitro endothelialization of 153 infrainguinal ePTFE grafts. Ann. Thorac. Surg. 71(5 Suppl):S327–S331, 2001.
Meinhart, J., M. Deutsch, and P. Zilla. Eight years of clinical endothelial cell transplantation. Closing the gap between prosthetic grafts and vein grafts. ASAIO J. 43(5):M515–M521, 1997.
Meyers, S. R., and M. W. Grinstaff. Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chem. Rev. 112(3):1615–1632, 2012.
Miller, M. R., and I. L. Megson. Review—recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 151(3):305–321, 2007.
Montini-Ballarin, F., D. Calvo, P. C. Caracciolo, F. Rojo, P. M. Frontini, G. A. Abraham, et al. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 60:220–233, 2016.
Motlagh, D., J. Yang, K. Y. Lui, A. R. Webb, and G. A. Ameer. Hemocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 27(24):4315–4324, 2006.
Mould, A. P., A. Komoriya, K. M. Yamada, and M. J. Humphries. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin alpha 4 beta 1. Inhibition of alpha 4 beta 1 function by RGD peptide homologues. J. Biol. Chem. 266(6):3579–3585, 1991.
Mulyasasmita, W., L. Cai, R. E. Dewi, A. Jha, S. D. Ullmann, R. H. Luong, et al. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J. Control. Release 191:71–81, 2014.
Munisso, M. C., A. Mahara, and T. Yamaoka. Design of in situ porcine closed-circuit system for assessing blood-contacting biomaterials. J. Artif. Org. 21(3):317–324, 2018.
Nagiah, N., R. Johnson, R. Anderson, W. Elliott, and W. Tan. Highly compliant vascular grafts with gelatin-sheathed coaxially structured nanofibers. Langmuir 31(47):12993–13002, 2015.
Nair, L. S., and C. T. Laurencin. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32(8):762–798, 2007.
Nehler, M. R., J. L. M. Taylor, R. W. Lee, G. L. Moneta, and J. M. Porter. Interposition grafting for reoperation on the common femoral artery. J. Vasc. Surg. 28(1):37–44, 1998.
Nemerson, Y. Tissue factor and hemostasis [published erratum appears in Blood 1988 Apr; 71(4):1178]. Blood 71(1):1–8, 1988.
Nesselmann, C., W. Li, N. Ma, and G. Steinhoff. Stem cell-mediated neovascularization in heart repair. Ther. Adv. Cardiovasc. Dis. 4(1):27–42, 2010.
Nickson, C. M., P. J. Doherty, and R. L. Williams. Novel polymeric coatings with the potential to control in-stent restenosis—an in vitro study. J. Biomater. Appl. 24(5):437–452, 2010.
Norde, W., and J. Lyklema. Why proteins prefer interfaces. J. Biomater. Sci. Polym. Ed. 2(3):183–202, 1991.
Norotte, C., F. S. Marga, L. E. Niklason, and G. Forgacs. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30(30):5910–5917, 2009.
Nottelet, B., E. Pektok, D. Mandracchia, J. C. Tille, B. Walpoth, R. Gurny, et al. Factorial design optimization and in vivo feasibility of poly(epsilon-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J. Biomed. Mater. Res. Part A 89(4):865–875, 2009.
Obiweluozor, F. O., A. P. Tiwari, J. H. Lee, T. Batgerel, J. Y. Kim, D. Lee, et al. Thromboresistant semi-IPN hydrogel coating: Towards improvement of the hemocompatibility/biocompatibility of metallic stent implants. Mater. Sci. Eng. C 99:1274–1288, 2019.
O’Connor, S., P. Andrew, M. Batt, and J. P. Becquemin. A systematic review and meta-analysis of treatments for aortic graft infection. J. Vasc. Surg. 44(1):38–45, 2006.
Olausson, M., P. B. Patil, V. K. Kuna, P. Chougule, N. Hernandez, K. Methe, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet 380(9838):230–237, 2012.
Pankajakshan, D., and D. K. Agrawal. Scaffolds in tissue engineering of blood vessels. Can. J. Physiol. Pharmacol. 88(9):855–873, 2010.
Pashneh-Tala, S., S. MacNeil, and F. Claeyssens. The tissue-engineered vascular graft-past, present, and future. Tissue Eng. Part B Rev. 22(1):68–100, 2016.
Patel, A., B. Fine, M. Sandig, and K. Mequanint. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc. Res. 71(1):40–49, 2006.
Pauker, S. G., and J. P. Kassirer. Therapeutic decision making: a cost-benefit analysis. N Engl J Med. 293(5):229–234, 1975.
Peck, M., N. Dusserre, T. N. McAllister, and N. L’Heureux. Tissue engineering by self-assembly. Mater. Today 14(5):218–224, 2011.
Peck, M., D. Gebhart, N. Dusserre, T. N. McAllister, and N. L’Heureux. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Org. 195(1–2):144–158, 2012.
Pektok, E., B. Nottelet, J. C. Tille, R. Gurny, A. Kalangos, M. Moeller, et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation 118(24):2563–2570, 2008.
Peng, H. F., J. Y. Liu, S. T. Andreadis, and D. D. Swartz. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng. Part A 17(7–8):981–990, 2011.
Persenaire, O., M. Alexandre, P. Degée, and P. Dubois. Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromol 2(1):288–294, 2001.
Piotrowski, J. J., B. L. McCroskey, and R. B. Rutherford. Selection of grafts currently available for repair of abdominal aortic aneurysms. Surg. Clin. N Am. 69(4):827–836, 1989.
Poldervaart, M. T., H. Gremmels, K. van Deventer, J. O. Fledderus, F. C. Oner, M. C. Verhaar, et al. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J. Controll. Release 184:58–66, 2014.
Poole-Warren, L. A., K. Schindhelm, A. R. Graham, P. R. Slowiaczek, and K. R. Noble. Performance of small diameter synthetic vascular prostheses with confluent autologous endothelial cell linings. J. Biomed. Mater. Res. 30(2):221–229, 1996.
Psaltis, P. J., A. Harbuzariu, S. Delacroix, E. W. Holroyd, and R. D. Simari. Resident vascular progenitor cells–diverse origins, phenotype, and function. J. Cardiovasc. Transl. Res. 4(2):161–176, 2011.
Pu, F. R., R. L. Williams, T. K. Markkula, and J. A. Hunt. Effects of plasma treated PET and PTFE on expression of adhesion molecules by human endothelial cells in vitro. Biomaterials 23(11):2411–2428, 2002.
Pugsley, M. K., and R. Tabrizchi. The vascular system: an overview of structure and function. J. Pharmacol. Toxicol. Methods 44(2):333–340, 2000.
Punnakitikashem, P., D. Truong, J. U. Menon, K. T. Nguyen, and Y. Hong. Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts. Acta Biomater. 10(11):4618–4628, 2014.
Rai, R., M. Tallawi, A. Grigore, and A. R. Boccaccini. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): a review. Prog. Polym. Sci. 37(8):1051–1078, 2012.
Ratcliffe, A. Tissue engineering of vascular grafts. Matrix Biol. 19(4):353–357, 2000.
Ren, X., Y. Feng, J. Guo, H. Wang, Q. Li, J. Yang, et al. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 44(15):5680–5742, 2015.
Rhee, K., and J. M. Tarbell. A study of the wall shear rate distribution near the end-to-end anastomosis of a rigid graft and a compliant artery. J. Biomech. 27(3):329–338, 1994.
Risau, W., H. Sariola, H. G. Zerwes, J. Sasse, P. Ekblom, R. Kemler, et al. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 102(3):471–478, 1988.
Roger, V. L., A. S. Go, D. M. Lloyd-Jones, R. J. Adams, J. D. Berry, T. M. Brown, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209, 2011.
Roll, S., J. Müller-Nordhorn, T. Keil, H. Scholz, D. Eidt, W. Greiner, et al. Dacron® vs. PTFE as bypass materials in peripheral vascular surgery—systematic review and meta-analysis. BMC Surg. 8:22, 2008.
Rosenberg, N., A. Martinez, P. N. Sawyer, S. A. Wesolowski, R. W. Postlethwait, and M. L. Dillon, Jr. Tanned collagen arterial prosthesis of bovine carotid origin in man. Preliminary studies of enzyme-treated heterografts. Ann. Surg. 164(2):247–256, 1966.
Salacinski, H. J., S. Goldner, A. Giudiceandrea, G. Hamilton, A. M. Seifalian, A. Edwards, et al. The mechanical behavior of vascular grafts: a review. J. Biomater. Appl. 15(3):241–278, 2001.
Sarkar, S., K. M. Sales, G. Hamilton, and A. M. Seifalian. Addressing thrombogenicity in vascular graft construction. J. Biomed. Mater. Res. B Appl. Biomater. 82(1):100–108, 2007.
Sarkar, S., T. Schmitz-Rixen, G. Hamilton, and A. M. Seifalian. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: a review. Med. Biol. Eng. Comput. 45(4):327–336, 2007.
Sauvage, L. R., R. Schloemer, S. J. Wood, and G. Logan. Successful interposition synthetic graft between aorta and right coronary artery. Angiographic follow-up to sixteen months. J. Thorac. Cardiovasc. Surg. 72(3):418–421, 1976.
Seifalian, A. M., A. Tiwari, G. Hamilton, and H. J. Salacinski. Improving the clinical patency of prosthetic vascular and coronary bypass grafts: the role of seeding and tissue engineering. Artif. Org. 26(4):307–320, 2002.
Seifu, D. G., A. Purnama, K. Mequanint, and D. Mantovani. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 10(7):410–421, 2013.
Seliktar, D., R. A. Black, R. P. Vito, and R. M. Nerem. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann. Biomed. Eng. 28(4):351–362, 2000.
Shimizu, M., S. Suzuki, S. Takaya, and K. Satoh. Replacement of the canine inferior vena cava with a seeded graft. Surg. Today 31(5):421–427, 2001.
Shin’oka, T., Y. Imai, and Y. Ikada. Transplantation of a tissue-engineered pulmonary artery. N Engl. J. Med. 344(7):532–533, 2001.
Sivalingam, G., S. P. Vijayalakshmi, and G. Madras. Enzymatic and thermal degradation of poly(ε-caprolactone), poly(d, l-lactide), and their blends. Ind. Eng. Chem. Res. 43(24):7702–7709, 2004.
Slevin, M., J. Krupinski, J. Gaffney, S. Matou, D. West, H. Delisser, et al. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 26(1):58–68, 2007.
Smith, M. J., M. J. McClure, S. A. Sell, C. P. Barnes, B. H. Walpoth, D. G. Simpson, et al. Suture-reinforced electrospun polydioxanone-elastin small-diameter tubes for use in vascular tissue engineering: a feasibility study. Acta Biomater. 4(1):58–66, 2008.
Soldani, G., P. Losi, M. Bernabei, S. Burchielli, D. Chiappino, S. Kull, et al. Long term performance of small-diameter vascular grafts made of a poly(ether)urethane-polydimethylsiloxane semi-interpenetrating polymeric network. Biomaterials 31(9):2592–2605, 2010.
Solouk, A., B. G. Cousins, H. Mirzadeh, M. Solati-Hashtjin, S. Najarian, and A. M. Seifalian. Surface modification of POSS-nanocomposite biomaterials using reactive oxygen plasma treatment for cardiovascular surgical implant applications. Biotechnol. Appl. Biochem. 58(3):147–161, 2011.
Stegemann, J. P., S. N. Kaszuba, and S. L. Rowe. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 13(11):2601–2613, 2007.
Strandness, D. E. S., and S. David. Hemodynamics for Surgeons. New York: Grune & Stratton, inc. Pub., 1975.
Swartz, D. D., J. A. Russell, and S. T. Andreadis. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 288(3):H1451–H1460, 2005.
Tan, H., and K. G. Marra. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 3(3):1746–1767, 2010.
The polysaccharide of the vitreous humor 1934. https://www.jbc.org/content/107/3/629.full.pdf.
Thomas, S., Y. Grohens, and N. Ninan. Nanotechnology applications for tissue engineering. Amterdam: Elsevier, 2015.
Tillman, B. W., S. K. Yazdani, S. J. Lee, R. L. Geary, A. Atala, and J. J. Yoo. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 30(4):583–588, 2009.
Uchida, N., H. Emoto, H. Kambic, H. Harasaki, J. F. Chen, S. H. Hsu, et al. Compliance effect on patency of small diameter vascular grafts. ASAIO Trans. 35(3):556–558, 1989.
Uchida, N., H. Kambic, H. Emoto, J. F. Chen, S. Hsu, S. Murabayshi, et al. Compliance effects on small diameter polyurethane graft patency. J. Biomed. Mater. Res. 27(10):1269–1279, 1993.
Unnikrishnan, M., P. R. Umashankar, S. Viswanathan, A. Savlania, R. Joseph, C. V. Muraleedharan, et al. Preclinical evaluation of hydrogel sealed fluropassivated indigenous vascular prosthesis. Indian J. Med. Res. 146(5):646–653, 2017.
van Wachem, P. B., T. Beugeling, J. Feijen, A. Bantjes, J. P. Detmers, and W. G. van Aken. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 6(6):403–408, 1985.
Vartanian, S. M., and M. S. Conte. Surgical intervention for peripheral arterial disease. Circ. Res. 116(9):1614–1628, 2015.
Vondrys, D., M. J. Elliott, C. A. McLaren, C. Noctor, and D. J. Roebuck. First experience with biodegradable airway stents in children. Ann. Thorac. Surg. 92(5):1870–1874, 2011.
Vrana, N. E. A. N., and P. Zorlutuna. Scaffolds for Tissue Engineering: Biological Design, Materials and Fabrication. Singapore: Pan Stanford Publishing Pte. Ltd, 2014.
Walluscheck, K. P., G. Steinhoff, S. Kelm, and A. Haverich. Improved endothelial cell attachment on ePTFE vascular grafts pretreated with synthetic RGD-containing peptides. Eur. J. Vasc. Endovasc. Surg. 12(3):321–330, 1996.
Wang, Y., G. A. Ameer, B. J. Sheppard, and R. Langer. A tough biodegradable elastomer. Nat. Biotechnol. 20(6):602–606, 2002.
Wang, X., P. Lin, Q. Yao, and C. Chen. Development of small-diameter vascular grafts. World J. Surg. 31(4):682–689, 2007.
Wang, S., L. Lu, C. Wang, C. Gao, and X. Wang. Polymeric biomaterials for tissue engineering applications 2011. Int. J. Polym. Sci., 2011.
Weinberg, C. B., and E. Bell. A blood vessel model constructed from collagen and cultured vascular cells. Science 231(4736):397–400, 1986.
Williams, D. F. On the mechanisms of biocompatibility. Biomaterials 29(20):2941–2953, 2008.
Wilson, G. J., H. Yeger, P. Klement, J. M. Lee, and D. W. Courtman. Acellular matrix allograft small caliber vascular prostheses. ASAIO Trans. 36(3):M340–M343, 1990.
Wise, S. G., M. J. Byrom, A. Waterhouse, P. G. Bannon, A. S. Weiss, and M. K. Ng. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 7(1):295–303, 2011.
Woodruff, M. A., and D. W. Hutmacher. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 35(10):1217–1256, 2010.
Wu, W., R. A. Allen, and Y. Wang. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med. 18(7):1148–1153, 2012.
Wu, H. C., T. W. Wang, P. L. Kang, Y. H. Tsuang, J. S. Sun, and F. H. Lin. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials 28(7):1385–1392, 2007.
Xie, X., A. Eberhart, R. Guidoin, Y. Marois, Y. Douville, and Z. Zhang. Five types of polyurethane vascular grafts in dogs: the importance of structural design and material selection. J. Biomater. Sci. Polym. Ed. 21(8–9):1239–1264, 2010.
Xu, C. Y., R. Inai, M. Kotaki, and S. Ramakrishna. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials 25(5):877–886, 2004.
Xu, W., F. Zhou, C. Ouyang, W. Ye, M. Yao, and B. Xu. Mechanical properties of small-diameter polyurethane vascular grafts reinforced by weft-knitted tubular fabric. J. Biomed. Mater. Res., Part A 92(1):1–8, 2010.
Xue, L., and H. P. Greisler. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 37(2):472–480, 2003.
Yang, X., L. Wang, G. Guan, M. W. King, Y. Li, L. Peng, et al. Preparation and evaluation of bicomponent and homogeneous polyester silk small diameter arterial prostheses. J. Biomater. Appl. 28(5):676–687, 2014.
Yang, H., G. Zhu, Z. Zhang, Z. Wang, J. Fang, and W. Xu. Influence of weft-knitted tubular fabric on radial mechanical property of coaxial three-layer small-diameter vascular graft. J. Biomed. Mater. Res. Part B Appl. Biomater. 100(2):342–349, 2012.
Yao, L., J. Liu, and S. T. Andreadis. Composite fibrin scaffolds increase mechanical strength and preserve contractility of tissue engineered blood vessels. Pharm. Res. 25(5):1212–1221, 2008.
Yaseen, M., X. Zhao, A. Freund, A. M. Seifalian, and J. R. Lu. Surface structural conformations of fibrinogen polypeptides for improved biocompatibility. Biomaterials 31(14):3781–3792, 2010.
Yeo, G. C., B. Aghaei-Ghareh-Bolagh, E. P. Brackenreg, M. A. Hiob, P. Lee, and A. S. Weiss. Fabricated elastin. Adv. Healthc. Mater. 4(16):2530–2556, 2015.
Yokota, T., H. Ichikawa, G. Matsumiya, T. Kuratani, T. Sakaguchi, S. Iwai, et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J. Thorac. Cardiovasc. Surg. 136(4):900–907, 2008.
You, Y., B.-M. Min, S. J. Lee, T. S. Lee, and W. H. Park. In vitro degradation behavior of electrospun polyglycolide, polylactide, and poly(lactide-co-glycolide). J. Appl. Polym. Sci. 95(2):193–200, 2005.
Zeugolis, D. I., S. T. Khew, E. S. Yew, A. K. Ekaputra, Y. W. Tong, L. Y. Yung, et al. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials 29(15):2293–2305, 2008.
Zhang, H., X. Jia, F. Han, J. Zhao, Y. Zhao, Y. Fan, et al. Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 34(9):2202–2212, 2013.
Zhang, Z., Y. Marois, R. G. Guidoin, P. Bull, M. Marois, T. How, et al. Vascugraft polyurethane arterial prosthesis as femoro-popliteal and femoro-peroneal bypasses in humans: pathological, structural and chemical analyses of four excised grafts. Biomaterials 18(2):113–124, 1997.
Zhang, Z., Z. Wang, S. Liu, and M. Kodama. Pore size, tissue ingrowth, and endothelialization of small-diameter microporous polyurethane vascular prostheses. Biomaterials 25(1):177–187, 2004.
Zhao, J., H. Qiu, D. L. Chen, W. X. Zhang, D. C. Zhang, and M. Li. Development of nanofibrous scaffolds for vascular tissue engineering. Int. J. Biol. Macromol. 56:106–113, 2013.
Zhu, C., D. Fan, and Y. Wang. Human-like collagen/hyaluronic acid 3D scaffolds for vascular tissue engineering. Mater. Sci. Eng. C 34:393–401, 2014.
Acknowledgments
This work was supported by Grants BCBI19045, BCRI19255 from Chonnam National University Hospital Biomedical Research Institute and a Grant (NRF-2019R1I1A1A01064115, and NRF-2019R1D1A3A03103899) from the Ministry of Education of the Republic of Korea and National research foundation of Korean.
Conflict of interest
No competing financial interests exist.
Ethical disclosure statement of human and animal studies, informed consent
Since our study does not involve the use of human or animal subject or data hence, there is no statement to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Jonathan Butcher oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obiweluozor, F.O., Emechebe, G.A., Kim, DW. et al. Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review. Cardiovasc Eng Tech 11, 495–521 (2020). https://doi.org/10.1007/s13239-020-00482-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00482-y