Abstract
Valve stiffening is a hallmark of aortic valve stenosis caused by excess extracellular matrix accumulation by myofibroblasts. We aimed to elucidate whether matrix stiffness regulates endothelial-to-mesenchymal transition (EndMT) of adult valvular endothelial cells (VECs) to myofibroblasts as a mechanism to further promote valve fibrosis. In addition, we specifically examined the role of the Wnt/β-catenin signaling pathway in the development of myofibroblasts during EndMT, as Wnt/β-catenin signaling has been implicated in EndMT during heart development, is reactivated in valve disease, and is required for mechanically-regulated myofibrogenesis of valve interstitial cells. Clonally derived porcine VECs were cultured on soft (5 kPa) or stiff (50 kPa) silicone Sylgard 527 substrates and treated with transforming growth factor (TGF)-β1 to induce EndMT. Immunofluorescent staining revealed that TGF-β1 preferentially promoted EndMT in VECs on stiffer substrates, evidenced by a decrease in the endothelial marker VE-cadherin and an increase in the myofibroblast marker α-smooth muscle actin (α-SMA). These changes were accompanied by β-catenin nuclear localization both in vitro and in vivo, assessed by immunostaining. Degradation of β-catenin with endostatin reduced VEC myofibroblast transition, as indicated by decreased α-SMA fiber expression. We conclude that TGF-β1-induced EndMT in aortic VECs is dependent on matrix stiffness and Wnt/β-catenin signaling promotes myofibrogenesis during EndMT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aortic valve stenosis (AVS), which affects approximately 3% of the population over the age of 75,13 is characterized by the stiffening of the aortic valve causing severe impairment in valve mobility. During the pathogenesis of AVS, valvular interstitial cells (VICs) are activated into myofibroblasts which synthesize and maladaptively remodel the extracellular matrix (ECM).25,26 In addition to activated VICs, valvular endothelial cells (VECs) that undergo endothelial-to-mesenchymal transition (EndMT) are thought to contribute to the adult myofibroblast population in AVS.1,12 EndMT is essential during valve development7 and is recapitulated in diseased human aortic valves.20 The EndMT process is characterized by increased cell motility and invasion, the reduction of endothelial markers (e.g., VE-cadherin), and the upregulation of myofibroblast markers (e.g., α-smooth muscle actin (α-SMA)).23
During aortic valve development, endocardial endothelial cells undergo EndMT to populate the cardiac cushions with mesenchymal cells.7 EndMT is regulated by multiple interacting pathways, including Notch, transforming growth factor (TGF)-β, and Wnt/β-catenin pathways.7 For example, TGF-β is a potent inducer of endocardial EndMT20,31 and is associated with activation of canonical Wnt/β-catenin signaling, as indicated by the translocation of β-catenin to the nucleus where it acts as a transcription factor.17 In this context, β-catenin is required for EndMT during valve development, as mice with endothelial cells deficient in β-catenin show lack of heart cushion formation in vivo and in vitro induction of endocardial EndMT by TGF-β2 shows reduced α-SMA expression and cell mobility in β-catenin-deficient endothelial relative to β-catenin-competent endothelial cells.17 Conversely, constitutive activation of Wnt/β-catenin signaling in zebrafish leads to EndMT throughout the endocardium instead of its normal restriction to the endocardial cushions.16
Notably, valve development is also regulated by hemodynamic forces,29 and many of the signaling pathways involved in EndMT and valve development are known to be shear stress- and stretch-sensitive suggesting a mechanism for force-driven morphogenesis.35 Mechanical regulation of EndMT has been shown in a variety of adult valve model systems. For example, low magnitude and oscillatory shear stress promoted EndMT by adult porcine VECs21; increased tissue strain induced by surgical constraint of mitral valves resulted in a significant increase in endothelial cells expressing α-SMA8; and cyclic strain promoted EndMT of adult VECs relative to static controls in vitro.1
In addition to altered shear stress and stretch, stiffening of the valvular extracellular matrix is a hallmark of AVS. Valve matrix stiffness regulates the pathological differentiation of VICs,5,14 with stiffer substrates promoting activation of VICs to α-SMA-positive myofibroblasts10,18,19,24,34 in a β-catenin-dependent manner.4 Myofibrogenic activation of VICs results in fibrosis to exacerbate tissue stiffening and putatively promote further fibrosis. Hypothetically, VEC EndMT could be similarly regulated to contribute to tissue fibrosis, but the roles of matrix stiffness and β-catenin signaling in adult valvular EndMT have not been investigated. In this study, we demonstrate that TGF-β-induced EndMT by adult VECs is enhanced on stiffer substrates in vitro. In vitro data and in vivo observations further suggest that β-catenin does not appear to be involved in initiating EndMT in VECs but is required for TGF-β1-induced activation of adult VECs to α-SMA-positive myofibroblasts.
Materials and Methods
Cell Culture
Primary porcine aortic VECs were isolated and clonally expanded as previously described.6 VECs at passage 3–7 were cultured in control medium consisting of M199 medium (Wisent Inc.) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in 5% CO2. VECs were seeded at 30,000 cells/cm2 on tissue culture-treated polystyrene (TCPS) 24-well plates coated with 0.35 mL of 75 μg/mL rat-tail collagen type I (Corning) overnight at 4 °C. To induce EndMT, VECs were treated with 10 ng/mL TGF-β1 (PeproTech), 30 ng/mL TNF-α (Invitrogen), or 25 μM H2O2 (Sigma-Aldrich) one day after seeding. Medium was changed every other day for the specified treatment length.
To inhibit β-catenin signaling, endostatin was used to degrade β-catenin and reduce its availability within the cell.9,15 VECs were seeded at 10,000 cell/cm2 in TCPS 24-well plates incubated with 0.35 mL of 75 μg/mL rat-tail collagen type I overnight at 4 °C. Two days post-seeding, cells were pre-treated with 15 μg/mL endostatin (Cedarlane) overnight before the addition of 10 μg/mL TGF-β1. Medium was changed every other day for a total of 5 days.
Sylgard 527 Substrate Preparation
To assay the role of matrix stiffness, cells were grown on 24-well plates cast with Sylgard 527 silicone substrates of 5 or 50 kPa stiffness, generated by mixing Sylgard 527 components A and B in prescribed ratios, as described.2,3 Substrate stiffnesses of 5 and 50 kPa were chosen to mimic the physiological ECM stiffness of normal valves and fibrotic valves, respectively.5 These substrates were plasma treated and functionalized using 0.5 mM Sulfo-SANPAH (CovaChem) treatment under a 368 nm UV lamp. The silicone substrates were coated with 0.35 mL of 75 μg/mL rat-tail collagen type 1 overnight in 4 °C before cell seeding at 30,000 cells/cm2.
Transwell Migration Assay
3 μM pore size Transwell insert membranes (Corning) were coated with a thin layer of type I collagen gel that presented a barrier to migration34 and seeded with VECs. 10 ng/mL TGF-β1 was added to both sides of the Transwell membranes for 3 days to induce the VECs to transdifferentiate and invade the collagen gel, after which non-invaded VECs on the top of the Transwell membrane were removed using a cotton swab. Invaded VECs located on the bottom side of the membrane were fixed with 10% neutral buffer formalin (NBF) (Sigma-Aldrich) and stained using crystal violet (Sigma Aldrich). Cell counts were performed on crystal violet-positive cells.
Immunofluorescence Analysis
After the specified treatment, VECs were fixed with 10% NBF for 10 min. Fixed cell samples were permeabilized with 0.1% Triton-X (Sigma-Aldrich) and blocked with 3% bovine serum albumin (BSA) (Sigma-Aldrich). Primary antibodies used for immunostaining included: anti-VE-cadherin (AbD Serotec, 1:100), anti-β-catenin (Abcam, 1:100), and anti-α-SMA conjugated with Cy3™ (Sigma-Aldrich, 1:100). Secondary antibodies Alexa Flour 488 goat anti-rabbit IgG (Life Technology, 1:200), Alexa Fluor 568 goat anti-rabbit IgG (Thermo Fisher Scientific, 1:200), and Alexa Fluor 488 goat anti-rat IgG (Thermo Fisher Scientific, 1:200) were diluted in 10% heat-inactivated goat serum (Sigma-Aldrich). All samples were stained with Hoechst 33342 (Thermo Scientific, 1:100) as a nuclear stain. Images were taken at 10× or 40× magnification on an Olympus IX71 microscope.
To quantify β-catenin nuclear intensity using ImageJ, nuclear masks were created by thresholding Hoechst 33342-stained images and then applied to the β-catenin-stained images to measure fluorescent intensity inside the nucleus.
Immunohistochemical Analysis
Paraffin embedded aortic valve tissue from ~ 5-month-old male Yorkshire barrows, as described by Sider et al.,28 was used for immunofluorescent staining. Antigen retrieval was performed using citrate buffer. Primary antibodies used for immunostaining include: anti-VE-cadherin (AbD Serotec, 1:20), anti-β-catenin (Abcam, 1:50), and anti-α-SMA (Sigma-Aldrich, 1:100). Secondary antibodies Alexa Fluor 488 goat anti-rat IgG (Thermo Fisher Scientific, 1:100), Alexa Fluor 568 goat anti-rabbit IgG (Thermo Fisher Scientific, 1:100) and Alexa Flour 647 goat anti-mouse IgG (Thermo Fisher Scientific, 1:100) were diluted in 10% heat-inactivated goat serum. All samples were stained with Hoechst 33342 (Thermo Scientific, 1:100) as a nuclear stain. Negative controls included no primary and IgG controls. Images were taken at 60× magnification on a Zeiss LSM700 confocal microscope.
Statistical Analyses
GraphPad Prism 5 was used to perform t-tests, two-way ANOVA and Bonferroni’s multiple comparison tests, as indicated. Data are presented as mean ± standard error of the mean (SEM).
Results
Clonally-Derived VECs Undergo TGF-β1-Induced EndMT
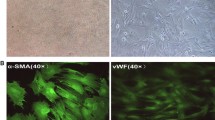
Twelve clonally-derived VEC populations were treated with TGF-β1, TNF-α, and H2O2 to identify which stimulus robustly induced EndMT characterized by morphological changes, decreased membrane VE-cadherin, formation of α-SMA positive fibers, and cell invasion. We found that TGF-β1 was capable of inducing EndMT in four of twelve VEC clones, but TNF-α and H2O2 did not in any of the clones (Fig. 1). The clone that responded the most robustly to TGF-β1 treatment was characterized and used for all subsequent experiments. Characteristic of EndMT, VECs changed from a cobblestone-like morphology (Fig. 1a) to a spindle-like morphology with TGF-β1 treatment (Fig. 1b). Immunofluorescent staining revealed disruption of VE-cadherin staining on the cell membrane and a significantly increased fraction of cells with α-SMA positive stress fibers (1.29 ± 0.31%) in the TGF-β1-treated condition (Figs. 1b and 1e) in contrast to the untreated cells, in which VE-cadherin staining remained on the cell membrane and there was little α-SMA staining (0.60 ± 0.12%; p ≤ 0.05 vs. TGF-β1-treated cells) (Figs. 1a and 1e). In a Transwell assay, cell invasion, an essential characteristic of EndMT, was also significantly increased in TGF-β1 treated VECs compared to the control condition (71.7 ± 14.8 vs. 10.3 ± 0.67 invaded cells, respectively; p < 0.05) (Fig. 1f). Based on these results, we concluded that TGF-β1 is a potent inducer of EndMT in aortic VECs characterized by changes in cell morphology, loss of membrane expression of the endothelial marker VE-cadherin, increased expression of the myofibroblast marker α-SMA in actin fibres, and increased motility.
TGF-β1 induces EndMT in clonally derived VECs. VECs were subjected to (a) growth media (control), or growth media supplemented with (b) 10 ng/mL TGF-β1, (c) 30 ng/mL TNF-α or (d) 25 μM H2O2 for 6 days. Immunofluorescent staining of VE-cadherin (green), α-SMA (red), and Hoechst (blue) showed that TGF-β1 induced EndMT in clonally derived VECs. Scale bar 100 μM. (e) The frequency of α-SMA positive cells increased with TGF-β1 treatment. n = 3. (f )The number of cells that migrated through a 3 μM pore sized Transwell membrane increased after 3 days of TGF-β1 induction. n = 3. *p ≤ 0.05 by unpaired t test.
Stiff Substrates Enhance TGF-β1-Induced EndMT in VECs
To test if EndMT-mediated VEC myofibroblast transformation is regulated by matrix stiffness, we used Sylgard 527 substrates with elastic moduli of 5 and 50 kPa to represent normal and fibrotic valve tissue stiffnesses respectively. In the absence of TGF-β1, VE-cadherin was localized to the membrane on both 5 and 50 kPa substrates, indicative of an endothelial phenotype and no EndMT, although VECs that also expressed α-SMA were occasionally observed on the stiffer 50 kPa substrates (0.16 ± 0.08%) but never on the softer 5 kPa substrates (p < 0.05) (Fig. 2). Meanwhile, TGF-β1 treatment induced significantly more α-SMA-positive cells than the control condition (p < 0.01; Fig. 2), in a substrate stiffness-dependent manner, with significantly more α-SMA positive cells on the 50 kPa substrates than on the 5 kPa substrates (0.55 ± 0.07% vs. 0.24 ± 0.12%, respectively; *p < 0.05) (Fig. 2b). This was accompanied by loss of membrane VE-cadherin expression on both stiff and soft substrates. These results suggest that EndMT of VECs is both matrix stiffness- and TGF-β1-dependent, with significantly more EndMT on stiffer substrates.
TGF-β1-induced EndMT in VECs is enhanced by matrix stiffness. (a) Immunostaining images of VE-cadherin (green), α-SMA (red) and Hoechst (blue) of VECs on 5 or 50 kPa silicone substrates. Scale bar 100 μM. (b) Frequency of α-SMA positive cells increased with matrix stiffness in TGF-β1 induced cells. n = 3. *p < 0.05 **p < 0.01 by two-way ANOVA test and Bonferroni’s post hoc test.
TGF-β1-Induced EndMT Alters β-Catenin Localization in VECs
We next examined the involvement of β-catenin signaling in TGF-β1-induced and matrix stiffness-dependent EndMT. In untreated cultures, β-catenin was localized to the cell membrane (Fig. 3a), with comparable levels of total β-catenin on the 5 and 50 kPa substrates (p > 0.05; Fig. 3b). TGF-β1 treatment induced EMT, evidenced by increased α-SMA expression and cell morphological changes (Fig. 3a), mobilized β-catenin localization from the cell membrane to the cytoplasm and nucleus (Fig. 3a), and significantly increased total β-catenin levels on both 5 and 50 kPa substrates over untreated controls (Fig. 3b; p < 0.001). Notably, there was a significant increase in nuclear β-catenin intensity with TGF-β1 treatment on the 50 kPa substrates (5.32 ± 0.25 fold change vs. untreated) vs. the 5 kPa substrates (4.52 ± 0.30 fold) (p < 0.05; Fig. 3c), but not in total cellular β-catenin (6.73 ± 0.71 (5 kPa) vs. 7.79 ± 0.67 (50 kPa); p > 0.05; Fig. 3c). These data suggest that stiff substrates promote β-catenin nuclear translocation during TGF-β1-induced EndMT.
TGF-β1-induced EndMT alters β-catenin localization in VECs in a matrix stiffness-dependent manner. (a) VECs co-stained with β-catenin (green), α-SMA (red) and Hoechst (blue). Arrows locate nuclear β-catenin localization. Scale bar 100 μM. (b) Total β-catenin intensity per cell increases with TGF-β1 treatment on 5 and 50 kPa substrates. Statistical significance was calculated using a two-way ANOVA test and Bonferroni’s post-test was used as a post hoc test. n = 6. (c) Nuclear and total β-catenin intensity in TGFβ-1 treated cells compared to control. Statistical significance was calculated using an unpaired t-test for nuclear and total β-catenin separately. n = 5. *p < 0.05 ***p < 0.001.
Degradation of β-Catenin Reduces the Frequency of TGF-β1-Induced EndMT
To test the functional role of β-catenin during TGF-β1-induced EndMT, VEC cultures were treated with endostatin. Endostatin treatment alone did not induce EndMT of VECs, as shown by similar membrane VE-cadherin and α-SMA expression levels as the control (p > 0.05; Fig. 4). In VECs treated with TGF-β1, endostatin did not prevent disruption of the cell monolayer, morphological changes associated with EndMT, or loss of VE-cadherin localization to the cell membrane (p > 0.05; Figs. 4a and 4b), but did significantly reduce the frequency of α-SMA-positive cells compared to TGF-β1 treatment alone (p < 0.01; Figs. 4a and 4c). These results suggest that β-catenin does not play a role in the initiating EndMT in VECs, but is required for TGF-β1-induced transformation of VECs to α-SMA+ myofibroblasts.
Endostatin reduces the frequency of TGF-β1-induced myofibroblast transformation. (a) Immunostaining images of VE-cadherin (green), α-SMA (red) and Hoechst (blue). Scale bar 100 μM. (b) Frequency of membrane VE-cadherin decreased with TGF-β1 independent of endostatin treatment. n = 4. (c) Endostatin blocked myofibroblast (α-SMA+) transformation induced by TGF-β1. n = 3. *p < 0.05 **p < 0.01 ***p < 0.001 by one-way ANOVA test and Bonferroni’s multiple comparison test.
EndMT in Porcine Aortic Valves
To confirm the role of β-catenin in EndMT in vivo, triple immunohistological staining of VE-cadherin, β-catenin and α-SMA was performed on porcine aortic valves. Staining revealed cells in the endothelial layer and in the leaflet interstitium that expressed VE-cadherin and α-SMA, indicative of EndMT. Notably, these cells were also positive for nuclear β-catenin (Fig. 5; arrows). In contrast, some cells in the interstitium were VE-cadherin-positive but α-SMA-negative, and these cells stained weakly for β-catenin in the cytoplasm and membrane, or not at all (Fig. 5; triangular arrowheads). These observations are consistent with our in vitro data indicating that β-catenin signaling (indicated here by intense nuclear translocation) is associated with α-SMA expression (Fig. S1; arrows) in EndMT and complete myofibroblast transformation, but does not appear to be required for initial VEC-to-mesenchymal transition.
EndMT in aortic side of the porcine aortic valve leaflets. Immunohistochemistry staining of VE-cadherin (green), β-catenin (red), α-SMA (white), and Hoechst (blue). Arrows locate triple-positive cells for VE-cadherin, α-SMA, and nuclear β-catenin, indicative of EndMT. (A merged image showing α-SMA and β-catenin staining only is shown in Fig. S1 for clarification). Triangular arrowheads locate VE-cadherin+/α-SMA- cells without nuclear β-catenin expression within the interstitial layer of the valve leaflet indicative of VEC-to-mesenchymal transition but not complete myofibroblast transformation. Scale bar 20 μM.
Discussion
There is increasing evidence that many of the processes involved in normal heart valve development also contribute to adult heart valve disease. In development, valve morphogenesis is regulated by biomechanical forces and critically involves endocardial endothelial cell EndMT regulated by β-catenin signaling. EndMT and β-catenin signaling are both reactivated within the stiffened extracellular matrix of sclerotic aortic valves, but the link between matrix mechanics, valvular EndMT, and β-catenin signaling had not been explored. Here we used a tunable substrate stiffness in vitro model system to show that EndMT of adult VECs was enhanced by TGF-β1 treatment and on stiff substrates (representing fibrotic tissue stiffness) relative to softer substrates with normal valve tissue stiffness. As in adult VICs, TGF-β1-induced transformation of VECs to α-SMA-positive myofibroblasts required β-catenin, but β-catenin was not required for initiation of EndMT. Consistent with these findings in vitro, α-SMA expression in VECs of sclerotic aortic valves was associated with nuclear β-catenin by immunostaining.
Adult valvular EndMT has been shown to be regulated by biomechanical forces associated with valve disease development, including disturbed shear stress21 and pathological stretch.1,8 Similarly, tissue stiffening due to fibrosis and calcification is a hallmark of AVS and has been shown to influence a variety of processes associated with valvular disease, including myofibrogenic differentiation of VICs (reviewed in Refs. 5 and 14). The current work suggests a new role for matrix mechanics in valve pathobiology via modulation of EndMT in response to TGF-β1. Matrix stiffness regulation of EndMT transition is not specific to the valve: stiff matrices upregulate epithelial-to-mesenchymal transition (EMT) in breast tumors through regulation of TWIST1 transcription factor nuclear translocation32 and soft matrices promote the reverse mesenchymal-epithelial transition (MET) in mouse embryonic fibroblast.11 These reports all support the view that the endothelial/epithelial and mesenchymal phenotypes are tightly regulated by matrix stiffness.
We observed that TGF-β1 promoted the morphological transformation of VECs to mesenchymal-like cells, as indicated by loss of VE-cadherin in the cell membrane and morphological changes, independent of the substrate stiffness. In contrast, complete transition to myofibroblasts expressing α-SMA-positive stress fibres was most frequent on stiff substrates (Fig. 2). Similarly, while total β-catenin levels increased with TGF-β1 treatment on soft and stiff substrates, β-catenin nuclear translocation was greater on stiff substrates (Fig. 3). Previously, we reported that TGF-β induced β-catenin nuclear translocation and α-SMA-positive stress fibres in VICs only on substrates with elastic moduli exceeding ~ 20 kPa.4 Further, as observed here, β-catenin signaling was essential for TGF-β-induced VIC myofibroblast differentiation, as its degradation by endostatin or silencing by siRNA abrogated all α-SMA-positive VICs regardless of substrate stiffness.4 Thus, the mechanisms that regulate activation of VICs to myofibroblasts, and their modulation by substrate stiffness, appear to also be involved in EndMT of VECs to fully differentiated myofibroblasts.
In contrast, TGF-β initiation of EndMT, including morphological changes and redistribution of VE-cadherin and β-catenin, was insensitive to substrate stiffness. The effects of other mechanical stimuli on TGF-β initiation of EndMT have not been studied. In other tissues and diseases, TGF-β initiates EMT via Smad pathway-dependent and –independent transcriptional regulation to repress epithelial marker gene expression and active mesenchymal gene expression.33 Adult VECs exposed to low magnitude and oscillatory shear stress demonstrate similar transcriptional profiles and increased TGF-β expression,21 suggesting that disturbed flow may aid in the initiation of EndMT, with potential involvement of TGF-β. Future studies should consider whether mechanical regulation of EndMT is two-step process, initiated by disturbed flow but requiring appropriately stiff matrix microenvironments for full mesenchymal transition.
It is notable that while almost all VECs changed their shape and lost VE-cadherin and β-catenin from the cell membrane in response to TGF-β1, only a small fraction of the cells completely transitioned to myofibroblasts expressing α-SMA-positive stress fibres, even on the stiffest substrates (1.29% of cells on tissue culture-treated plastic; Fig. 1). The low frequency of complete EndMT is in part explained by the stringent criterion we applied to identify fully transitioned cells, i.e., that they express α-SMA in stress fibres (indicative of a fully differentiated myofibroblast) and not simply have upregulated diffuse α-SMA expression (indicative of a proto-myofibroblast30). Further, the EndMT frequency we measured here likely underestimates that actual transition frequency, as we observed that some motile cells undergoing EndMT detached from the tunable stiffness substrates. The inability of cells to invade or remodel the Sylgard 527 silicone substrate, which are key characteristics of EndMT, is a limitation of our system. Collagen gel-based 3D systems12 address this limitation, but are not well-suited for studying matrix stiffness effects, as altering collagen gel stiffness leads to changes in porosity which could confound invasion frequencies.22 Finally, and perhaps most interestingly, non-uniform EndMT suggests that there are heterogeneous VEC subpopulations with differential potentials for mesenchymal transition, as has been reported previously for TNF-α-induced EndMT of VECs.12 While we would expect the clonally-derived VEC populations used here to more homogenous than pooled populations, heterogeneity would be expected due to phenotypic drift with expansion. It is interesting that we also observed heterogeneity in vivo, with only a portion of migrated VE-cadherin-positive cells also being positive for α-SMA and β-catenin. This heterogeneity in vivo may represent cellular heterogeneity, but also may reflect heterogeneity of the local microenvironment. For example, in light of our in vitro results demonstrating matrix stiffness sensitivity of VEC myofibroblast transition, it would be interesting to test whether migrated VECs that are triple-positive for VE-cadherin, α-SMA, and β-catenin colocalize with locally stiff regions of the extracellular matrix, which could be measured in tissue sections by atomic force microscopy.27
In conclusion, we found that matrix stiffness regulates TGF-β1 induction of adult aortic VECs transition to myofibroblasts in a β-catenin-dependent manner, but does not influence the initial morphological changes that occur with EndMT. These novel findings contribute to our understanding of the role of matrix stiffness in aortic stenosis pathogenesis and inform mechanical considerations in valve tissue engineering strategies that aim to recapitulate development. These findings also contribute to growing evidence that the Wnt/β-catenin signaling pathway is a potential therapeutic target for the management of AVS.4,11
References
Balachandran, K., P. W. Alford, J. Wylie-Sears, J. A. Goss, A. Grosberg, J. Bischoff, et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc. Natl. Acad. Sci. USA 108:19943–19948, 2011.
Calve, S., and H. G. Simon. Extracellular control of limb regeneration. Dordrecht: Springer, pp. 257–266, 2010.
Calve, S., and H.-G. Simon. Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 26:2538–2545, 2012.
Chen, J. H., W. L. K. Chen, K. L. Sider, C. Y. Y. Yip, and C. A. Simmons. β-Catenin mediates mechanically regulated, transforming growth factor-β1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 31:590–597, 2011.
Chen, J. H., and C. A. Simmons. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 108:1510–1524, 2011.
Cheung, W.-Y., E. W. K. Young, and C. A. Simmons. Techniques for isolating and purifying porcine aortic valve endothelial cells. J. Heart Valve Dis. 17:674–681, 2008.
Combs, M. D., and K. E. Yutzey. Heart valve development: regulatory networks in development and disease. Circ. Res. 105:408–421, 2009.
Dal-Bianco, J. P., E. Aikawa, J. Bischoff, J. L. Guerrero, M. D. Handschumacher, S. Sullivan, et al. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 120:334–342, 2009.
Dixelius, J., M. J. Cross, T. Matsumoto, and L. Claesson-Welsh. Endostatin action and intracellular signaling: beta-catenin as a potential target? Cancer Lett. 196:1–12, 2003.
Duan, B., Z. Yin, L. Hockaday Kang, R. L. Magin, and J. T. Butcher. Active tissue stiffness modulation controls valve interstitial cell phenotype and osteogenic potential in 3D culture. Acta Biomater. 36:42–54, 2016.
Fang, M., C. M. Alfieri, A. Hulin, S. J. Conway, and K. E. Yutzey. Loss of beta-catenin promotes chondrogenic differentiation of aortic valve interstitial cells. Arter. Thromb Vasc Biol. 34:2601–2608, 2014.
Farrar, E. J., and J. T. Butcher. Heterogeneous susceptibility of valve endothelial cells to mesenchymal transformation in response to TNFα. Ann. Biomed. Eng. 42:149–161, 2014.
Go, A. S., D. Mozaffarian, V. L. Roger, E. J. Benjamin, J. D. Berry, W. B. Borden, et al. Heart disease and stroke statistics-2013 update: a Report from the American Heart Association. Circulation 27(1):e6–e245, 2013.
Gould, S. T., S. Srigunapalan, C. A. Simmons, and K. S. Anseth. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 113:186–197, 2013.
Hanai, J. I., J. Gloy, S. Ananth Karumanchi, S. Kale, J. Tang, G. Hu, et al. Endostatin is a potential inhibitor of Wnt signaling. J. Cell Biol. 158:529–539, 2002.
Hurlstone, A. F., A. P. Haramis, E. Wienholds, H. Begthel, J. Korving, F. Van Eeden, et al. The Wnt/β-catenin pathway regulates cardiac valve formation. Nature. 425:633–637, 2003.
Liebner, S., A. Cattelino, R. Gallini, N. Rudini, M. Iurlaro, S. Piccolo, et al. β-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 166:359–367, 2004.
Ma, H., A. R. Killaars, F. W. DelRio, C. Yang, and K. S. Anseth. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials. 131:131–144, 2017.
Mabry, K. M., R. L. Lawrence, and K. S. Anseth. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials. 49:47–56, 2015.
Mahler, G. J., E. J. Farrar, and J. T. Butcher. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33:121–130, 2013.
Mahler, G. J., C. M. Frendl, Q. Cao, and J. T. Butcher. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol. Bioeng. 111:2326–2337, 2014.
Miron-Mendoza, M., J. Seemann, and F. Grinnell. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 31:6425–6435, 2010.
Paranya, G., S. Vineberg, E. Dvorin, S. Kaushal, S. J. Roth, E. Rabkin, et al. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am. J. Pathol. 159:1335–1343, 2001.
Quinlan, A. M. T., and K. L. Billiar. Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J. Biomed. Mater. Res. Part A. 100:2474–2482, 2012.
Rabkin, E., M. Aikawa, J. R. Stone, Y. Fukumoto, P. Libby, and F. J. Schoen. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104:2525–2532, 2001.
Rabkin-Aikawa, E., M. Farber, M. Aikawa, and F. J. Schoen. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 13:841–847, 2004.
Sewell-Loftin, M.-K., C. B. Brown, H. S. Baldwin, and W. D. Merryman. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 21:513–520, 2012.
Sider, K. L., C. Zhu, A. V. Kwong, Z. Mirzaei, C. F. M. De Langé, and C. A. Simmons. Evaluation of a porcine model of early aortic valve sclerosis. Cardiovasc. Pathol. 23:289–297, 2014.
Steed, E., F. Boselli, and J. Vermot. Hemodynamics driven cardiac valve morphogenesis. Biochim. Biophys. 1863:1760–1766, 2016.
Tomasek, J. J., G. Gabbiani, B. Hinz, C. Chaponnier, and R. A. Brown. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3:349–363, 2002.
Walker, G. A., K. S. Masters, D. N. Shah, K. S. Anseth, and L. A. Leinwand. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 95:253–260, 2004.
Wei, S. C., L. Fattet, J. H. Tsai, Y. Guo, V. H. Pai, H. E. Majeski, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17:678–688, 2015.
Xu, J., S. Lamouille, and R. Derynck. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 19:156–172, 2009.
Yip, C. Y. Y., J. H. Chen, R. Zhao, and C. A. Simmons. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 29:936–942, 2009.
Zhong, A., and C. A. Simmons. Heart valve mechanobiology in development and disease. New York: Springer, pp. 255–276, 2016.
Funding
This study was funded by Canadian Institutes of Health Research Operating Grants MOP-102721 and MOP-302041.
Conflict of interest
Aileen Zhong declares that she has no conflict of interest. Zahra Mirzaei declares that she has no conflict of interest. Craig Simmons declares that he has no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Hanjoong Jo and Ajit P. Yoganathan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhong, A., Mirzaei, Z. & Simmons, C.A. The Roles of Matrix Stiffness and ß-Catenin Signaling in Endothelial-to-Mesenchymal Transition of Aortic Valve Endothelial Cells. Cardiovasc Eng Tech 9, 158–167 (2018). https://doi.org/10.1007/s13239-018-0363-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-018-0363-0