Abstract
Karyotype characterizing the phenotypic aspects of the chromosome complement represents structural and functional organization of the nuclear genome. Its constancy ensures transfer of the same genetic material to the next generation, while variation enables ecological differentiation and adaptation. Superimposition of karyotype information onto a phylogenetic framework has immense utility in elucidating direction of evolutionary change and delineation of taxonomic hierarchy. This article attempts to provide an illustrated description of the chromosomal features that are useful in discerning differences and affinities between species and taxa. A detailed account of experimental parameters useful in chromosome identification and evaluation of karyotype asymmetry is furnished citing suitable examples. In addition to various karyotypic indices, special emphasis is given to the quantitative parameter of “chromosome dispersion index (DI)” that promises phylogenetic differentiation of closely related karyotypes, since most genera of herbaceous angiosperms display interspecific differences in chromosome size and symmetry, if not number. Karyo-evolutionary trends involve both change in chromosome number, morphology/karyotype symmetry, ploidy and total haploid length. The pattern of DNA addition/deletion across the chromosome complement has been found to be variable. Such change in DNA is either equally shared by all the chromosomes or is proportionately shared commensurate to chromosome size, leading to differential pace of change in karyotype asymmetry across the taxa. The large data accumulating on chromosome number over the years offer opportunities to utilize them as additional tools in taxonomy. The basic chromosome number (ancestral haploid number) in angiosperms has been suggested as n = 7, and an ancestral 1C of 1.73 pg, which is characteristic of the major groups with slight deviation in certain orders. The average ‘holoploid genome size’ i.e. 1C, for the Angiosperms is inferred to be 5870 Mb/6.0 pg. However, the 1C-value data available for ca.10800 species of Angiosperms reveal genome size diversity ranging from 61 Mb/0.0648 pg in the carnivorous plant Genlisea tuberosa (Lentibulariaceae) to 1,49,000 Mb/152.23 pg in Paris japonica (Melanthiaceae) suggesting over 2400 fold variation across the angiosperms; and 230 fold variation within the family (0.66 pg in Schoenocaulon texanum vs. P. japonica with highest C value). Metaphase chromosome size is estimated to range from the shortest ~ 0.3 µm in Genlisea aurea, and at family level from ~ 0.8 µm in Chamaelirium luteum to ~ 30 µm in Paris japonica. Chromosome number ranges from n = 2 to 320 across the angiosperms, at family level from n = 4 to 120 in the Brassicaceae, and at genus level from n = 2 to 45 in Brachyscome (Compositae).The evolution has mostly been at the diploid level. It is generally believed that the chromosome size of monocots is larger than dicots and the chromosome size of temperate plants is larger than tropical plants. For a broader sense and larger scale understanding, the evolutional conception of karyotype is principally based on the thoughts that: (i) symmetrical karyotype is more primitive than asymmetrical ones, (ii) longer chromosomes are primitive than shorter ones, (iii) median centromeres with chromosome arms of equal length are more primitive than chromosome arms of unequal length, (iv) low basic numbers had given rise to higher ones, and the taxa with variable chromosome number are considered young and still in evolutionary flux, (v) species with one NOR site per haploid genome are considered advanced than multiple NOR sites, (vi) ancient species had less heterochromatin (repetitive DNA), the primitive species accredited heterochromatin, followed by gradual shedding of excess heterochromatin with evolutionary specialization.
However, discovery of ancient episodes of Whole Genome Duplication (WGD) events said to have happened as an escape to the 5th mass extinction at the end of Cretaceous as a survival strategy has opened newer possibilities. A new thinking is beginning to emerge that concomitant with the climate change happening at a fast pace in the Anthropocene, it is likely that if global climate undergoes major change in coming centuries then auto-polyploidization could be the important player leading to increase in chromosome number.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The word “Chromosome” introduced in a review in 1888 by German anatomist Heinrich Wilhelm Gottfried Waldeyer has survived one full century, because it succinctly defines what early cytologists were able to see with the most modern instrument of their time, a light microscope [127]. The German proposal of the word ‘chromosome’ by Waldeyer in 1888 [122] was translated in English by William B. Benham which reads as ‘I must beg leave to propose a separate technical name ‘ chromosome’ for those things which have been called by Boveri ‘chromatic elements’, in which there occurs one of the most important acts in karyokinesis, viz. the longitudinal splitting. They are so important that a special and shorter name appears useful. If the term I propose is practically applicable it will become familiar, otherwise it will soon sink into oblivion’–cited in Zacharias [127]. With the advancement of our knowledge on super-chromosomal organization, we now know that about 4 cms. long DNA fiber is compacted to form a chromosome 1 µm in diameter and 10 µm in length [64], with estimated 1 µm long continuous string of B DNA equivalent to 3.27 kb [19]. Further, there is a critical upper limit to chromosome size that a cell can tolerate i.e. the longest chromosome arm must not exceed half of the average length of the spindle axis at telophase [94], and also there is lower limit which should be > 1% of the host genome to ensure proper kinetochore function during anaphase necessary for meiotic stability [90].
At metaphase, the chromosomes are so highly condensed (nearly 10,000 fold) that their morphology can be easily studied under the light microscope. Phenotypes of the chromosome complement technically denoted as 'karyotype' are not just another taxonomic character like number of petals/leaf shape, etc. but quite important in discerning evolutionary patterns. But their use has not been rigorous and calls for an integrated approach combining tools of comparative cytogenetics [2]. The commonly used diagnostic chromosomal landmarks used in comparative analyses are chromosome number, size, symmetry, position of centromere, secondary constriction and heterochromatic regions.
Cytological indices based on chromosome number and form have long been recognized as definitive species character, providing an exceedingly useful tool in the hands of the taxonomists to define species relationships. Cytotaxonomic data have provided meaningful insights into the evolutionary relationships within natural groups of species to reinforce conclusions based on morphological criteria. Chromosomal diversification within genera has been a focal point of plant evolutionary studies, primarily because: (i) chromosomal change imparts partial or complete barrier to interspecific gene exchange, and (ii) chromosomal traits could provide clues to species interrelationships [reviewed in 40]. Raven [81] provides an extensive account of chromosome numbers in angiosperms in accordance with the classificatory system of Cronquist. Based on a probabilistic approach to haploid chromosome number (n) changes along a phylogeny embracing more than 10,000 taxa, and genome size sampling over 5,000 taxa from the Plant DNA C-values database (https://cvalues.science.kew.org/search/angiosperm), Carta et al. [8] underscores an ancestral haploid chromosome number for angiosperms of n = 7, and ancestral 1C = 1.73 pg. Carta et al. [8] further suggest that WGD did not occur for the first 30 million years, instead 57% nodes experienced genome downsizing and prominent pattern of chromosomal reduction by dysploidy. As such, the WGDs were concentrated only towards the tips of the tree. It is further inferred that in the absence of chromosome fusion and genome downsizing during post-polyploid diploidization, both chromosome numbers and genome sizes in plants would be dramatically higher than they are today [17].
Heslop-Harrison [25], Heslop-Harrison and Schwarzacher [26] and Weiss-Schneeweiss and Schneewiiss [126] provide comprehensive account on plant genome organization including amplification/loss of DNA sequences and mechanism of chromosome change and diversity in chromosome number and structure. In addition to variation in chromosome number and morphology of the conventionally stained karyotype, Lavania and Sharma [47], Greilhuber [21] provide an exhaustive account of patterns in linear distribution and amount of constitutive heterochromatin by various techniques to unravel phylogenetic affinities between species and genera.
While discussing the subject of chromosome evolution it may be worth pointing out that habitat divergence plays a driving role in speciation. Severe environmental fluctuations and/or stress may bring about unreduced gamete formation in germline or mitotic errors in somatic tissues, enabling the establishment of new genomic states. However, fixation of any such variation should involve mechanisms that produce reproductively isolated lineages. This could be realized either through polyploidy enabling alteration in genomic constitution involving chromosome duplication and/or numerical/structural chromosome alterations or by hybridization/homoploidy. Polyploidy may lead to stable genomic states owing to their tolerance to post-polyploid genetic modification. Although, interspecies hybridization is often maladaptive but can also promote speciation through generation of homoploid hybrids (i.e. hybridization without polyploidy) enabling reproductive isolation of evolving hybrids. However, homoploidy as a speciation mechanism is considered only as a minor contributor in evolution, but polyploidy, more particularly autopolyploidy stands apart as a major evolutionary force owing to its wide occurrence and short generation time. Therefore, polyploidy/autopolyploidy shall continue to be effective speciation mechanism to sustain habitat disturbance emanating from rigors of climate change [44].
Levin [52] argues that in future the autopolyploid speciation shall be the main type of plant speciation because production of unreduced gametes that facilitates autopolyploidization is positively correlated with nutrient, humidity, temperature variation and high levels of herbivory, a situation likely to be encountered under climate change regime. Allopolyploidy on account of greater ecological tolerance and greater invasive potential would be another form of speciation. But its frequency of occurrence shall be second most important because it involves the additional step of participation of more than one species in its formation and subsequent stabilization, as well as formation of chromosomal rearrangements. Visualizing the likely pattern of speciation vis-à-vis climate changes in the next 500 years, Levin [52] opines that if global climate undergoes major changes, then these major changes will inevitably lead to an increase in the number of plant chromosomes. This in effect could increase the current proportion of polyploids in angiosperms to 35–50%, and an overall proportion up to 50% of the Earth’s plant species as polyploids. It is argued that euploidization in herbaceous plants is 40–70 times more likely than aneuploidization, and evolution of short-statured herbaceous plants is more likely in response to major climatic changes in future. This is consistent with the incidence of chromosome changes occurring during in vitro passage of sub-cultures akin to stressed environment [44]. However, Gao et al. [20] argue that plant extinction shall excel plant speciation in Anthropocene (i.e. the present era influenced by anthropogenic interventions) except in protected facilities such as cities, polar regions and botanical gardens where new plant species might be acceleratingly formed through autopolyploid speciation and hybridization.
From phylogenetic viewpoint the cytological data featuring chromosomal details constitute an important aid to decipher the differences and affinities, more particularly in the related taxa. The area is treated under cytotaxonomy or karyosystematics. This has been the focus of this article with examples drawn for Angiosperms. The subject is dealt here under 2 major heads: (A) Principles of karyosystematics, (B) Applications as an aid in solving systematic problems.
A. Principles of Karyosystematics
Concept of “Karyotype” and chromosome markers of linear differentiation
The commonest form of the plant nuclear chromosome is the one with localized centromere, which is usually at the site of the major (i.e. primary) chromosomal constriction. To a cytotaxonomist, the localized centromere provides the all-important marker to classify chromosomes on the basis of the sizes of the two arms of a chromosome marked by the position of the centromere. For a classical cytologist, the conventionally stained chromosome preparations from root-tips comprise the hallmark of essential information on the chromosome complement of individuals. The first necessity in any chromosome study, therefore, is a view of the chromosomes based on preparations of the highest possible quality.
Lewitsky 1931 [57] proposed the term ‘karyotype’ to define the phenotypic appearance of a somatic chromosome complement. Three terms namely, karyotype, karyogram and idiogram are often used for description and identification of chromosomes. Karyotype analysis is usually based on chromosome measurements with maximum chromosome condensation with clearly discernible primary and secondary constrictions for optimum analysis. Occasionally conventional staining may not distinguish chromosomes of similar morphological features. In such situation linear differentiation based on C-, N-, Q-banding, Fluorescence In Situ Hybridization (FISH) could facilitate differentiation. Further for small chromosome Pachynema (Pachytene) stage could be better for differentiation, see [107] for more details. A description of the karyotype of a species includes: (i) chromosome number, (ii) total length of chromosome complement (genome size), (iii) absolute and relative sizes of chromosomes within the complement, (iv) symmetry of chromosome complement that takes into account the position of centromere of each chromosome, (v) number and position of non-centromeric constriction (i.e. secondary constriction/satellite), (vi) distribution of heterochromatic segments. Karyotype characteristics are generally species specific, although in certain instances variation in chromosome size is known to be occurring relating to ontogenetic development (e.g. in Allium fistulosum L., the chromosomes are usually much shorter in the pollen than in the root tips, and in Papaver rhoeas L., the chromosomes from leaf meristems are about the half the length and half the thickness of the metaphase chromosomes in root tips [30]. The karyotype can be studied from somatic metaphase in root-tips, shoot-tips, pollen mitosis, pachytene of meiosis, etc., but the preferred and standard karyotyping is based on mitotic metaphases from root-tips of comparable age. The chromosome complement of an individual is diagrammatically represented as ‘idiogram’ that defines both the number and morphological features of the chromosomes. The standard way of displaying the chromosomes of an individual is to line up the chromosomes in pairs (in diploids) in decreasing order of size starting with the largest and with the short arm pointing to the top.
The study of chromosome constitution of related taxa provides a means of determining the degree of similarity of karyotypes and the nature of mechanisms that have brought about any detectable changes. The attendant karyomorphological features to construct a karyotype for a given species involves a description of the position of primary (centromeric) and secondary (non-centromeric) constrictions, relative arm lengths and total chromosome sizes. On the basis of the position of centromere, the individual chromosomes can be categorized as metacentric (V-shaped), submetacentric (L-shaped), telocentic (i-shaped) and acrocentric (rod-shaped). Levan et al. 1964 [50] proposed a classification of chromosomes based on relative ratio between the two chromosome arms differentiated by the position of centromere. Chromosome form based on position of centromere and relative arm ratio is summarized in the Table 1 below, in accordance to the classification of Levan et al. [50].
Further based on absolute chromosome length, the individual chromosomes within the complement could be categorized into various groups denoted by symbols: A, B, C, D, …..etc.. Citing example of the genus Papaver where the length of metaphase chromosomes across the species ranges from over 1 μm to 8 μm [49], the chromosomes within the genus could be grouped into various classes based on individual chromosome length i.e. A = more than 7 μm–up to 8 μm, B = more than 6 μm up to 7 μm, C = more than 5 μm up to 6 μm, D = more than 4 μm up to 5 μm, E = more than 3 μm up to 4 μm, F = more than 2 μm up to 3 μm, and G = more than 1 μm up to 2 μm. However, to differentiate the chromosomes falling in the same class with respect to size, the position of centromere is the decisive factor to delineate the chromosome order in the karyotype i.e. chromosomes with increasing difference in the two arms shall be placed in descending order in the karyotype. Using above parameters, a representative idiogram drawn from arbitrary data for chromosome arm length (as given in Table 2) is shown in Fig. 1a. The karyotype formula derived from the data shown in Table 2, could be depicted as follows:
a. Idiogram showing representative karyomorphological features, b. Linear differentiation of morphologically similar chromosomes by banding pattern [40]
Karyotype formula for the idiogram in Fig. 1a: 1AMsc + 1AM + 1Bm + 1Bsm + 1Csmst + 1Est + 1FT + 1Gt. Here sc and st depict secondary constriction and satellite, respectively.
However, there are several other chromosomal indices based on centromere position, such as arm ratio, centromere index, total form index etc. that are in vogue for classifying the chromosomes.
Huziwara 1962 [29] gives a simple equation to define Total Form (TF) % of the Karyotype, where:
TF % = Total sum of short arm length/Total sum of chromosome length × 100.
i.e. for the data given in Table 2, TF% = 15.25∕44.5 × 100 = 34.27%
Arano 1963 [1] introduced another karyotype symmetry index Ask% considering the sum of long arm as opposed to above, where:
Ask % = Total sum of long arm length/Total sum of chromosome length x 100.
i.e. for the data given in Table 2, Ask% = 29.25∕44.5 × 100 = 65.73%
Subsequently, to evaluate Karyotype Asymmetry, Greilhuber and Speta [22] developed two indices: (i) Index of Karyotype Asymmetry, and (ii) Index of Chromosomal Size Resemblance, that were later called as Syi Index and Rec Index, respectively by Venora et. al. [120]. The Syi value indicates the ratio of the mean length of the short arms against the mean length of the long arms in the chromosome complement. The Rec Index expresses the mean of the ratios of the length of each chromosome to that of the longest one.
Syi Index = Mean length of the short arms/Mean length of the long arms × 100.
i.e. for the data given in Table 2, Syi Index = 15.25∕29.25 × 100 = 51.14%
Rec Index = Mean length of each chromosome (CL)/Mean length of the longest chromosome (LC) ÷ Haploid number of chromosome set (n) × 100;
in short-CL/LC ÷ n × 100.
i.e. for the data given in Table 2, Rec Index = 44.5∕8 ÷ 8 × 100 = 69.53%
Some more formulations have also been advanced-see Table 6.
With the advent of chromosome banding techniques reviewed in [41, 47], it has been made possible to realize further differentiation of chromosomes in the form of dark staining heterochromatin and light staining euchromatin segments. This provides supplementary linear chromosome markers in addition to the conventional primary and secondary constrictions. Thus, the otherwise similar looking chromosomes could be distinguished further because of their banding patterns (Fig. 1b). With such details of chromosome morphology, it is possible to describe the extent of the differences and similarities between the karyotypes. Considerable amount of diversity is encountered in the karyotypes across the taxa, that can form the basis of scoring taxonomic relationships. Mentioned below are the major components of karyotype variation having value in understanding chromosomal diversification and speciation, that can supplement the taxonomic data.
(a) Variation in chromosome number
Chromosome number is the simplest stable karyotype parameter not influenced by external conditions, age and developmental phases. Of course, incidences of occurrence of additional ‘B’ chromosomes are reported in certain taxa [34]. As such, occurrence of variation in chromosome number across the taxa offers substantial information about the genome of the species and constitutes a valuable parameter in the hands of cyto-taxonomists [23]. The basic chromosome number (ancestral haploid number) in angiosperms has been suggested as n = 7, and an ancestral 1C of 1.73 pg, which is characteristic of major groups with slight deviation in certain orders [8].
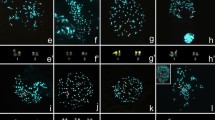
Chromosome numbers are extraordinarily variable in angiosperms, ranging from n = 2 to n = 320 [87]. Many genera and families exhibit huge variation in base number of an array of magnitude (The Chromosome Count database—http://ccdb.tau.ac.il/) and chromosome size. At family level Brassicaceae has been found to exhibit highest range of variation in chromosome number varying from n = 4 to n = 120 [61, 123]. At genus level highest variation is recorded in Brachyscome from n = 2 in B. dichromosomatica to n = 45 in B. radicata [65]-Fig. 2c–d. In angiosperms, highest recorded chromosome number is n = 320 in Sedum suaveolens Kimnach (Crassulaceae) for dicots and n = 298 in Voanioala gerardii J.Dransf. (Palmae) for monocots [31], and for other land plants it ranges up to n = 720 in a fern Ophioglosum reticulatum [37]. So far, no plant taxon has been described with n = l (although reported in animals: in ant-Myrmecia pilosula and threadworm -Parascaris univalens), but n = 2 is known in six angiosperms, dicots-Haplopappus gracilis (Nutt.) Gray (syn.Xanthisma gracile (Nutt.)D.R.Morgan & R.L.Hartm.) (Asteraceae), Brachyscome dichromosomatica C.R.Carter (Asteraceae), and monocots -Ornithogalum tenuifolium Delaroche (syn. Albuca virens (Lindl.) J.C. Manning & Goldblatt. (Liliaceae), Zingeria biebersteiniana (Claus) P. Smirnov (Poaceae), Colpodium versicolor (Stev.) Schmalh (Poaceae) [10], and Rhynchospora tenuis Link (Cyperaceae) [9]–the latter with holocentric chromosomes. Such variation in chromosome number might have occurred via inversion, interchange, fission, fusion, deletion, duplication and genomic multiplication that are encountered as the processes of evolutionary changes [26, 126]. All this is succinctly described here.
-
(i)
through mere genomic multiplication i.e. autopolyploidy (Medicago sativa, Solanum tuberosum, 2n = 4x = 48) or after intercrossing between the two related taxa followed by amphidiploidization i.e. allopolyploids e.g. Triticum aestivum (2n = 6x = 42), and their combination. In both the situations the related species could be arranged in a polyploid series exhibiting change of certain base numbers. The base number is denoted by ‘x’ whereas the gametic number by ‘n’.
-
(ii)
through multiple translocations: Stebbins [113] has described progressions leading to an increase and decrease of chromosome number without a change in genome size through two translocations arising in single plant followed by production of n + 1 and n – 1 gametes. In contrast to Stebbins' model, Schubert and Rieger [95] propose that new numbers may arise from the crossing of two plants each with a unique translocation followed by the production of n + 1 and n – 1 gametes.
-
(iii)
through structural alterations between the chromosomes via Robertsonian fusion or centric fission [33]. Robertsonian fusion is a process wherein two nonhomologous acrocentric chromosomes each break at the centromere. This is followed by the fusion of the long chromosome arms and the fusion of the short chromosome arms, resulting into a long metacentric and short metacentric chromosome. Fusion of gametes carrying both chromosomal novelties produces a homozygote with original chromosome number but altered karyotype. Frequently, the fusion chromosome incorporating the short arms of the broken chromosomes is lost, thus yielding a homozygote whose base number is one less than the original. However, the sums of the total number of long arms remain the same. Progressive fusions can produce a steady decline in chromosome number—see, Levin [51]. Fissions involve chromosome breakage at the centromere. They are most likely to occur in chromosomes with two relatively long arms. Each fission will increase the base number one by one—for more details see, Levin [51]. Fissions are associated with heterochromatin rich metacentric chromosomes, whereas fusions tend to occur preferentially between chromosomes with less heterochromatin [36]. The species alliance of Gibasis linearis (Commelinaceae) shows most species with 2n = 12, but one has 2n = 10, having an additional metacentric in its basic set. This relationship is suggestive of Robertsonian fusion. Similarly, within G. schiedeana (syn. G. pellucida (M. Martens & Galeotti) D.R. Hunt, there are diploids (2n = 10, x = 5) and tetraploids (2n = 16, x = 4) whose chromosome sets show a Roberstsonian relationship [32]. Thus, three different base numbers, namely x = 4,5,6 exist in Gibasis.
-
(iv)
by intergenomic interchanges involving hybridization and chromosomal rearrangements between hybridizing genomes via aneuallopolyploidy. In the genus Papaver (Papavaraceae) a range of chromosome numbers are found viz. 2n = 12, 14, 22, 28, 42 and 44 [49]. Whereas most species support 2n number representing a polyploid series of 2n = 14, 28 and 42, with a base no. of x = 7, but certain species have 2n = 12, 22 and 44. The occurrence of 2n = 12 in such situation is supposed to have taken place by simple Robertsonian translocation (i.e. by fusion between two acrocentric chromosomes to give way to one metacentric chromosome) as also supported by the presence of a pair of large metacentric chromosomes in Papaver pavoninum [49], but the occurrence of 2n = 22, P. somniferum or 2n = 44, P. setigerum, is difficult to interpret. It is presumable that in such situation a deviant chromosome no. of 2n = 22 may have arisen from a triploid hybrid followed by chromosome fission and / or other structural changes emanating from interspecific hybridization between two ancestral species having 2n = 14 and 2n = 28. Although, such incidences are not so common, but unique means of speciation involving interspecies introgression and chromosomal alterations.
-
(v)
by non-disjunction assisted aneuploid series: Nondisjunction of chromosomes at anaphase may give way to aneuploidy. Many genera have a series of chromosome numbers generated by the addition or subtraction of single chromosome. Levin [51] has provided a reasonable account about the occurrence of such aneuploid series over a range of taxa. Incidence of ascending and descending aneuploid series both have been reported in literature, for more details see Levin [51]. The examples of occurrence of more bizarre aneuploid series worth mentioning are Claytonia virginica L. (Montiaceae) with 2n = 12, 14, 17–20, 22, 24, 26, 28, 30–32, 34, 36, 41, 48, 72 [54, 55, 89], representing both ascending and descending series, with original x = 8. Descending aneuploidy occurs in the genus Calycadenia (Asteraceae) having n = 4–7 [5]. Among the species growing in India, the somatic chromosome number in Nymphaea alba (Nymphaeaceae) ranges between 48 and 160, in N. rubra between 42 and 112, and in Sprekelia formosissima (Amaryllidaceae) between 27 and 180, for more details see, Koul [39].
-
(vi)
by aneusomaty via participation of variant nuclei in the formation of daughter shoots in asexual species: In several asexually reproducing species, mostly belonging to monocot families viz. Agavaceae, Helleboreae, Liliaceae, Amaryllidaceae, Araceae, Zingiberaceae, Dioscoreaceae etc. where vegetative reproduction is either obligatory or profuse, their somatic tissues / shoot apex often represent a chromosome mosaic, where in addition to the normal karyotype, varying complements occur regularly although with lesser frequency. This was first reported in Caladium bicolor (Araceae). Such chromosome mosaicism is genotypically controlled and most arise out of nondisjunction and partial endoreduplication. Participation of variant nuclei in the formation of new daughter shoots leads to the origin of an individual with a hereditary constitution quite different from the parent one, suggesting a new means of speciation in obligate asexuals [98, 100, 101].

reproduced from: a- Tanaka 2020 [118], b- Pellicer et al. 2010 [74], c. Levin 2002 [51]. d. Murray et al. 2013 [65]
Showing range of variation in chromosome size and number. a-b. chromosome size within the family (Melanthiaceae): shortest 0.8 µm in Chamaelirium luteum (a) vis-a-vis largest 30 µm in Paris japonica (b); C-D. chromosome number within the genus, Brachyscome (Compositae): lowest 2n = 4 in B. dichromosomatica (c) vis-a-vis highest 2n = 90 in B. radicata (d). Figures
(b) Karyotype symmetry: an index of variation in chromosome size and diversity in karyo- morphology
Chromosome size
As mentioned under the introduction there is a critical lower and upper limit of chromosome size for normal development and meiotic stability. The lower limit is determined such that it ensures proper functioning of kinetochore [90], and the upper limit is confined to the largest chromosome arm that should not exceed half of the average length of spindle axis at telophase to realize meiotic stability [94]. Based on a survey conducted on 856 angiosperm species, Levin and Funderberg [53] opined that in general the chromosome size of monocots is larger than dicots and the chromosome size of temperate plants is larger than tropical plants. The length of shortest to largest chromosome at metaphase in Angiosperms is known to vary from ~ 0.3 to ~ 30 µm, and more precisely from ~ 0.2—0.4 µm in Genlisea aurea (Lentibulariaceae) [18] to 30 µm in Paris japonica (Melanthiaceae) [74]. At the family level largest range in chromosome size is reported in Melanthiaceae from ~ 0.8 µm in Chamaelirium luteum [118] to ~ 30 µm in Paris japonica—the species with the largest known eukaryotic genome [74]. This is depicted in Fig. 2a–b.
Karyotype symmetry and biomodal karyotype
In order to elucidate karyotypic differentiation vis-à-vis evolutionary specialization, Stebbins [113] defined the terms symmetric and asymmetric karyotypes. Whereas symmetric type is characterized by mainly metacentric and sub-metacentric chromosomes of approximately equal size, the asymmetric ones depict shift in centromere position towards telomere i.e. intrachromosomal and /or addition or deletion of chromatin in chromosome arms affecting differences in size between the largest and smallest chromosomes i.e. interchromosomal. Subsequently, Stebbins [115] provided the classification of karyotypes into 12 categories, ranging from an extreme symmetrical to an asymmetrical karyotype, based on relative proportions of chromosome morpho-types in a given karyotype (Table 3). Accordingly, the karyotypes which consist of chromosomes essentially similar in size and all with median or submedian centromeres may be called “symmetric”, whereas those with chromosomes differing greatly in size and/or partly with subterminal or terminal centromeres are called “asymmetric”. It is possible to arrange the closely related species in an evolutionary order in a given taxa based on the class of karyotype symmetry. The prevailing concept is that during evolution karyotype has undergone change from symmetry to asymmetry with evolutionary specialization. Diversification in chromosome morphology may arise by alteration in chromosome symmetry through intra-genomic translocations, pericentric inversions, fusion / fission, that may or may not affect alteration in genome size.
Whereas, differences in genome size may arise either by accretion or deletion of redundant DNA or genomic duplication, there are instances of incidence of special form of asymmetry characterized by presence of two distinct sets of chromosomes of contrasting size in a chromosome complement i.e. ‘biomodal karyotype'—what could be called as ‘together but different'. Such biomodal karyotypes may originate either by fusion for the formation of large chromosome set and by fission for the small set, or differential accumulation of repetitive sequences, or by allopolyploid combination of species sporting different chromosome sizes [4]. Such bimodal karyotypes are common in monocot families such as Asphodelaceae (Aloe, Haworthia, Gasteria), Agavaceae (Agave, Yucca) [126].
Quantitative differentiation of closely related karyotypes within the same class of asymmetry
In certain instances, it may be possible that closely related species may not show significant differences in their karyotypes despite having clear distinction between the species on exo-morphological account. In such situations, there remains a need to further differentiate closely related karyotypes falling within the same class of karyotype asymmetry, to erect affinities from evolutionary view point between the closely related species. The following measures may be useful to differentiate the closely related karyotypes:
(i) Chromosome "Dispersion Index" i.e. DI: Lavania and Srivastava [48] developed an index called Chromosome “Dispersion Index” depicting statistical gradient of centromere position within the karyotype to facilitate differentiation of closely related karyotypes falling within the same “asymmetry” class of Stebbins.
The “Dispersion Index” could be calculated from the following equations:
-
(1)
Centromeric gradient (CG) = Length of median short arm/Length of median chromosome × 100
-
(2)
Coefficient of variation (CV) for chromosome length = S.D./Mean × 100
-
(3)
Dispersion Index (DI) = Proportionate measure of CG with respect to CV
For the sake of convenience of the readers and chromosome workers this is explained by taking the example of Papaver bracteatum for which karyomorpholgical details are provided in Lavania and Srivastava [49], and the same is reproduced above in the Table 4.
From the above example the values for Dispersion Index (DI) could be estimated as follows:
Dispersion Index (DI) = Proportionate measure of CG with respect to CV i.e. 22.62% of 39.2 = 8.87.
The inferences for this Index were based on detailed examination of chromosome morphology of 21 species of Papaver having established affinities from exo-morphological and phytogeographical viewpoint. The criterion of “Dispersion Index” has been utilized effectively to differentiate closely related karyotype asymmetry in an evolutionary order, facilitating development of species cladogram of Papaver [49].
(ii) Asymmetry Index i.e. AI: Paszko [73] provides two coefficients of variation (CVs) to evaluate intrachromosomal and interchromosomal asymmetry:
(1) CVCI index i.e. Coefficient of variation (CV) of the centromeric index (centromeric index is the ratio of the length of the short arm to that of the total chromosome length). This evaluates differences in centromere position for each chromosome in the karyotype and provides a measure of intrachromosomal asymmetry.
(2) CVCL index i.e. Coefficient of variation (CV) of chromosome lengths. This gives a measure of interchromosomal asymmetry as it reflects how variable the chromosome sizes are in the karyotype. In fact this index is exactly the same as already given by Lavania and Srivastava [48, 49] for estimating Coefficient of variation (CV) for chromosome lengths.
wherein Asymmetry Index (AI) = (CVCI x CVCL) ÷ 100.
In both cases, the larger the value the greater the asymmetry in the karyotype.
For the sake of convenience of the readers and chromosome workers this is explained taking again the example of Papaver bracteatum for which karyomorpholgical details are provided in Table 4 above. As per the requirement of Paszko [73], the same value are modified, and are given below in Table 5, in order to estimate the values of AI.
Based on the values given in Table 5, the values for CVCI and CVCL could be estimated as follows:
wherein Asymmetry Index (AI) = (CVCL × CVCI) ÷ 100 = (22.62 × 26.47) ÷ 100 = 5.987.
In addition to TF %, Ask%, Syi and Rec Index discussed above under the section on ‘Concept of Karyotype’, and the Dispersion Index [48] explained above having specific significance in differentiating closely related karyotypes within the same group of Karyotype Asymmetry categories of Stebbins [115], many other parameters to estimate Karyotype Asymmetry have since been published. These are briefly outlined here to update the reader on this topic. Romero-Zarco [88] provided another alternative method by using quantitative and graphic representation based on Intra-chromosomal and Inter-chromosomal—Asymmetry Index. Further in order to accurately characterize variation in intrachromosomal asymmetry Zuo and Yuan [130] termed such asymmetry estimate as ‘Heterogeneity of the Centromeric Index’. A detailed discussion on the different Indices to estimate karyotype asymmetry is available in Paszko [73], Zuo and Yuan [130]. All such parameters have been recently used collectively in analysis to differentiate Musa varieties by Dehery et al. [12]. A summarized account of all such indices has been provided by Zuo and Yuan [130]. These are given here through Table 6 for the benefit of advanced learners.
Most genera of herbaceous angiosperms display interspecific differences in chromosome size and symmetry, if not number. Two most studied genera that depict desperate karyotypes with vast variation in chromosome size, symmetry and base chromosome number are Crepis, x = 3 to 6 [15], and Brachyscome, x = 2 to 9 [124]. In Brachyscome, mean level of chromosome symmetry varies from 0.09 to 0.50 (a value of zero indicates perfect symmetry i.e. both chromosome arms have the same length); the value increases as the centromere moves toward one end of the chromosome, reaching its maximum near 0.5 when centromeres are near the ends of the chromosomes, for more details see Levin [51]. Application of karyotypic features are of immense utility in such situation to elucidate phylogenetic affinities and direction of evolutionary change.
(c) Variation in NOR regions
Normally, one pair of chromosomes in a somatic chromosome complement contains additional sites of constriction other than the site of centromere. This additional constriction is called as secondary constriction (i.e. noncentromeric constriction), which is generally located in the subtelomeric regions of one of the chromosome arms (usually short arm), or at times in the terminal region (in the form of a satellite body). These secondary constriction sites are also rich in ribosomal DNA and are responsible for organization of the nucleolus. Therefore, the secondary constriction sites are also called as nucleolar organizer region (NOR) in a broad sense. Of course the accuracy of NOR sites could be judged only after specific silver staining techniques/or more recently by in situ hybridization based localization with ribosomal RNA specific DNA probes. It is observed that most of the diploid species have one pair of ribosomal DNA site (i.e. NOR) in a somatic complement [63], although some diploids may have multiple sites [45]. The data on the occurrence of number of secondary constrictions and NOR sites has been utilized to establish phylogenetic affinities in family Apiaceae [24] and Plantago [104]. Taxa with one nucleolar chromosome per haploid genome are presumed to be advanced over those exhibiting nucleolar chromosome plurality [39].
(d) Linear differentiation
The power of karyotype analysis expanded in late 1960s with the development of banding techniques [41]. In addition to conventional chromosome markers that morphologically differentiate chromosomes longitudinally on the basis of the position of centromere (primary constriction), secondary constriction, relative arm ratio, the techniques of chromosome banding provide further linear differentiation of chromosomes, facilitating differentiation between the morphologically similar chromosome linkage groups within the karyotype (Fig. 1b). Lavania and Sharma [47], Greilhuber [21] provide an exhaustive account on the distribution of heterochromatin banding patterns over a range of plant taxa with respect to application of chromosome banding as a tool in plant taxonomy. A representative example is depicted in Fig. 3.This “new karyosystematics” has its impact mainly at the infrageneric level and, at any rate, in the “diffuse” zone, where it becomes difficult to separate by traditional karyological and other methods [21]. 1980s brought even more powerful tool of fluorescence in situ hybridization by which it is possible to identify genomes, chromosomes and chromosome segments [42] on account of localization of homologous DNA sites on the chromosomes when hybridized in situ by specific DNA probes i.e. FISH or even total genomic DNA i.e. GISH [79] (Fig. 4). Further, even the micro-structural diversity within the chromosome complement that may be occurring at the population level in the hot spot areas could be linearly delineated by in situ hybridization of the target chromosome complements with the total genomic DNA of a reference species, e.g. rice or Arabidopsis DNA [43].
Giemsa C-banded idiograms in Lathyrus species: A. L. angulatus, B. L. clymenum, C. L. aphaca, D. L. articulatus, E. L. cicera, F. L. hirsutus, G. L. ochrus, H. L. odoratus, I. L. sativus, J. L. sphaericus, K. L. tingitanus, L. L. latifolius, M. L. maritimus, N. L. sylvestris, O. L. tuberosus. Note—Here the species are arranged in phylogenetic order following morphological criteria and breeding habit. The banding pattern is species specific showing definitive change in C-patterns with evolutionary specialization i.e. gradual reduction / diminution in C-bands from intercalary to telomeric bands, adopted from Lavania and Sharma [46, 47]

reproduced from Lavania and Srivastava [49]
a. Somatic chromosomes of Aloe vera (2n = 14) showing bimodal karyotype, 4 pairs of large chromosomes + 3 pairs of small chromosomes; b. DAPI stained Fluorochrome-banding in Aloe vera showing localization of heterochromatin in centromeric region; c. Localization of r-DNA sites in Papaver somniferum by FISH—note presence of three such pairs suggesting multi-genomic origin of opium poppy; d. FISH based localization of telomere specific repetitive DNA families on somatic chromosomes of rye; e. Cytological differentiation of wheat and rye chromosomes in the synthetic hybrid Triticale (tetraploid wheat x diploid rye) differentiated by genomic in situ hybridization with rye specific DNA probe: all rye chromosomes show red hybridization signals in the telomeric region. Figure 'c' is
(e) Repetitive sequences in evolution of chromosome structure
The structure and shape of chromosome could be altered by chromosome rearrangements, including insertion, duplication, deletion, fusion, inversion and translocation [91]. Information available in Plant DNA C-value Database (https://cvalues.science.kew.org/) and on genome sequencing clearly suggests that there has been huge accumulation of repetitive DNA sequences over time leading to changes in genome size [75, 77]. Such changes have played multiple generative roles in structural chromosomal repatterning in shaping the karyotype in plants [26, 58, 126]. The repetitive DNA sequences frequently constitute cytologically detectable heterochromatic regions that can be microscopically discerned to elucidate pattern of change. Therefore, it is desirable that interpretation of karyotype evolution must consider chromosomal structural constraints [93], and the degree to which persistent epigenetic chromatin alterations are based [92].
(f) Repetitive elements in formation of sex chromosomes
Separate sexes and sex chromosomes are rare in plants. Even in dioecious plants heterogametic sex chromosomes are not common unlike animals where they are distinctly manifested. The Y chromosome is a unique part of the genome that does not recombine over some or most of its length, and represents male limited transmission [3]. The main stages of Y chromosome evolution are manifested by establishment of the sex determining region, local suppression of recombination, accumulation of repeats, degeneration of genes and shrinkage by deletions [27]. In plants sex chromosomes have been identified in 40 species but heteromorphic sex chromosomes have been detected in just 19 species. Papaya is unique for its trioecious sex chromosomes system with XX female, XY male, and XYh hermaphrodite [59, 128]. Even plants with homomorphic sex chromosomes reveal structural changes in non-recombining regions and accumulation of repeats that are absent in autosomes and have no homology with other plant species. It has been observed that the chloroplast DNA has frequently accumulated on the Y chromosomes in plants, possibly by nuclear insertion colonizing the non-recombined regions. Further, accumulation of satellite DNA that exhibit discrete chromosomal localization has been shown to be accompanying evolution of sex chromosomes in some species with heterochromatic sex chromosomes – most prominent example is of Rumex acetosa where heteromorphic regions are formed by tandem repeats that are clearly distinguishable—see Fig. 5, and further details in Hobza et al. [27].

reproduced from Hobza et al. [27]
Localization of satellites on metaphase chromosomes of Rumex acetosa using FISH. The name of the satellite or number of satellite cluster is inside each figure,
(g) Meiotic behaviour
Where polyploidy is a major source of specialization, the karyotype analysis becomes quite limiting to determine chromosomal changes responsible for evolutionary divergence, although dense genetic maps / chromosome markers such as heterochromatin bands and knobs discernible at pachytene could be of some help. Therefore, study of meiotic behaviour of chromosomes is highly desirable part of karyotype analysis for it is this which discloses the structural homologies whilst the consequences reflect on the relative degree of genic similarities of chromosome sets. It is from these observations that we can be confident of the presence and nature of structural heterozygosities, hybridity and polyploidy.
It goes without saying that examination of hybrid meiosis is of special value in comparing karyotypes of distinct entities for it is only when these are brought together into the same cell that we can assess their structural and genic homologies. But it also becomes apparent that the effectiveness of such an analysis depends very largely on the degree of relatedness of the individuals concerned. Naturally, when they are long separated in evolution, hybridization may not be possible and the benefits of hybrid meiosis are unavailable. Even, where there is no such barrier, mechanisms of change are detectable only when they are in relative terms simple. There is no difficulty in spotting the similarity or differences on account of interchanges or inversions.
(h) DNA content/genome size
Initial surveys conducted on nuclear DNA content in angiosperms revealed the occurrence of DNA variation raging from lC value of 0.16 pg (157 Mb) in Arabidopsis thaliana, 2n = 10 [7] to 63.7 pg in Fritillaria assyriaca, 2n = 24 [6]. The DNA estimates available at that time were mainly based on feulgen microdensitometry. The technology has since been far refined making use of flowcytometry. The C-value data base is now available for ca.10800 species of Angiosperms (https://cvalues.science.kew.org/). An array of genome size diversity with 1C value ranging from 61 Mb/0.0648 pg in the carnivorous plant Genlisea tuberosa (Lentibulariaceae) -to-1,49,000 Mb/152.23 pg in Paris japonica (Melanthiaceae) suggesting over 2400 fold variation across the angiosperms is revealed [77]. In dicots highest estimates for DNA amount of 1C = 102.9 pg is recorded for Viscum album 2n = 20 (Santalaceae) [129], thus depicting 1600 fold variation over Genlisea tuberosa. However, a strong skewed distribution is observed in genome size towards small and very small genomes in Angiosperms. Accordingly, the average ‘holoploid genome size’ i.e. 1C, for the Angiosperms considering the modal value is = 5870 Mb/6.0 pg, and the mean value is 5020 Mb/5.132 pg [75]. Further, in terms of variation in genome size within the genus and family, it is found to be 25 fold in the genus Genlisea i.e. from 0.065 pg in G. tuberosa—to-1.76 pg G. lobata—a record holder genus for the smallest genome size [18], but in the family that sports largest genome size i.e. family Melanthiaceae there is 230 fold variation within the family ranging from 0.66 pg in Schoenocaulon texanum (2n = 2x = 16) to 152.23 pg in Paris japonica (2n = 8x = 40). At the 1Cx (i.e. monoploid 'X'), this drops down to c. 86‐fold in Paris forrestii (2n = 2x = 10) with 1C = 56.59 pg. [76]. Large-scale variations have been reported for species within the same genus: fourfold in Lathyrus [67], fivefold in Papaver [109], sevenfold in Vicia [80], ninefold in Crepis [35], and 25 fold in Genlisea [18]. Perennials have been found to contain more nuclear DNA than annuals suggesting that there occurs reduction in DNA amount with the course of evolutionary specialization. A summarized account of record holders for DNA amounts, as well as chromosome number and size is given here in Table 7 as a ready reckoner.
In terms of molecular organization, the DNA composition of various species could be analyzed by determining the content of unique, moderately repetitive and highly repetitive nucleotide sequences to supplement the karyotypic affinities. Further, autologous DNA: DNA hybridization could decipher the nucleotide homologies in the given species. Data to such effect have been effectively utilized for Lathyrus to elucidate the species affinities in Lathyrus [70] and Scilla [13].
(i) Polyploid complexes
Polyploid evolution has been a source of considerable interest and controversy. Numerous reviews have discussed the aspect of polyploidy as a speciation mechanism [56]. It is estimated that polyploid state is present in upto 80% plants, representing 2–4% of speciation events in flowering plants and 7% in ferns [62, 72]. Polyploidy may originate either through repeated cycles of endoreduplication/unreduced gamete formation, and/or intergenomic hybridization followed by amphidiploidization to ensure stabilization of allopolyploids. As such, there is no direct way to measure the pattern of chromosome change based on karyological data, except to get an idea about the incidence of polyploid series in a given taxa. Therefore, the most accurate way to determine the origin of polyploidy is to analyze related diploids and polyploids, to undertake extensive crosses between potential ancestors, and finally to cross the synthetic and natural ones.
However, the advent of the molecular cytogenetic technique of Genomic in situ hybridization (GISH) that was first demonstrated in synthetic cereal hybrids for identification for parental genomes has made direct insights in our understanding about origin and evolution of polyploid genomes. It is now possible to directly visualize the component genomes in a synthetic or natural polyploid on a microscope slide by GISH techniques [42, 79], see Fig. 4. Application of GISH and chromosome painting has unraveled origin of polyploid genomes and unequivocal identification of genomic ancestry, as well as identification of individual chromosomes using chromosome specific probes [126].
Karyotype diversity and evolutionary trends
Joint studies of chromosome complements and DNA contents have helped our understanding of how increases and decreases in genome size are distributed among chromosomes -see Levin [51]. Observations recorded over a range of species in: Vicia [80], Lathyrus [68, 69], Papaver [49, 109], explicitly suggest that the changes in nuclear DNA amounts within the genera are typically achieved by equal changes (increase / decrease) i.e. small chromosomes acquire or shed same amount of DNA as large chromosomes, with an exception of Aloe (Asphodelaceae) that has a bimodal karyotype (Fig. 4 a) composed of four long and three short chromosomes [51]. Therefore, in order to elucidate significance of karyomorphological changes in speciation, it is important to know whether there are patterns of karyotype evolution within genera. Stebbins [115, 116] provides evidence that there are patterns, although they are not universal. In some genera, increasing specialization has been achieved by reduction in genome size with a shift from perennial to annual growth habit or a shorter growing season, such as: Crepis [35], Artemisia [66], Lathyrus [46, 85], Papaver [49], Asphodelus [14], Allium [71], Helianthus [105], Ranunculus [108], Podolepis [38], Arachis [106]. Conversely, increasing genome size ostensibly has accompanied the shift to the annual habit or shorter growing season in Lolium [86], Anthemis and Anacyclus [66]. In some genera, ecological shifts also have been associated with changes in chromosome number, e.g. the shift from perennial to annual habit in arid land races appears to have been accompanied by reduction in chromosome number in Calotis [111, 112], Podolepis [38], for further details, see Levin [51].
Evolutional conception of Karyotype
The evolutionary trends in the karyotype also involve symmetry. Lewitsky who proposed the karyotype concept noted that chromosomes become more asymmetrical with evolutionary specialization in Helleboreae (Ranunculaceae). Taking cues from Lewitsky’s observations, Stebbins [115] contended that in Asteraceae the development of asymmetric karyotypes is associated with entrance into pioneer habitats and often with a shift from the perennial to annual habit. Accordingly, Stebbins [115] proposed that increasing asymmetry might be achieved through natural selection for an accumulation of adaptive clusters of linked genes on one chromosome arm, which would tend to lengthen that arm at the expense of other. Levin [51] proposed an alternative hypothesis for an increasing asymmetry based on associated decline in genome size. Taking cues from the data of Srivastava and Lavania [109] on DNA content of individual chromosome arms within the karyotype over the range of species in Papaver, Levin [51] has proposed “if same amount of DNA was removed from both chromosome arms, asymmetrical chromosomes would become more asymmetrical. A correlate to this would be that increasing genome size would be accompanied by greater chromosome symmetry”. This is consistent with the observations recorded across the family Liliaceae (sub-groups—Tricyrtis, Lilioideae and Tulipeae) wherein an increase in genome size is accompanied by decreasing size differences between chromosomes of the karyotype i.e. a more symmetrical karyotype [78]. A third pattern of ‘unequal increase’, between longer and shorter chromosome arms leading to an overall increase in karyotype asymmetry with increase in genome size has been noted in species with large chromosome size, e.g. Fritillaria and Tulipeae [78]. Further, pericentric inversions and/or differential translocation of DNA between larger and smaller chromosomes without change in chromosome number, and Robertsonian fissions and fusions accompanied by changes in chromosome number are the other means that could lead to change in karyotypic symmetry without change in genome size—reviewed by Schubert [91]. Such changes serve as valuable resources to elucidate karyo-evolution occurring on account of structural / numerical chromosome variation in certain situations.
As such, no universal patterns could be surmised. Nevertheless, the knowledge of the karyotype could certainly be utilized as an adjunct to solve taxonomic hierarchy. Based on comparison of karyotypes of known relative antiquity as determined from classical taxonomy, a larger scale understanding is developed on evolutional conception of karyotype. In a broad sense this is based on the idea that: (i) symmetrical karyotype is more primitive than asymmetrical ones, (ii) longer chromosomes are primitive than shorter ones, (iii) median centromeres with chromosome arms of equal length are more primitive than chromosome arms of unequal length, (iv) low basic numbers had given rise to higher ones, and the taxa with variable chromosome number are considered young and still in evolutionary flux, (v) species with one NOR site per haploid genome are considered advanced than multiple NOR sites (vi) ancient species had less heterochromatin (repetitive DNA), the primitive species accredited heterochromatin followed by gradual shedding of excess heterochromatin with evolutionary specialization. Further, during the process of shedding of excess heterochromatin, the intercalary heterochromatin is dispensed earlier, followed by telomeric fraction, as surmised by Lavania and Sharma [47] based on the application of cytological data taken in conjunction with other morphological taxonomic parameters, applied over several plant taxa of relatively known antiquity-representative example is shown in the Fig. 3. Accordingly, Lavania and Sharma [47] have postulated that “during the course of eukaryotic evolution, especially plants, ancient species were practically devoid of heterochromatic excess DNA and the mechanism of DNA amplification and replication vis-à-vis geo-climatic conditions brought them accumulation of additional genetic elements. Once the particular taxon has attained more than the optimum amount of repetitive DNA through progressive increase, there is a tendency of gradual shedding of additional heterochromatic segments”.
B. Application of karyological data as an aid to systematic problems:
Tremendous variation in chromosome number found in Angiosperms is said to have contributed remarkable diversity and evolution of the flowering plants [116]. Chromosome form and number characterizing the chromosome complement-the Karyotype, represents the highest level of structural and functional organization of the nuclear genome; and its constancy ensures the transfer of the same genetic material to the next generation [110, 116]. Dobigny et al. [16] have critically analysed the various methods employed in chromosome analysis to score conventional and molecular cytogenetic information and underpinned the significance of chromosome change as a character and its absence or presence as a character state. They emphasized the need to first posit an ancestral karyotype as a common character and then apply its utility to reconstruct phylogeny in a group of species from a cladistics perspective.
Accumulation of large data on chromosome number and morphology has provided the possibility to utilize them as additional tools in taxonomy. Sharma [99] projected the importance of chromosomes in taxonomy, and Cronquist [11] duly recognized the significance of chromosome morphology in solving problems of taxonomic dispute. The basic chromosome number in angiosperms has been suggested as x = 7, which is characteristic of major groups with slight deviation in certain orders, and evolution has mostly been at the diploid level [84, 114]. Liliaceae is a major exception where basic number is suggested as x = 8 [78]. Simultaneously, on the basis of high chromosome numbers in some of the perennial woody species of the Annoniflorae and Hamamelidiflorae, it is claimed that polyploidy was present in some of the ancestral forms of the surviving families, though not so prevalent in the basal order of Dillenidae [82, 84]. Valuable Cytotaxonomic data have been generated on the orders Helobiales, Butomales, Commelinales, Eriocaulales, Bromeliales, Zingiberales, Liliales, Graminales, Orchidales, Palmales, Arales, Cyperales and Juncales, Iridales, Typhales, Amaryllidales, Agavales, from among monocots and Rosales, Leguminales, Araliales, Urticales, Cucurbitales, Malvales, Apocyanales, Rubiales, Verbenales, Laminales, Geraniales, among dicots from India—for detailed account see [39, 102, 103]. Peruzzi et al. [78] have made extensive analysis on chromosome diversity and evolution in Liliaceae from phylogenetic perspective based on extensive literature survey featuring karyo-morphometric parameters across 217 species belonging to all genera in Liliaceae sensu the APG (Angiosperm Phylogeny Group) superimposing the karyological data onto a phylogenetic framework.
As such analysis and interpretation of considerable information available on the chromosome evolution has been presented by various workers against a background of taxonomic classification in which assignment of status of taxa is based on their phylogeny. Examples given below are based on classificatory system popular at that time. Sharma [102] has provided exhaustive analysis on the evolution of monocotyledons in the background of classificatory system given by Hutchinson [28]. Some of the important examples dealing with cytological applications in plant systematics worth mentioning are:
-
(i)
Family Ranunculaceae has been classified into three subfamilies i.e. Helleboreae, Anemoneae and Clematideae by the classical taxonomists, on the basis of occurrence of number of carpels and the number of ovules per carpel. But karyotype studies suggest the separation of genera of this family only in two groups: (a) with small chromosomes and prominent heterochromatic centromeres with x = 6, e.g. Hydrastis, Coptis, Aquilegia of Helleboreae, and Thalictrum and Anemone of Anemoneae, and (b) with large chromosomes having x = 6, 7 or 8, e.g. members of Clematideae, Anemoneae and remaining 9 genera of Helleboreae [100].
-
(ii)
Engler and Prantl considered the three genera Pandanus, Typha and Sparganium constituting the Pandanales, as the ancient progenitors of derived monocots. But Hutchinson separated them into two groups based on aquatic and terrestrial habit comprising Typha and Sparganium in the former and Pandanus in the latter. But karyomorphological evidences do not support such splitting since all the three genera consist of almost similar looking small chromosomes with x = 15 [100].
-
(iii)
Engler and Prantl grouped the primitive orders Alismatales and Butomales under Helloboreae. Although, cytological evidence, especially for the genus Alisma confirms its primitive nature in view of its large chromosomes with symmetrical karyotype and low base number x = 5, with potentiality of giving rise to all the other orders of monocotyledons, but this is not true for all the members of Butomales. For example there are certain genera such as Hydrocharis in Hydrocharitaceae of Butomales showing extreme size differences in the chromosome complement. The size difference is so extreme that it finds parallel only in Agavaceae [96].
-
(iv)
Engler and Prantl included Liliaceae and Amaryllidaceae under an advanced series Lilifloreae. These families were distinguished on the basis of superior (Liliaceae) and inferior (Amaryllidaceae) ovary by them, whereas Hutchinson considered inflorescence as an important distinguishing character thus created a new order Agavales on the basis of occurrence of long spicate inflorescence coupled with arborescent habit out of members of Liliales (e.g. Agave, Yucca, Dracaena and Funkia) and of Amaryllidales (e.g. Polianthes). Hutchinson's contention of Agavales has been supported by cytological data [100]. Since all of them possess extremely bimodal asymmetrical karyotype with x = 30. However, the cytological data suggest the placement of Dracaena in Liliaceae under Ophiopogoneae, for lack of bimodal karyotype.
-
(v)
Ophiopogon is regarded as an intermediate step in the evolution from the Liliales to Juncales by Hutchinson [28], notwithstanding the fact that Juncales, Bromeliales, and Commelinales have been derived directly from the Asphodeloideae by Takhatajan [117]. In Juncus there are different series of chromosome numbers, the predominant ones being 8 and 9, and in Luzula it is x = 3. If Ophiopogon is considered as the progenitor of Juncales, it is difficult to visualize the evolution of the long chromosomes of Luzula with diffuse centromere from the medium sized ones with localized centromere of Ophiopogon. Further studies on chromosome evolution may suggest that the Juncales, Cyperales and Restionales may have a common ancestry as proposed by Cronquist [11]. In Hutchinson’s system Graminales is an offshoot from Juncales with basic chromosome sets of n = 5 and 10.
-
(vi)
Occurrence of primitive form of karyotypes in Ruscaceae has facilitated its differentiation from Asparagaceae and Liliaceae. Ruscaceae differs from Liliacae in having united stamens, with highly specialized morphology. In Asparagus, though root-stock is rhizomatous, vegetative structure is reduced to a spiny cladode. In Ruscaceae too, slightly woody nature is seen and the branchlets are very much modified and flattened. The basic chromosome numbers are also related, being x = 10 in Asparagaceae as 2n = 20 in Ruscaceae. This family has been merged within Liliaceae by Cronquist [11] and in Asparagaceae by Takhtajan [117]. The more primitive karyotypes of Ruscus justify its inclusion in a tribe more primitive than, but allied to the Asparagaceae [102].
-
(vii)
Weins [125] has provided cytological evidence for separation of Viscaceae from Loranthaceae. While Loranthaceae is unified by the existence of single base number x = 9, whereas Viscaceae is characterized by a series of aneuploid numbers ranging from x = 10–14.
-
(viii)
An exhaustive account on chromosome evolution in Liliaceae is provided by Peruzzi et al. [78]. Superimposing karyomorphometric features in a phylogenetic frame work they have reconstructed the most likely patterns of chromosome evolution for the 217 species representing 15 genera in the family Liliaceae. Using 13 different parameters to measure karyotype asymmetry they underscore the significance of change in chromosome form and number in elucidating phylogeny. For further details, see Peruzzi et al. [78].
-
(ix)
Karyotype analysis has shown the role of chromosomal alterations in the evolution of two separate lines within the Commelinaceae, one with x = 6 and long chromosomes, and the other with higher numbers and shorter chromosomes, but Callisia fragrans comprises of an intermediate type with a mix of both long and short [97], suggesting the significance of chromosome analysis in discerning species differentiation.
-
(x)
Presence of chromosome polymorphism i.e. different numbers in the two individuals of the same population, incidence of reverse trend from asymmetry to symmetry in some cases on account of fission of chromosomes followed by fusion, and occurrence of polysomaty in vegetatively propagating plants have opened newer possibilities to understand speciation from cytotaxonomic perspective.
-
(xi)
Various chromosome indices developed over time have facilitated discerning affinities between closely relate species. Lavania and Srivastava [49] have effectively utilized parameter of ‘Chromosome Dispersion Index (DI)’ to quantitatively differentiate closely related karyotypes falling in a given class of asymmetry. Superimposing such data on morpho-phylogenetic framework, they were able to develop a karyotype assisted cladogram in the genus Papaver. This is considered as the first case of its kind on the application of karyotypic data in cladistics.
Concluding remarks
The present authors, long associated with the teaching of graduate students, have often been receiving queries from the students of plant systematics about the description of their findings in the perspective of chromosome analysis from taxonomic and species differentiation angle. Therefore, we teamed-up to address the curiosities of the students and young teachers to provide hands on information and ready reckoner in the area as well as generate interest for the outliers in subject of plant sciences in its general outreach. As such the present article provides an overview of the chromosome change in evolutionary perspective detailing out study tools and state of art in the area.
Some key events that shaped the area of plant chromosome analysis from cytotaxonomic perspective vis-a-vis chromosome change and karyotype differentiation are: (a) formulation of karyotype concept in systematics by Lewitsky, 1931 [57], (b) nomenclature of chromosomes on the basis of centromere position by Levan et al., 1964 [50], (c) concept of karyotype symmetry by Stebbins,1971 [115], (d) chromosome morphology and number in resolving problems of taxonomical disputes, defined as ‘Cytotaxonomy’ by Sharma, 1964 [99], followed by their acceptance in the classificatory system by Cronquist, 1968 [11], (e) importance of chromosome number in evolutionary differentiation of taxa by Raven, 1975 [81] in accordance to the prevailing classificatory system. Further, the two classic books: (i) Chromosomal evolution in higher plants by Stebbins,1971 [115], and (ii) The role of chromosomal change in plant evolution by Levin, 2002 [51], provided a great deal of analytical treatment from systematic and evolutionary perspective. This projected the significance of chromosome change at a very high pedestal from an evolutionary view point; although both the authors had reasonably diverse views in terms of evolutionary patterns. Side by side efforts were made to draw relationship between species and genera based on chromosome form vis-a-vis karyotype symmetry. However, a need was felt to differentiate the closely related karyotypes falling within the same class of asymmetry. To address this a new parameter of ‘Chromosome Dispersion Index' was developed by Lavania and Srivastava, 1992 [48] taking into account the dispersion of centromere within the karyotype using the statistical gradient. Subsequently there was a wave to develop a series of indices to delineate intrachromosomal and bimodal symmetry by various authors. Therefore, a need was felt to deal with all these indices in one place for their possible use by researchers suiting to their specific requirement to deduce karyotype affinities. The science of chromosome identification has much progressed since then providing additional means for linear differentiation of chromosomes far beyond the initial markers of primary and secondary constriction. Intervention of banding techniques, Fluorescence In Situ Hybridization, comparative DNA: DNA hybridization, and lately the DNA systematics have added new dimensions to comparative cytogenetics. An up-to-date account on chromosome number and DNA amount is now available at one place in the form of The Chromosome Counts Database (CCDB: http://ccdb.tau.ac.il/), and The Plant DNA C‐values database: http://cvalues.science.kew.org/.
This article is an attempt to provide an overview of these developments with the hope that readers would find this useful for general and specific understanding of the subject area as well as to help advance their knowledge further by developing over this foundation.
References
Arano H. Cytological studies in subfamily Carduoideae (Compositae) of Japan IX The karyotype analysis and phylogenic consideration of Pertya and Ainsliaea. Bot Mag. 1963;76:32–9.
Astuti G, Roma-Marzio F, Peruzzi L. Traditional karyomorphological studies: can they still provide a solid basis in plant systematics? Fl Medit. 2017;27:91–8. https://doi.org/10.7320/FlMedit27.091.
Bachtrog D. Y-chromosome evolution : emerging insights into process of Y-chromosome degeneration. Nat Rev Genet. 2013;14:113–24.
Báez M, Vaio M, Dreissig S, Schubert V, Houben A, Pedrosa-Harand A. Together but different: the subgenomes of the bimodal Eleutherine karyotypes are differentially organized. Front Plant Sci. 2019;10:1170. https://doi.org/10.3389/fpls.2019.01170PMCID:PMC6791338.
Baldwin BG. Molecular phylogenetics of Calycadenia (Compositae) based on its sequences of nuclear ribosomal DNA : chromosomal and morphological evolution reexamined. Am J Bot. 1993;80:222–38.
Bennett MD, Leitch IJ. Nuclear DNA amounts in angiosperms. Ann Bot. 1995;76:113–76.
Bennett MD, Leitch IJ, Price HJ, Johnston JS. Comparisons with Caenorhabditis (~100 Mb) and Drosophila (~157 Mb) using flow cytometry show genome size in Arabidopsis to be ~157 MB and thus 25% larger than the Arabidopsis genome initiative estimate of ~125 Mb. Ann Bot. 2003;91:547–57.
Carta A, Bedini G, Peruzzi L. A deep dive into the ancestral chromosome number and genome size of flowering plants. New Phytol. 2020;228:1097–106. https://doi.org/10.1111/nph.16668.
Castiglione MR, Cremonini R. (2012) A fascinating island: 2n = 4. Plant Biosystems. 2012;146:711–26. https://doi.org/10.1080/11263504.2012.714806.
Cremonini R. Low chromosome number angiosperms. Caryologia. 2005;58:403–9.
Cronquist A. The evolution and classification of flowering plants. Boston: Houghton Mifflin; 1968. p. 396.
Dehery SK, Panda E, Saha PR, Sinha PR, Das AB. Chromosome diversity and karyotype asymmetry analysis in four cultivated triploid and three diploid wild genotypes of Musa from North-east India. Nucleus. 2020. https://doi.org/10.1007/s13237-020-00334-z.
Deumling B, Greilhuber J. Characterization of heterochromatin in different species of Scilla siberica group (Liliaceae) by in situ hybridization of satellite DNAs and fluorochrome banding. Chromosoma. 1982;84:535–55.
Diaz-Lifante Z. A karyological study of Asphodelus L. (Asphodelaceae) from western Mediterranean. Bot J Linn Soc. 1996;121:285–344.
Dimitrova D, Greilhuber J. Karyotype and DNA content in ten species of Crepis (Asteraceae) distributed in Bulgaria. Bot J Linn Soc. 2000;132:281–97.
Dobigny G, Ducroz JF, Robinson TJ, Volobouev V. Cytogenetics and cladistics. Syst Biol. 2004;53:470–84. https://doi.org/10.1080/10635150490445698.
Escudero M, Wendel JF. The grand sweep of chromosomal evolution in angiosperms. New Phytol. 2020;228:805–8. https://doi.org/10.1111/nph.16802.
Fleischmann A, Michael TP, Rivadavia F, Sousa A, Wang W, Temsch EM, Greilhuber J, Müller KF, Heubl G. Evolution of genome size and chromosome number in the carnivorous plant genus Genlisea (Lentibulariaceae), with a new estimate of the minimum genome size in angiosperms. Ann Bot. 2014;114:1651–63. https://doi.org/10.1093/aob/mcu189.
Fransz PF, Alonso-Blanco C, Liharska TB, Peeters AJM, Zabel P, deJong JH. High resolution physical mapping in Arabidopsis thaliana and tomato by fluorescence in situ hybridization to extended DNA fibers. Plant J. 1996;9:421–30.
Gao J-G, Liu H, Wang N, Yang J, Zhang X-L. Plant extinction excels plant speciation in the Anthropocene. BMC Plant Biol. 2020;20:430. https://doi.org/10.1186/s12870-020-02646-3.
Greilhuber J. Chapter 9. Chromosomal evidence in taxonomy. In: Heywood VH, Moore DM, editors. Current concepts in plant taxonomy. London and Orlando: Academic Press; 1984. p. 157–80.
Greilhuber J, Speta F. C-banded karyotype in the Scilla hohenackeri group, S. persica and Puschkinia (Liliaceae). Pl Syst Evol. 1976;126:149–88.
Guerra M. Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genome Res. 2008;120:339–50. https://doi.org/10.1159/000121083.
Hamal IA, Langer A, Koul AK. Nucleolar organizing regions in the Apiaceae (Umbellifereae). Pl Syst Evol. 1986;154:11–30.
Heslop-Harrison JS. Genome evolution: extinction, continuation or explosion? Curr Opinion Plant Biol. 2012;15:115–21. https://doi.org/10.1016/j.pbi.2012.03.006.
Heslop-Harrison JS, Schwarzacher T. Organisation of the plant genome in chromosomes. Plant J. 2011;66:18–33. https://doi.org/10.1111/j.1365-313X.2011.04544.x.
Hobza R, Cegan R, Jesionek W, Kejnovsky E, Vyskot B, Kubat Z. Impact of repetitive elements on the Y chromosomes formation in plants. Genes. 2017;8:302. https://doi.org/10.3390/genes8110302.
Hutchinson J. The families of flowering plants, vol. 2. Oxford: Clarendon Press; 1959. p. 511.
Huziwara Y. Karyotype analysis in some genera of compositae VIII further studies on the chromosomes of Aster. Amer J Bot. 1962;49:116–9.
John B, Lewis KR. The chromosome complement Protoplasmatologia VI AF1. Wein: Springer-Verlag; 1968.
Johnson MAT, Kenton AY, Bennett MD, Brandham PE. Voanioala gerardii has the highest known chromosome number in the monocotyledons. Genome. 1989;32:328–33.
Jones K. Mechanisms of chromosome change. Nucleus. 1978;21:152–7.
Jones K. Robertsonian fusion and centric fission in karyotype evolution in higher plants. Bot Rev. 1998;64:273–89.
Jones N. New species with B chromosomes discovered since 1980. Nucleus. 2017;60:263–81. https://doi.org/10.1007/s13237-017-0215-6.
Jones RN, Brown LM. Chromosome evolution and DNA variation in Crepis. Heredity. 1976;36:91–104.
Kenton A, Parokonny AS, Gleba YY, Bennett MD. Characterization of the Nicotiana tabacum L. genome by molecular cytogenetics. Mol Gen Genet. 1993;240:159–69.
Khandelwal S. Chromosome evolution in the genus Ophioglossum L. Bot J Linn Soc. 1990;102:205–17. https://doi.org/10.1111/j.1095-8339.1990.tb01876.x.
Konishi N, Watanabe K, Kosunge K. Molecular systematics of Australian Podolepis (Asteraceae : Gnaphalieae): evidence from DNA sequences of the nuclear ITS region and the chloroplast matK gene. Austral Syst Bot. 2000;13:709–27.
Koul AK. Chapter 31. Cytology and cytogenetics of flowering plants. In, BM Johri (ed.) Botany in India – History and Progress. 1995; Vol II : 189-208. Oxford & IBH Publishing Co. Pvt. Ltd., New Delhi.
Lavania S, Lavania UC. Chapter 10 Chromosomes in speciation and implications in plant systematics. In: Chauhan SVS, et al., editors. Plant Reproduction Biology and Biotechnology 2007. Jaipur: Aviskar Publishers; 2007. p. 126–44.
Lavania UC. Differential staining and plant chromosomes–a progress in cytogenetics. Curr Sci. 1978;47:255–60.
Lavania UC. Fluorescence in situ hybridization in genome, chromosome and gene identification in plants. Curr Sci. 1998;74:126–33.
Lavania UC. Chromosome diversity in population: defining conservation units and their micro-identification through genomic in situ painting. Curr Sci. 2002;83:124–7.
Lavania UC. Plant speciation and polyploidy: in habitat divergence and environmental perspective. Nucleus. 2020;63:1–5. https://doi.org/10.1007/s13237-020-00311-6.
Lavania UC, Basu S, Srivastava S, Mukai Y, Lavania S. In situ chromosomal localization of rDNA sites in “Safed Musli” Chlorophytum Ker-Gawl and their measurement by Fiber FISH. J Heredity. 2005;96:155–60.
Lavania UC, Sharma AK. Giemsa C banding in Lathyrus L. Bot Gaz. 1980;199:199–203.
Lavania UC, Sharma AK. Chromosome banding and evolutionary plant cytogenetics. Proc Indian Acad Sci (Pl Sci). 1983;92:51–79.
Lavania UC, Srivastava S. A simple parameter of dispersion index that serves as an adjunct to karyotype asymmetry. J Biosci. 1992;17:179–82.
Lavania UC, Srivastava S. Quantitative delineation of karyotype variation in Papaver as a measure of phylogenetic differentiation and origin. Curr Sci. 1999;77:429–35.
Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:201–20.
Levin DA. The role of chromosomal change in plant evolution. New York: Oxford University Press; 2002.
Levin DA. Plant speciation in the age of climate change. Ann Bot. 2019;124:769–75. https://doi.org/10.1093/aob/mcz108.
Levin DA, SW, Funderburg. Genome size in angiosperms: temperate versus tropical species. Am Nat. 1979;114:784–5.
Lewis WH. Chromosome phylogeny and habitat preference of Clarkia. Evolution. 1953;7:102–9.
Lewis WH. Chromosomal drift, a new phenomenon in plants. Science. 1970;168:1115–6.
Lewis WH, editor. Polyploidy – Biological relevance. Plenum: New York; 1980. https://doi.org/10.1007/978-1-4613-3069-1
Lewitsky GA. The ‘karyotype’ in systematics. Bull Appl Bot Genet Plant Breed. 1931;27:220–40.
Li S-F, Su T, Cheng G-Q, Wang B-X, Li X, Deng C-L, Gao W-J. Chromosome evolution in connection with repetitive sequences and epigenetics in plants. Genes. 2017;8:290. https://doi.org/10.3390/genes8100290.
Liu Z, Moore PH, Ma H, et al. A primitive Y chromosome in papaya marks incipient sex chromosome evolution. Nature. 2004;427:348–52.
Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IJ. The dynamic ups and downs of genome size evolution in Brassicaceae. Mol Biol Evol. 2009;26:85–98.
Lysak MA, Koch MA, Pecinka A, Schubert I. Chromosome triplication found across the tribe Brassicaceae. Genome Res. 2005;15:516–25.
Masterson J. Stomatal size in fossil plants : evidence for polyploidy in majority of angiosperms. Science. 1994;264:421–3.
Mishima M, Ohmido N, Fukai K, Yahara T. Trends in site-number change of rDNA loci during polyploid evolution in Sanguisorba (Rosaceae). Chromosoma. 2002;110:550–8.
Murray AW. How to compact DNA. Science. 1998;282:425–7.
Murray BG, Molloy BPJ, Lange PJ. Chromosome studies in New Zealand species of Brachyscome (Asteraceae). NZ J Bot. 2013;2013(51):139–44. https://doi.org/10.1080/0028825X.2013.778299.
Nagl W. Mitotic cycle time in perennial and annual plants with various amounts of DNA and heterochromatin. Dev Biol. 1974;39:342–6.
Narayan RKJ. Discontinuous DNA variation in the evolution of plant species. The genus Lathyrus Evolution. 1982;36:877–91.
Narayan RKJ. Evolutionary significance of DNA variation in plants. Evol Trends Plants. 1988;2:121–30.
Narayan RKJ, Durrant A. DNA distribution in chromosomes of Lathyrus species. Genetics. 1983;61:47–53.
Narayan RKJ, Rees H. Nuclear DNA variation in Lathyrus. Chromosoma. 1976;54:141–54.
Ohri D, Fritsch RM, Hanelt P. Evolution of genome size in Allium (Alliaceae). Pl Syst Evol. 1998;210:57–86.
Otto SP, Whiton J. Polyploid incidence and evolution. Ann Rev Genet. 2000;34:401–37.
Paszko B. A critical review and a new proposal of karyotype asymmetry indices. Pl Syst Evol. 2006;258:39–48.
Pellicer J, Fay MF, Leitch I. The largest eukaryotic genome of them all? Bot J Linn Soc. 2010;164:10–5. https://doi.org/10.1111/j.1095-8339.2010.01072.x.
Pellicer J, Hidalgo O, Dodsworth S, Leitch IJ. Genome size diversity and its impact on the evolution of land plants. Genes. 2018;9(2):88. https://doi.org/10.3390/genes9020088.
Pellicer J, Kelly LJ, Leitch IJ, Zomlefer WB, Fay MF. Universe of dwarfs and giants: genome size and chromosome evolution in the monocot family Melanthiaceae. New Phytol. 2014;201:1484–97. https://doi.org/10.1111/nph.12617.
Pellicer J, Leitch IJ. The Plant DNA C-values database (release 7.1): an updated online repository of plant genome size data for comparative studies. New Phytol. 2020;226(2):301–5. https://doi.org/10.1111/nph.16261.
Peruzzi L, Leitch IJ, Caparelli KF. Chromosome diversity and evolution in Liliaceae. Ann Bot. 2009;103(3):459–75. https://doi.org/10.1093/aob/mcn230.
Raina SN, Rani V. GISH technology in plant genome research. Methods Cell Sci. 2001;23:83–104.
Raina SN, Rees H. DNA variation between and within chromosome complements of Vicia species. Heredity. 1983;51:335–46.
Raven PH. The basis of angiosperms phylogeny: cytology. Ann Missouri Bot Gard. 1975;62:724–64.
Raven PH, Axelrod DI. Angiosperm biogeography and past continental movements. Ann Missouri Bot Gard. 1974;61:539–673.
Raven PH, Kyhos DW. New evidence concerning the original basic chromosome number in angiosperms. Evolution. 1965;19:244–8.
Raven PH, Kyhos DW, Cave MS. Chromosome numbers and relationships in annoniflorae. Taxon. 1971;20:479–83.
Rees H, Hazarika MH. Chromosome evolution in Lathyrus. Chromosome Today. 1969;2:158–65.
Rees H, Jones GH. Chromosome evolution in Lolium. Heredity. 1967;22:1–18.
Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I. The chromosome counts database (CCDB) – a community resource of plant chromosome numbers. New Phytol. 2015;206:19–26. https://doi.org/10.1111/nph.13191.
Romero-Zarco C. A new method for estimating karyotype asymmetry. Taxon. 1986;35:526–30.
Rothwell NV. Aneuploidy in Claytonia virginica. Am J Bot. 1959;46:353–60.
Schubert I. Alteration of chromosome numbers by generation of minichromosomes – Is there a lower limit of chromosome size for stable segregation? Cytogenet Cell Genet. 2001;93:175–81. https://doi.org/10.1159/000056981.
Schubert I. Chromosome evolution current opinion. Plant Biol. 2007;10:109–15.
Schubert I. Some past developments and open questions in understanding the biology of nucleus. Nucleus. 2017;60:247–9. https://doi.org/10.1007/s13237-017-0217-4.
Schubert I, Lysak MA. Interpretation of karyotype evolution should consider chromosome structural constraints. Trends Genet. 2011;27:207–16.
Schubert I, Oud JL. There is an upper limit of chromosome size for normal development of an organism. Cell. 1997;88:515–20.
Schubert I, Rieger R. A new mechanism for altering chromosome number during karyotype evolution. Theor Appl Genet. 1985;70:213–21.
Sharma A. The chromosomes. New Delhi: Oxford IBH publishers; 1975.
Sharma A. Taxonomy as related to genetic diversity in plants. J Indian Bot Soc. 1990;69:1–3.
Sharma AK. A new concept of speciation in plants. Caryologia. 1956;9:93–130.
Sharma AK. Cytology as an aid in taxonomy. Bull Bot Soc Bengal. 1964;18:1–4.
Sharma AK, Plant cytogenetics. Cell Nucleus. . 2: 263–291. San Francisco: Academic Press Inc.; 1974.
Sharma AK. Change in chromosome concept. Proc Indian Acad Sci. 1978;87:161–90.
Sharma AK. Chapter 7: Chromosome evolution in the monocotyledons – an overview. In: Sharma AK, Sharma A, editors. Chromosomes in evolution of eukaryotic groups, vol. 2. Boca Raton, Florida: CRC Press; 1984. p. 169–87.
Sharma AK, Sharma A. Chapter 9 Trends of chromosome evolution in the plant kingdom. In: Sharma AK, Sharma A, editors. Chromosomes in evolution of eukaryotic groups, vol. 2. Boca Raton, Florida: CRC Press; 1984. p. 227–39.
Sharma PK, Langer A, Koul AK. Studies on nucleolus and nucleolar chromosomes in Angiosperms. XI Plantago L. Ann Bot. 1986;57:145–54.
Sims LE, Price HJ. Nuclear DNA content variation in Helianthus (Asteraceae). Am J Bot. 1985;72:1213–9.
Singh KP, Raina SN, Singh AK. Variation in chromosomal DNA associated with evolution of Arachis species. Genome. 1996;39:890–7.
Singh RJ. Plant Cytogenetics 3. Florida: CRC Press; 2017.
Smith DM, Bennett MD. DNA variation in genus Ranunculus. Heredity. 1975;35:231–9.
Srivastava S, Lavania UC. Evolutionary DNA variation in Papaver L. Genome. 1991;34:763–8.
Stace CA. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon. 2000;49:451–77.
Stace HM. Cytoevolution in the genus Calotis R Br (Compositae : Astereae). Austral J Bot. 1978;26:287–307.
Stace HM. Calotis (Compositae), a Pliocene arid zone genus. In: Barker WR, Greenslade PJM, editors. Evolution of the Flora of Arid Australia. Australia: Peacock Publications; 1982. p. 357–67.
Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950.
Stebbins GL. Chromosome variation and evolution. Science. 1966;152:1463–569.
Stebbins GL. Chromosomal evolution in higher plants. London: Edward Arnold; 1971.
Stebbins GL. Chromosomes, DNA, and plant evolution. Evol Biol. 1976;9:1–34.
Takhtajan A. Flowering plants : origin and dispersal (translated by Jeffrey, C). Washington, DC: Smithsonian Institution Press; 1969. p. 310.
Tanaka N. Chromosomal traits of Chamaelirium luteum (Melanthiaceae) with particular focus on the large heterochromatic centromeres. Taiwania. 2020;65:286–94. https://doi.org/10.6165/tai.2020.65.286.
Tran TD, Šimková H, Schmidt R, Doležel J, Schubert I, Fuchs J. Chromosome identification for the carnivorous plant Genlisea margaretae. Chromosoma. 2017;126:389–97. https://doi.org/10.1007/s00412-016-0599-0.
Venora G, Blangiforti S, Ruffini CM, Pignone D, Lpsavio F, Cremonini R. Chromatin organization and computer aided karyotyping of Triticum durum Desf. cv. Timilia. Caryologia. 2002;55:91–8.
Verma SC. Cytogenetics and evolution. In: Chopra VL, editor. Genetic manipulation for crop improvement. New Delhi: Oxford and IBH; 1985. p. 101–26.
Waldeyer W. Ueber Karyokinese und ihre Beziehungen zu den Befruchtungsvorgängen. Arch Mikr Anat. 1888;32:1–122.
Warwick SI, Al-Shehbaz IA. Brassicaceae: Chromosome number index and database on CD-Rom. Pl Syst Evol. 2006;259:237–48. https://doi.org/10.1007/s00606-006-0421-1.
Watanabe K, Yahara T, Denda T, Kosuge K. Chromosomal evolution in the genus Brachyscome (Asteraceae, Astereae): statistical tests regarding correlation between changes in karyotype and habitat using phylogenetic information. J Plant Res. 1999;112:145–61.
Wiens D. Chromosome numbers in African and Madagascan Loranthaceae and Viscaceae. Bot J Linn Soc. 1975;71:295–310.
Weiss-Schneeweiss H, Schneewiiss GM. Karyotye diversity and evolutionary trends in Angiosperms (2013). In: Leitch IJ et al (eds) Chapter 13 - Plant Genome Diversity. Springer-Verlag Wein. pp 209–230. https://doi.org/10.1007/978-3-7091-1160-4_13
Zacharias H. Key word : Chromosome. Chromosome Res. 2001;9:345–55.
Zhang W, Wang X, Yu Q, Ming R, Jiang J. DNA methylation and heterochromatinization in the male-specific region of the primitive Y chromosome of papaya. Genome Res. 2008;18:1938–43.
Zonneveld BJM. New record holders for maximum genome size in Eudicots and Monocots. J Botany. 2010;2010(527357):4. https://doi.org/10.1155/2010/527357.
Zuo L, Yuan Q. The difference between the heterogeneity of the centromeric index and intrachromosomal asymmetry. Pl Syst Evol. 2011;297:141–5. https://doi.org/10.1007/s00606-011-0528-x.
Acknowledgement
This work is supported by INSA Senior Scientist grant to UCL. Authors are grateful to Noriyuki Tanaka (Hachioji, Tokyo), Ilia Leitch (RBG, Kew), Donald Levin (University of Texas, Austin) and Brian Murray (University of Auckland, New Zealand) for kind permission for the pictures, reused in composing the Figure 2 in this article with due citation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All authors have read the manuscript. Necessary permission has been obtained to reuse the material from other sources citing due reference to the original source. Authors have drawn a part of the text from their own work [40].
Additional information
This article is dedicated to the memory of (late) Prof YS Murty for his inspiring interest to the authors towards plant systematics
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Anita Mukherjee; Reviewers : Anath Bandhu Das, SR Yadav, Veenu Kaul.
Rights and permissions
About this article
Cite this article
Vimala, Y., Lavania, S. & Lavania, U.C. Chromosome change and karyotype differentiation–implications in speciation and plant systematics. Nucleus 64, 33–54 (2021). https://doi.org/10.1007/s13237-020-00343-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-020-00343-y