Abstract
Two asynaptic plants were identified during meiosis from 0.2 % colchicine treated inflorescences for 12 h in Allium cepa. The asynaptic behavior could be noticed in early as well as at late diakinesis / metaphase-I. The chiasma frequency was very low as compared to control. At telophase-I/II, univalents created high abnormality. Unequal distributions, laggards, tripolar and micronuclei were found in asynaptic plants. Pollen fertility was found to be very low (55.1 and 52.6 %). Observations suggest that colchicine treatment impacts the meiosis of Allium cepa, suggesting that chromosomal associations in asynaptic plants are independent of meiotic behavior in the control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the meiotic cycle, homologous chromosome pairing is one of the most important events that start at the early stages of prophase-I and continues until the homologous chromosomes separate, each one moving to its pole at anaphase [6]. Synapsis, the homologous chromosomes pairing, characteristic of normal meiosis, is gene controlled. Mutations of malfunctioning of gene controlling synapsis obviate reduction in the pairing process, whereby absence or failure of synapsis leads to asynapsis [8].
Allium cepa L. is one of the most important vegetable crops worldwide. The predominant basic chromosome number is x = 8. In the present study, Allium cepa L. has been selected for its low chromosome number, relatively large size and susceptibility to cytological manipulations [13]. Various physical and chemical mutagenic agents are used to induce favorable mutations at high frequency in plants [5]. Colchicine (C22H25O6N) is a potent chemical mutagen. It is responsible for induction of polyploidy as well as chromosomal mutants. Colchicine acts by binding to the tubulin dimmers, preventing the formation of microtubules on the spindle fibers during cell division, so that the chromosomes get duplicated during the mitotic process in the absence cell division per se leading to polyploid cell formation. Another work showed that colchicine inhibited formation of synaptonemal complex as well as suppression of chromosomal pairing [2]. During the present investigation, two plants exhibited asynaptic behavior in the colchicine treated inflorescences. The asynaptic behavior was observed during meiosis where univalents could be noted in early as well as at late diakinesis / metaphase-I.
Materials and methods
Young inflorescences were treated with 0.2 % colchicine solution for 6, 12, 18, 24 h using “cotton swab method” [26]. After treatment, inflorescences were thoroughly washed with water. For meiotic studies, young flower buds of appropriate size were collected and fixed in Carnoy’s fluid (3 Absolute Alcohol: 1 Acetic Acid). Anthers were separated, teased in a drop of 2 % iron acetocarmine on a clean slide and squashed under a cover glass. PMCs were analyzed for suitable stages of meiosis. Slides with well spread cells and clear chromosomes were selected for scoring. The pollen fertility was calculated from 2 % iron acetocarmine stained pollen grains. The statistical tool Analysis of Variance was applied to examine the relationship among chromosomal associations [18].
Results
Meiosis in normal plant
Allium cepa L. has chromosome number (n = 8). Meiosis in the normal plant was regular with 8 bivalents (Fig. 1). Chromosomes were equally distributed (8:8) at each pole at anaphase-I. Pollen fertility was 93.2 % (Table 4).
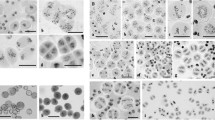
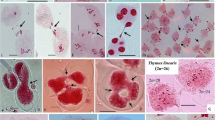
Meiotic division in control and colchicine induced asynaptic plants in Allium cepa L. 1. Meiosis in control. Diakinesis, 8II. 2–12. Meiosis in asynaptic plants.2. 7II + 2I. 3. 6II + 4I. 4. 5II + 6I. 5. 4II + 8I. 6. 3II + 10I. 7. 2II + 12I. 8. 1II + 14I. 9. 16I at diakinesis/metaphase-I. 10. Micronuclei at telophase-I. 11. Tripolar telophase-II. 12. Micronuclei at telophase-II (Scale bar =4 μm)
Colchicine induced asynaptic plants in Allium cepa
Two asynaptic plants (AS1 and AS2) were identified during meiosis from 0.2 % colchicine treated inflorescences for 12 h. The asynaptic behavior was observed in the meiosis where univalents could be noted in early as well as at late diakinesis/metaphase-I. At diakinesis/metaphase-I, 83 % cells were abnormal in AS1 and 86 % cells in AS2. Out of 100 cells analyzed in each plant, 17 were normal, 2 had 7II + 2I, 1 had 6II + 4I, 17 had 5II + 6I, 18 had 4II + 8I, 21 had 3II + 10I, 12 had 2II + 12I, 9 had 1II + 14I and 3 had 16I of chromosomal configurations in AS1. However, AS2 showed 14 normal cells, 3 had 7II + 2I, 2 had 6II + 4I, 23 had 5II + 6I, 25 had 4II + 8I, 10 had 3II + 10I, 14 had 2II + 12I, 8 had 1II + 14I and 1 had 16I of chromosomal configurations (Table 1; Figs. 2–9).
In AS1, the number of chiasmata per cell ranged from 0 to 25, mean number being 7.58, out of which 4.61 were terminalized giving terminalization coefficient of 0.64 and 2.97 chiasmata were unterminalized. In AS2, the number of chiasmata per cell ranged from 0 to 23, mean number being 7.36, out of which 4.59 were terminalized giving terminalization coefficient of 0.60 and 2.77 chiasmata were unterminalized. The chiasma frequency was very low as (0.64, 0.60) compared to control (0.83) (Table 2).
At anaphase/telophase-I/II, univalents created high abnormality. Out of 70 cells analyzed 20 had normal, 5 had unequal distribution, 24 had laggards, 5 had bridges, 4 had tripolars and 12 had micronuclei in AS1 and out of 70 cells, 16 had normal, 13 had unequal distribution, 8 had laggards, 8 had bridges 2 had tripolars and 23 had micronuclei in AS2 (Table 3, Figs. 10–12). Pollen fertility was found to be relatively low (55.1 %) in AS1 and (52.6 %) in AS2 (Table 4). ANOVA was applied among of chromosomal associations in asynaptic plants. The table value for V1 = 8 and V2 = 9 at 5 % level of significance was 3.23 whereas calculated value was 43.05. Results showed highly significant differences (Table 5).
Discussion
In all sexually reproducing organisms, the homologous chromosomes synapse longitudinally during zygotene and pachytene, forming bivalents. The phenomenon of failure of pairing during the prophase-I is referred to as asynapsis or desynapsis. Synaptic mutants have been observed in 126 species belonging to 93 genera [21]. Asynaptic mutants of spontaneous origin have also been isolated from natural populations. Similar mutations may also be induced by mutagenesis [10]. Spontaneous asynaptic mutants have been found in Allium ascalonicum by Darlington and Hague [1]; in Glycine max by Palmer and Kaul [14]; in Secale cereal by Fedotova et al. [3]; in Sorghum vulgare by Stephens and Schertz [23]. Induced asynaptic mutants have been reported in various plant species as in Allium cepa by Konvicka and Gottschalk [11]; in Capsicum annuum by Panda et al. [15], Gulfishan et al. [6]; in Crotalaria juncea by Verma and Raina [25]; in Hordeum vulgare by Singh [20]; in Lathyrus odoratus by Khawaja and Ellis [9]; in Panax sikkimensis by Sharma et al. [19]; in Vicia faba by Sjodin [22], Joshi and Verma [7].
In the present study, two asynaptic plants were identified during meiosis. The synaptic plants showed high frequency of univalents (83 % in AS-1 and 86 % in AS-2) at diakinesis/metaphase-I. Prakken [17] classified three type of asynaptic mutants (weak, medium strong and compalate) according to frequency of univalents at metaphase-I. Weak asynapsis is characterized by a few univalents in some of the cells; medium strong asynapsis by many univalents in most of the cells; and complete asynapsis by univalents only. In the present investigation, the plants were medium strong asynaptic according to Prakken [17].
La Cour and Wells [12] examined suppression of zygotene and pachytene pairing and revealed the absence of synaptonemal complex in synaptic mutants of Triticum durum. Effect of colchicine on meiotic division also had been investigated by several workers as in Secale cereale by Pena et al. [16] and in Rhoeo by Verma et al. [26], Verma and Ahirwar [24]. They observed meiotic division after the colchicine treatment and found failure in chromosome pairing at diakinesis/metaphase-I. In the present study, 2–16 univalents were observed at the diakinesis/metaphase-I of the meiotic division. The results indicate that perhaps colchicine lead to inhibited chromosome pairing. Chiasma frequency in asynaptic plants was very low. The correlations between univalent formation and reduction of chiasmata per cell have been recorded. Sjödin [22] suggested that it is affected by asynaptic genes.
At the molecular level, several genes are responsible for the formation of synaptonemal complex and chromosomes paring. Two groups of genes namely As and Ds genes, control the homologous chromosome pairing and, if present in recessive state, result in failure of pairing. The As genes inhibit the synapsis during zygotene and the condition is known as asynapsis. The Ds genes act on paired chromosomes at diplotene-diakinesis stages, causing a reduction or total absence of chiasma formation leading to desynapsis i.e., formation of univalents [4]. Chromosome disjunction at telophase-I was highly irregular in asynaptic plants. Meiotic division-II also was irregular because cells inherit chromosomal abnormalities from first meiotic division. Asynapsis produces chromosomally unbalanced gametes, resulting in high pollen sterility [21]. In the present investigation, at telophase-I/II, univalents created high abnormality and revealed unequal distributions, laggards and micronucleus. Univalents sometime move to the poles at random without dividing, while in other case, they divide equally.
Univalent formation with high pollen sterility had been reported in Panax sikkimensis by Sharma et al. [19] and in Vicia faba by Joshi and Verma [7]. In the present investigation, pollen fertility was very low in asynaptic plants. Unequal distribution of chromosomes and the subsequent production of micronuclei lead to the formation of aborted pollen grains with varying sizes. ANOVA was applied on various chromosomal associations in colchicine induced asynaptic plants. The calculated value (43.05) was greater than the tabulated value (3.23) at the 5 % level of significance in F test. Therefore, the differences in the mean of chromosomal association of the asynaptic cells are most significant in both asynaptic plants. It is concluded that colchicine treatment effected the meiosis of Allium cepa and chromosomal associations independently in asynaptic plants.
References
Darlington CD, Hague A. The timing of mitosis and meiosis in Allium ascalonicum. A problem of differentiation. Heredity. 1955;9:117–27.
El-Morsy SI, Dorra MDM, Elham AA, El-Hady A, Atef AAH, Ahmed YM. Comparative studies on diploid and tetraploid levels of Nicotiana alata. Acad J Pl Sci. 2009;2:182–8.
Fedotova YS, Bogdanov YF, Gadzhiyeva SA, Sosnikhina SA, Smirnov VG, Mikhailova EI. Meiotic mutants of rye Secale cereale II. The nonhomologous synapsis in desynaptic mutants sy 7 and sy 10. Theor Appl Genet. 1994;88:1029–36.
Gottschalk W, Klein HD. The influence of mutated genes in sporogenesis. A survey on the genetic control of meiosis in Pisum sativum. Theor Appl Genet. 1976;48:23–34.
Goyal S, Khan SA. Comparative study of chromosomal aberrations in Vigna mungo induced by ethyl methane sulphonate and hydrazine hydrate. Thail J Agric Sci. 2009;42:177–82.
Gulfishan M, Jafri IF, Khan AH, Bhat TA. Methyl methane sulphonate induced desynaptic mutants in Capsicum annuum L. Chromosom Bot. 2013;8:59–62.
Joshi P, Verma RC. Ethyl Methane Sulphonate (EMS) induced (Partial) Asynaptic mutant in Faba bean (Vicia faba L.). Cytologia. 2005;70:143–7.
Kaul MLH, Nirmala C. Male sterility in pea II. Male sex specific dys-synapsis. Cytologia. 1993;58:67–76.
Khawaja HII, Ellis JR. Colchicine-induced desynaptic mutations in Lathyrus odoratus L. and L. pratensis L. Genome. 1987;29:859–66.
Koduru PRK, Rao MK. Cytogenetics of synaptic mutants in higher plants. Theor Appl Genet. 1981;59:197–214.
Konvicka O, Gottschalk W. Untersuchngen an der meiosis steriler mutanten von Allium cepa. Angew Botanik. 1974;48:9–19.
LaCour LP, Wells B. Meiotic prophase in anthers of asynaptic Wheat. A light and electron microscopic study. Chromosoma. 1970;29:419–27.
Mahandjiev A, Kosturkova G, Mihov M. Enrichment of Pisum sativum gene resources through combined use of physical and chemical mutagens. Isr J Plant Sci. 2001;49:279–84.
Palmer RG, Kaul MLH. Genetics, cytology and linkage studies of a desynaptic soybean mutant. J Hered. 1983;74:260–4.
Panda RC, Kumar OA, Rao KGR. Desynaptic mutant in chili pepper. J Hered. 1987;78:101–4.
Pena ADL, Puertas MC, Cermeno MC, Girraldez R. Evidence of crossing-over inhibition in Rye anthers culture with colchicine. Chromosoma. 1979;72:151–5.
Prakken R. Studies of asynapsis in Rye. Hereditas. 1943;29:475–95.
Ramakrisnan P. Biostatistics. Nagercoil: Saras Publications; 2005.
Sharma SK, Bisht MS, Pandit MK. Synaptic mutation-driven male sterility in Panax sikkimensis Ban. (Araliaceae) from Eastern Himalaya, India. Pl Syst Evol. 2010;287:29–36.
Singh MR. Cytogenetic studies of induced desynaptic mutants in Barley (Hordeum vulgare L.). Cytologia. 2002;67:129–33.
Singh RJ. Plant cytogenetics. 2nd ed. New York: CRC press Boca Raton London; 2003.
Sjodin J. Induced asynaptic mutants in Vicia faba L. Hereditas. 1970;66:215–32.
Stephens JC, Schertz KF. Asynapsis and its inheritance in Sorghum vulgare. Pers Crop Sci. 1965;5:337–9.
Verma RC, Ahirwar R. Chromosomal behavior in Rhoeo discolor against colchicine treatment. Cytologia. 2012;77:17–21.
Verma RC, Raina SN. Cytogenetics of Crotalaria VI. Chiasma frequency and position, and univalent behavior in a (Partially) asynaptic mutant of C. juncea. Genetica. 1982;58:65–70.
Verma RC, Vyas P, Raina SN. The effect of colchicine on meiosis in the Rhoeo discolor stabile complex translocation heterozygote. Genome. 1992;35:611–3.
Acknowledgments
The authors thankfully acknowledge to Madhya Pradesh Council of Science and Technology, Bhopal for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahirwar, R., Verma, R.C. Colchicine induced asynaptic chromosomal behavior at meiosis in Allium cepa L.. Nucleus 58, 47–51 (2015). https://doi.org/10.1007/s13237-015-0133-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-015-0133-4