Abstract
This is a multidimensional review of mangrove fungi occurring as saprobes, pathogens and endophytes of a wide range of host substrates and those isolated from the water columns and sediments in mangroves. Eight-hundred and fifty taxa including 658 that are supported by both morphology and molecular data and 192 with only morphological data are listed. These constitute Ascomycota, the dominant group with 773 species, and 58 Basidiomycota, one Blastocladiomycota, five Chytridiomycota, and 13 Mucoromycota. This study also includes data on mangrove yeasts 103 Ascomycota, 39 Basidiomycota and 193 taxa isolated from sediments. Endophytes isolated from submerged parts of mangrove plants total 38. The most specious orders of mangrove fungi are Pleosporales 133, Saccharomycetales 102, Microascales 101, Eurotiales 87, Hypocreales 60 and Xylariales 54. Speciose genera include Candida 39, Aspergillus 53, Penicillium 17 and Corollospora 16. The highest number of mangrove fungi have been recorded from the Pacific Ocean 553, which is the largest ocean, followed by Indian 408 and Atlantic Oceans 259. Geographical distribution of mangrove fungi varied from ocean to ocean with only 109 taxa common to the Atlantic, Indian and Pacific Oceans. Of the various countries reported for mangrove fungi, India accommodates the highest number (339) followed by Thailand 303, Malaysia 171, Florida Everglades, USA 134 and Brunei 134. A total of 60 different mangrove plants and their associates have been surveyed for mangrove fungi. These results are discussed and compared with previous studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction to mangroves and mangrove fungi
In this manuscript we use the term ‘mangrove fungi’ to refer to those marine fungi documented from various mangrove subtrates, thus avoiding the need to state that all taxa listed are marine. Approximately 75% of the world’s tropical coastlines between 25° N and 25° S, support a rich diversity of mangrove plants, while other areas having a lower diversity further from the equator, for example the east coasts of Africa, Australia, and New Zealand where mangroves occur 10–15° further south. On the northern side of the equator, mangroves extend to 5–7° which includes the coastlines of Japan, Florida, Bermuda, and the Red Sea. Mangroves in the Indo-West Pacific are the most diverse with more than 30 plant species, while those in Saudi Arabia and Florida comprise one and plant tree species, respectively. Australia and New Zealand comprise six and one plant species, respectively (https://www.floridamuseum.ufl.edu/southflorida/habitats/mangroves/geographical-distribution/).
Mangroves are salt-tolerant evergreen forests found along sheltered coastlines, shallow-water lagoons, estuaries, rivers or deltas in 124–127 tropical and subtropical countries and areas mainly growing on soft muddy substrates (FAO 2007). Mangroves are distributed along the equator encompassing the Atlantic, Indian and Pacific Oceans. Fungi occurring on mangrove forest have been reported since the 1920’s (Stevens 1920), while Cribb and Cribb (1955) studied mangrove fungi on Australian mangrove plants and introduced a number of new taxa. Kohlmeyer (1969) listed 75 mangrove fungi of which 39 were ascomycetes, 27 asexual morphs and nine basidiomycetes, along with their host preferences. Hyde and Jones (1988) reported 90 species of mangrove fungi from the intertidal zone collected from 26 different mangrove trees. These early studies involved the description of new taxa based on morphology with observation on their ecological distribution. Schmit and Shearer (2003) detailed a historical account of the occurrence of mangrove fungi, when they listed 625 species, but many of these included freshwater and terrestrial taxa. Only 287 species could be regarded as growing on submerged to intertidal mangrove substrata (Alias and Jones 2009; Sridhar et al. 2012; Loilong et al. 2012).
Marine fungi have been well-documented, especially over the past 50 years, when numbers ranged from 174 species (Kohlmeyer 1969) to 530 species (Jones et al. 2009) and later to 1112 species (Jones et al. 2015) and currently total 1692 species (Jones et al. 2019, www.marinefungi.org 12/05/2020). A significant number of marine fungi from mangroves account for this increase. Over the past 25 years there has been great activity in documenting mangrove fungi from around the world (Fig. 1), with studies in the Pacific (Jones and Abdel-Wahab 2005; Pang et al. 2011), Indian (Hyde and Jones 1986; Sarma et al. 2001; Sridhar et al. 2012) and Atlantic Oceans (Jones and Puglisi 2006; Fell et al. 2011). Seas sampled for mangrove fungi include the Red Sea (Abdel-Wahab 2005; Abdel-Wahab et al. 2014, 2019a), Arabian Sea (Chinnaraj 1993; Ananda and Sridhar 2004; Maria and Sridhar 2003) and Bay of Bengal (Ravikumar and Vittal 1996; Sarma and Vittal 1998–99, 2000; Devadatha et al. 2017, 2018a, b, 2019). Our understanding of mangrove fungal diversity and discovery rate of new mangrove fungal species are growing rapidly. The current taxonomic approaches include powerful molecular methods to reveal the evolutionary relationships of marine fungi (Hongsanan et al. 2017, 2020a, b). A total of 850 mangrove fungi are included in our list of which 649 are known by both morphology and molecular phylogeny, while the remaining have only morphological information.
Spatafora and Blackwell (1994) were the first to study the phylogeny of the marine fungus, Halosphaeriopsis mediosetigera (Halosphaeriaceae). Subsequently, phylogenetic analyses examined the relationships of a number of marine fungal taxa at the ordinal level and at the genus/species level. The order Halosphaeriales was introduced by Hawksworth and Eriksson (1986) and then accepted by Spatafora et al. (1998), Sakayaroj et al. (2005), Zhang et al. (2006) and Tang et al. (2007). Hibbett et al. (2007) rejected the ordinal position of Halosphaeriales and referred it as a family Halosphaeriaceae in Microascales. Over the past two decades, the application of molecular techniques to study the evolutionary relationship of organisms has advanced considerably with the higher order classification of the group supported by molecular data (Jones et al. 2009, 2015, 2019a, b; Hongsanan et al. 2017, 2020a, b). Hongsanan et al. (2017) updated backbone phylogenetic tree for Sordariomycetes and suggested changes to this class based on divergence times. Hyde et al. (2020) listed 45 orders, 167 families and 1499 genera and provided an updated phylogenetic tree for Sordariomycetes. Liu et al. (2017) updated strategies of using divergence estimates in the classification of Dothideomycetes. Hongsanan et al. (2020a) accepted three orders with 25 families in Dothideomycetidae and four orders with 94 families in Pleosporomycetidae based on a multigene phylogenetic tree. Hongsanan et al. (2020b) listed 31 orders comprising 50 families as orders incertae sedis in Dothideomycetes and 41 families are assigned as families incertae sedis due to lack of morphological and molecular evidence. Chemotaxonomy utilizes secondary metabolite profiling for differentiation among filamentous fungi (Frisvad et al. 2008). Species of filamentous fungi in most genera of ascomycetes and basidiomycetes, produce highly species-specific profiles of secondary metabolites (Frisvad et al. 2008). Chemotaxonomy appears to be a decent source for fungal classification, generally in combination with polyphasic methods and multi-locus phylogeny (Frisvad 2015). Pyrosequencing and metagenomics have added another dimension in the characterization of the marine fungal communities, although much remains to be done in coastal waters and particularly mangrove ecosystems (Richards et al. 2012; Arfi et al. 2012a, b; Amend et al. 2019).
Sampling methods, strategies and limitations
Samples commonly collected for mangrove fungi include: algae, corals, dead animals, driftwood, living and decaying leaves, roots and submerged branches, mangrove wood, soil or sediment and water (Kohlmeyer and Kohlmeyer 1979; Jones et al. 2009). There are limitations to the sampling methods used to document the occurrence and distribution of mangrove fungi. Generally, they are a one-off collection at a location and a limited number of samples taken e.g. Southern China (Vrijmoed et al. 1996). However, a number of locations have been repeatedly studied: Australia (Hyde 1990a; Kohlmeyer and Volkmann-Kohlmeyer 1991), Kampong Danu and Kampong Kapok mangroves, Brunei (Hyde 1990b, 1991), Kuala Selangor and Morib mangroves, Malaysia (Alias and Jones 2000a, Alias et al. 2010), Mai Po, Hong Kong (Vrijmoed et al. 1994a, b; Sadaba et al. 1995), Seychelles (Hyde and Jones 1989), India (Sarma et al. 2001; Devadatha et al. 2017, 2019) and Thailand (Sangtiean et al. 2014; Suetrong et al. 2016), however, in most cases only lignicolous substrates were sampled.
Numerous factors determine the biodiversity of mangrove fungi: sample size; diversity and size of the mangrove forest, availability of decayed wood; nature of the host tissue; time exposed to seawater; tidal amplitude, succession on the substrate; fungal interaction and horizontal or vertical zonation of fungi, physical and chemical parameters such as hydrostatic pressure, light, osmotic effects, oxygen level, pH, pollutants, salinity and temperature (Kohlmeyer and Volkmann-Kohlmeyer 1989; Jones 2000; Schmit and Shearer 2004). Kohlmeyer and Kohlmeyer (1979) recommended to collect the wood samples that have been in a marine habitat for many weeks as indicated by the attack of marine fouling or boring organisms. Jones and Hyde (1988) discussed the disadvantage of random collections, namely only sporulating species can be identified, the method does not give good qualitative data, and host substrate is not known unless subjected to anatomical examination of the host substrate. However, in the case of identifying unknown living substrates, molecular methods are accurate. Most of the studies on mangrove fungi involve the collection of the trapped or decayed wood, all variable in sample number and size (Kohlmeyer and Kohlmeyer 1979; Hyde 1990b; Alias and Jones 2000a). Besitulo et al. (2002) advocated 400 samples for each site to establish the total diversity at that location. Sarma and Hyde (2001) have suggested a protocol for sampling of marine fungi in a mangrove, namely all the key mangrove trees should be sampled by collecting equal number of samples, with collections from three vertical zones, three salinity zones and three seasons resulting in the need to make 270 samples per tree species. They further advised that the minimum samples over a 2-year (seasons) period should be of the order of 1000 to 2000. However, only a few studies on the occurrence of mangrove fungi come any way near to this total number of samples due to the logistics involved.
Mangrove sediments or soil samples are rich in organic matter and are collected with a soil-hole borer from a depth of 2 cm, placed in sterile plastic bags and stored at 4 °C until use (Rai et al. 1969; Nayak et al. 2011). Water specimens under the surface can be collected in sterile plastic bottles at the intertidal zone, at a distance of 1 m from the shore and depth of 0.5 m (Nayak et al. 2011).
Meyers and Reynolds (1958) were the first to advocate baiting techniques to investigate the colonization and succession of mangrove fungi, which was later followed by Jones (1963) and Byrne and Jones (1974). The advantage of baiting includes: sequence of colonization and sporulating stages can be followed; fungi present at specific locations and species or type of substrate can be determined and physical and physiological activities of fungi can be measured. However, Kohlmeyer and Kohlmeyer (1979), Jones and Hyde (1988) and Tan et al. (1989a) noted that fewer species are identified from baits than randomly collected samples. Clearly, a number of sampling strategies are required in the study of the ecology of marine fungi (Amend et al. 2019; Overy et al. 2019).
Studies on frequency of mangrove fungi have differences in the number of samples collected and examined. A study with a larger number of samples results in a distinct percentage of occurrence than a study with a smaller number of samples. Sarma and Hyde (2001) proposed that a minimum 540–1060 samples should be collected for one season and 1000–2000 for 2 seasons (wet and dry). However, the number of samples collected will depend on the accessibility of substrata and other logistics. Different mangrove plant samples should be collected in order to avoid duplication and uniform sample size should be followed. The discovery of new species rate increases with examination of more samples, different substrata collected, mangrove plant species, site with local environmental factors and microcosms (Sarma and Hyde 2001).
Succession studies of mangrove fungi
A number of studies have exposed submerged test panels/blocks of mangrove wood of fixed dimensions in the field and their recovery at regular intervals up to 60 weeks (Tan et al. 1989a; Leong et al. 1991; Sadaba et al. 1995; Alias and Jones 2000a). For example, Leong et al. (1991) exposed wood samples by splitting young Bruguiera cylindrica and Rhizophora apiculata stems (5–7 cm girth, 8 cm length), into quarters so that each sample contained two sides of wood pieces with intact bark or exposed wood on surfaces, at Mandai mangrove, Singapore. Some species were early colonisers (6–18 weeks) e.g. Verruculina enalia (as Didymosphaeria enalia, ≡ Lojkania enalia), Lignincola laevis, Payosphaeria minuta, others appeared in the intermediate phase (22–32 weeks) e.g. Halosarpheia marina, Halorosellinia oceanica (as Hypoxlyon oceanicum), Savoryella paucispora, while others were late colonisers (37–60 weeks) e.g. Nais inornata, Sclerococcum haliotrephum (as Dactylospora haliotrepha) and Aigialus mangrovis on B. cylindrica.
Tan et al. (1989a) also noted that dominant species on two other timbers (Avicennia alba and A. lanata) were V. enalia, Lulworthia sp.1, and Lignincola laevis. The mangrove fungi inhabiting the two timbers exhibited a pattern of succession, with L. laevis as an early colonizer, V. enalia and Lulworthia sp. 1 as intermediate colonizers and A. parvus a late colonizer (Tan et al. 1989a). These studies showed a succession in the sporulation of the fungi with distinct differences between the timbers investigated. Fewer species and a lower occurrence were recorded on the Avicennia timber samples (21 species) with 32 fungi colonising the B. cylindrica and Rh. apiculata timbers. Random collections of 188 drift mangrove wood at the same site yielded 42 species with Natantispora retorquens and Lignincola laevis the most common taxa (Tan et al. 1989a).
Alias and Jones (2000a) conducted a similar study by exposing test blocks (5 × 1 × 1 cm) of Avicennia marina and Bruguiera parviflora at Kuala Selangor mangrove, Malaysia, and their retrieval at intervals of 6–18 weeks. Sixty-one taxa were identified from 486 test blocks. The percentage occurrence of fungi on wood was very high, 50% colonization in the early stage (6–18 weeks) and 100% at the intermediate stage (26–54 weeks) and the late stage (60–96 weeks). The number of fungi per sample was lower at the early stage 1.8–4.2; with 6–8 at the intermediate stage and 4–7 during the late stages. Total number of species on Avicennia marina and Bruguiera parviflora were 45 and 54 species, respectively, with little evidence of host specificity. There was a clear pattern of colonisation on both timbers, with Halosarpheia marina, Lignincola laevis, Natantispora retorquens, Neptunella longirostris and Sammeyersia grandispora as early colonizers. Intermediate colonisers were Halocyphina villosa, Saagaromyces ratnagiriensis, Savoryella lignicola and Verruculina enalia, while late colonisers included Aigialus parvus, Dyfrolomyces marinospora (as Saccardoella marinospora) and Quintaria lignatilis. There were clear differences between the fungi colonising timbers at Kuala Selangor and those on twigs exposed at Mandai (Singapore) mangrove.
Alias and Jones (unpublished data) compared the fungal colonization of Rhizophora apiculata twigs (with and without bark-decorticated) exposed at Kuala Selangor mangrove stand (Malaysia) for up to 92 weeks. The sequence of colonization was divided into three arbitrary stages: early (6–18 weeks of exposure), intermediate (26–54 weeks) and late colonization (60–96 weeks). They showed that the presence of bark on the wood markedly affected the development of fungal communities. Not only were there more fungi present on the barked wood, but percentage colonization was also much higher in contrast with the decorticated wood (Fig. 2). However, there was no evidence that these were restricted to the barked substratum as these fungi have also been collected on other woody tissues. Hyde (1991) also noted that more fungi were present on the bark of R. apiculata poles exposed in Kampong Kapok mangrove, Brunei, while the fungi on Xylocarpus granatum were more numerous on the exposed xylem tissue.
a Species diversity on exposed barked and debarked Rhizophora apiculata twigs ECWB early colonizers with bark; ECWOB early colonizers without bark; ICWB intermediate colonizers with bark; ICWOB intermediate colonizers without bark; LCWB late colonizers with bark: LCWOB late colonizers without bark, b Twigs after exposure showing a loose bark layer surrounding a central xylem core
The healthy growth of mangrove tree seedlings is vital for the regeneration of forests, especially those destroyed by hurricanes, and discarded fish and prawn ponds. Twenty-one fungi are reported from mangrove seedlings (Table 1). However, the most comprehensive study on Rhizophora mangle seedlings is that of Newell (1976) who listed 84 fungi, but not all were identified to species level and many occurred only very infrequently. The three most common fungi were Cladosporium cladosporioides, Pestalotia sp., and Sammeyersia grandispora (Newell 1976). Newell (1976) identified four seral stages in the colonisation of Rhizophora seedlings: Seral stage 1: Phyloplane fungi as on the parent trees; seral stage 2: a decrease in the number of phylloplane fungi and emergence of a number of new species: Septonema sp., Aspergillus repens, Cephalosporium sp., Colletotrichum sp. and a Phoma sp. None of these were present on the pre-abscission seedlings; seral stage 3: Sammeyersia grandispora and Lulwoana uniseptata (as Zalerion maritima) appeared on the seedlings and rose to dominant frequency; seral stage 4: the dominant species in stage 3 showed a marked increase in frequency with the appearance of two new species: Trichoderma viride and Penicillium roseopurpureum. In the initial phases phylloplane and terrestrial asexual fungi were dominant. In the last phases these fungi disappeared and were substituted by facultative and obligate marine fungi (Newell 1976). The percentage of occurrence of sexual and asexual morphs according to the succession stages were not studied yet based on the earlier studies. However, frequent mangrove fungi on different stages of succession of mangrove wood were recorded in various studies. Alias and Jones (2000a) reported the succession patterns of mangrove fungi on Avicennia marina and Bruguiera parviflora wood: early and late colonization of sexual morphs and intermediate colonization of both sexual and asexual morphs. Leong et al. (1991) reported the colonization of sexual morphs in different stages of succession in the decomposition of Avicennia alba wood. Both sexual and asexual morphs are involved in the different stages of succession of Avicennia officinalis wood (Maria and Sridhar 2003). Poonyth et al. (2001) found both sexual and asexual morphs during the various stages of decomposition of Bruguiera gymnorrhiza and Rhizophora mucronata. Sarma and Vittal (2017) have studied the seasonal occurrence of sexual and asexual morphs of marine fungi in Godavari and Krishna mangroves. They found that the percentage occurrence of sexual morphs is higher during dry season and that of asexual morphs in wet season. Fungi colonising various mangrove seeds are illustrated in Fig. 3a–d.
a Prop roots of Rhizophora showing colonisation by Pyrenographa xylographoides and exclusion of other fungi, b, c Rhizophora mucronata seedlings colonised by Halocyphina villosa and Nectria sp., respectively, d Sonneratia sp. pneumatophore colonized by fungi and attacked by marine borers. e, f Sonneratia forest at Morib mangrove, Malaysia with pneumatophores, g, h Rhizophora sp. with prop roots, i, j Acanthus ilicifolius with stems from Mai Po mangrove, Hong Kong, k Fern Acrostichum donaefolium
Vertical and horizontal zonation of mangrove fungi
Another variable to consider when documenting the occurrence of mangrove fungi is whether they are vertically distributed. Alias and Jones (2000b) studied vertical distribution of mangrove fungi on Rhizophora apiculata trees at two mangroves in Malaysia. Prop roots, subterranean roots and overhanging branches of R. apiculata were collected at three intertidal levels: upper (high water mark), middle and lower. There was evidence that fungi were vertically distributed with Pyrenographa xylographoides, Halojullela avicenniae and Aigialus grandis occurring in the upper level and with Sammeyersia grandispora, Hydea pygmea (as Cirrenalia pygmea) and Verruculina enalia more prevalent at the lower level. Some species, such as Leptosphaeria australiensis and Halocyphina villosa occurring at all levels. These differences can be attributed to several factors and the topic requires further study. Sadaba et al. (1995) observed vertical distribution of fungi on Acanthus ilicifolius at Mai Po mangrove, Hong Kong (Fig. 3i, j). The apical portions were colonised by typical terrestrial fungi (Acremonium sp, Colletotrichum gloeosoprioides Corynespora cassiicola, Fusarium sp., Phialophora sp., Tubercularia sp.) and the basal portions by mangrove fungi (Aniptodera chesapeakensis, Halosarphea marina, Halosphaeriopsis mediosetigera, Lignincola laevis, Natantispora retorquens). They found that the asexual morphs (32) are dominant in contrast to sexual morphs (12) (Sadaba et al. 1995). This was attributed to tissue type and varying degree of exposure to tidal inundation governing species distribution along the vertical line. Similar observations have been made for the intertidal estuarine fungi on Phragmites communis, also in Hong Kong (Poon and Hyde 1998). Those occurring in the intertidal level of mangroves can discharge their ascospores forcibly, especially those with bitunicate asci: Halojullela avicenniae, Pyrenographa xylographoides and Verruculina enalia, while those with deliquescing asci discharge their ascospores passively: Antennospora quadricornuta and Torpedospora radiata. Fungi growing above mean tide are exposed to harsher conditions and they are exposed for long periods and subject to desiccation and sunlight, as well as variation in salinity. During dry periods the salinity is higher, but in the monsoon season, mangroves are subject to freshwater.
Hyde et al. (1990) also investigated the vertical distribution of intertidal fungi on Rhizophora apiculata at Ranong mangrove and found Kallichroma tethys, Morosphaeria ramunculicola (= Massarina ramunculicola), Phialophorophoma cf. litoralis and Savoryella longispora as the ‘most common’ species above mean tide, and Phomopsis mangrovei, Sammeyersia grandispora and Verruculina enalia as ‘common’ below mean tide. Twenty-two taxa were found restricted to above mean tide, 13 occurred throughout the tidal range and 3 were confined to below mean tide. The presence of bark was also important in determining the fungal community found on Rhizophora samples: above mean tide, young roots with bark were colonized by Dyfrolomyces mangrovei (= Saccardoella mangrovei), Morosphaeria ramunculicola and Rhizophila marina while below mean tide Sammeyersia grandispora and a Phomopsis species dominated. Hyde (1988) collected intertidal prop roots, subterranean roots and overhanging branches of Rhizophora apiculata and R. mucronata at three levels in Kampong Danau mangrove, Brunei (upper level, mid level and low level). Forty-one taxa were identified with some species present at all levels viz., Halocyphina villosa, Leptosphaeria australiensis and Sammeyersia grandispora. Others appeared to be vertically distributed: upper level Cytospora rhizophorae and Lignincola tropica; mid-level Aigialus parvus, Morosphaeria velatopsora and lower level Antennospora quadricornuta and Trichocladium alopallonella. This distribution pattern was attributed to difference in submergence to seawater and exposure to air, especially at the upper level. A similar study was undertaken at Kampong Kapok mangrove, Brunei at four levels. Vertical zonation was less apparent, but Dictyosporium pelagicum and Halorosellinia oceanica were only recorded at the upper level and A. quadriconuta and Thalassogena sphaerica present only at the lower level.
Sarma and Vittal (2002) investigated the vertical distribution of fungi on the prop roots of Rhizophora apiculata and found that certain fungi have an affinity towards a particular level. Thus Lulworthia sp., Hydea pygmea and Halocyphina villosa had higher percentage occurrence at submerged level, while a Halosarpheia sp., L. australiensis and S. ratnagiriensis in the intertidal region and Epicoccum purpurascens and Trimmatostroma sp. at high tide level were recorded in more numbers at the respective levels. Some fungi had their occurrence throughout the tidal range, for example, Hysterium sp., Sclerococcum haliotrephum, Massarina sp., Periconia prolifica, Phoma sp. and Monodictys pelagica with differences in percentage occurrence. Besitulo et al. (2002) investigating the vertical distribution on Rhizophora spp. found more diversity at the upper tidal level i.e. 25 specie (representing the intertidal level) while only 17 species were found in the lower tidal level (representing the submerged samples). They also investigated the vertical zonation of fungi on Xylocarpon granatum. Samples from the lower tidal level supported a higher number of fungal species (17) when compared to the upper tidal level (8). This is in contrast to Rhizophora sp. where more species were recorded at upper tidal level. Acrocordiopsis patilii and Lignincola tropica were confined to the lower level. Species common to both levels were Coronopapilla mangrovei, Passeriniella savoryellopsis, Phialophorophoma litoralis and Swampomyces sp.
Hyde and Sarma (2006) investigated vertical distribution of fungi on fronds and leaves of Nypa fruticans at three levels: submerged, intertidal and terrestrial. The greatest number of species (32) were recorded in the submerged zone, followed by intertidal zone (25) and terrestrial zone (9).
Spatial variations in the form of horizontal distribution in aquatic ecosystems are rare and often from none mangrove studies (Jones and Oliver 1964; Shearer 1972; Byrne and Jones 1975; Fryar et al. 2004; Tsui and Hyde 2004). Hyde and Lee (1995) opined that very few investigations consider the effect of salinity on the mycota in mangrove forests. In a study of mangrove fungi on decaying wood samples along five sites on the Tutong River and its tributary, the Sungai Kelakas, Fryar et al. (2004) found that species distributions were correlated to the salinity gradient. Seven species viz., Annulatascus velatisporus, Aquaticola longicola, Cancellidium applanatum, Fluviatispora reticulata, Lasiosphaeria sp.1, Sporidesmium cf. anglicum and Sungaiicola brachydesmiella occurred at all sites, 11 species were unique to brackish water sites and 11 to freshwater sites. Hyde and Sarma (2006) reported on the fungi colonizing Nypa fruticans samples in Brunei collected at four different sites including freshwater, brackish water and sea water with mangrove fungi found at all sites. However, many fungi are unique to Nypa and this can be attributed to both the host itself and the often-low salinities where the palm grows (Loilong et al. 2012). Clearly mangrove fungi have wide tolerance to variation in salinity and further experimental studies are warranted.
Seasonal variation of mangrove fungi
Spatio-temporal variations among mangrove fungi has been little investigated and relies on casual observations of collections made at different localities. Aleem (1980) found seasonal periodicity of mangrove fungi in Sierra Leone with more numbers and growth intensity in wet season (May–November). Species such as Haligena viscidula, Leptosphaeria australiensis, L. avicenniae, Halorosellinia oceanica and Torpedospora radiata were more frequently recorded towards the end of rainy season. Besitulo et al. (2002) observed the occurrence of mangrove fungi over a 10-month period covering wet and dry seasons in Siargao Island, Philippines by collecting Nypa fruticans samples at bimonthly intervals. The highest percentage occurrence of fungi was greatest in October than the other months. Most of the frequently occurring fungi e.g. Astrosphaeriella striatispora, Caryosporella rhizophorae, Sclerococcum haliotrephum and Linocarpon appendiculatum were collected in all months.
Sarma and Vittal (1998–1999) studied seasonal occurrence of marine fungi in mangroves of Godavary and Krishna deltas colonizing Rhizophora apiculata and Avicennia spp. by conducting bimonthly samplings for 2 years from January 1994 to November 1995. The east coast of Inda experiences two monsoons: south west monsoon (July to August) and North East monsoon (October to November). The number of marine fungal species dropped during summer (May) but increased to maximum during rainy season (November) in both deltas and on both hosts. The reason could be the extreme environmental factors experienced during summer (May) including very high atmospheric temperatures, surface water temperature and high salinity and lack of rain. On the contrary low temperature, low salinity as a result of heavy rains during November increased the occurrence of mangrove fungi in the November collection. A similar increase in species diversity was also found in July (South west monsoon) but not to the extent of November (North East monsoon). This also shows that most of the fungi colonizing mangrove substrata prefer moderate salinity and temperatures. Some marine mangrove fungi such as Verruculina enalia, Halocryptosphaeria bathurstensis (= Eutypa bathurstensis), Lophiostoma mangrovei, Hypoxylon sp., Rhizophila marina, Saccardoella rhizophorae on Rhizophora apiculata occurred in almost all the samplings indicating that these fungi can tolerate a wide range of temperature and salinity regimes. In contrast, the following fungi: Halorosellinia oceanica, Halocyphina villosa, Lulworthia sp., Sclerococcum haliotrephum showed seasonality in terms of their percentage occurrence. This may suggest that these fungi may not be equally tolerant when compared to the other fungi mentioned above which were recorded in all the samplings with high percentage occurrence. When the seasonal occurrence of sexual and asexual morphs of marine mangrove fungi was observed on Rhizophora apiculata at Godavary and Krishna deltas (Sarma and Vittal 1998–1999), asexual morphs showed more diversity and occurrence during July, November and January months at both deltaic mangroves. On Avicennia spp., however, no definite pattern was observed. Excepting rainfall, no other parameter seems to have a definitive effect on the distribution of sexual and asexual morph occurrence during different months of sampling. Though a few leads are available from this study, the seasonality of marine mangrove fungi requires long term observations involving multiple year examination to get any definitive conclusions on the effect of different environmental factors (Sarma and Vittal 2017).
These studies relied on field collections and documentation of sporulation of fungi on the substrate, ignoring that other taxa may already be present in the host. The subject also lacks critical experimental work by the exposure of substrates and their recovery over dry/wet seasons.
Ecological interactions of mangrove fungi
There has been enormous interest in fungal community structures as such studies help in understanding the ecosystem dynamics, such as mutualism, commensalism and antagonism (Cooke and Rayner 1984), but few examples have been reported for mangrove fungi. Sarma and Raghukumar (2013) found three different fungal assemblages of mangrove fungi viz., commensalistic, mutualistic and antagonistic life styles at Chorao mangroves, Goa, India. Commensalistic assemblages were typified by Aigialus grandis, Morosphaeria ramunculicola and Halocyphina villosa, fungi that occurred both in association with others, as well as singly. Mutualistic association was characterized by Trichocladium achrasporum and Verruculina enalia, fungi that occurred almost always associated with other fungi suggesting a mutualistic occurrence. Rimora mangrovei always occurred singly, suggesting a possible antagonistic life style (Sarma and Raghukumar 2013). In these three life styles, commensalism signifies a tolerance to other speices, while mutualism indicates a dependency on other species in a mutualistic manner to act as a community in breaking down specific parts of the substrate which is then used according to the requirement of each individual species (Weber 2006; Pouska et al. 2013; Sarma and Raghukumar 2013).
Lichens growing on mangrove wood, branches and bark of the mangrove plants are named as manglicolous lichens (Raja et al. 2012). The diversity of lichens in mangroves are few in contrast to the lichens of terrestrial environment, as their growth is halted by high level of salinity and moisture (Logesh et al. 2013). However, these lichens occur in the aerial parts of the mangrove plants and are not intertidal. Some of them may fall on to the ground but they cannot be considered as marine and hence are not included.
Sarma and Hyde (2018) investigated the fungal species consortia on Nypa fruticans substrata and found three different assemblages. Astrosphaeriella striatispora, Linocarpon nypae and Oxydothis nypae were found to be commensalists as they occurred both singly and also in consortia with other fungi. Linocarpon appendiculatum and Linocarpon bipolaris were mutualistic which suggests they may be dependent upon other fungi for their colonization. Anthostomella eructans, Anthostomella sp., and Trichocladium sp. were found occurring singly on N. fruticans thus indicating their antagonistic potential. However, these observations need to be tested experimentally as shown by the studies of Cooke and Rayner (1984) for terrestrial fungi. Studies based on reproductive structures of fungi found on natural samples reveal limited information on causal relationships in their occurrence (Pouska et al. 2013) as the mycelial state of a fungus gives a better understanding of their functional roles (Cooke and Rayner 1984).
Few experimental studies have been undertaken on mangrove fungi to determine the interactions between taxa and their affect in the colonisation of substrates. Tan et al. (1995) investigated the interactions between mangrove fungi under laboratory conditions. Three mangrove fungi, Aigialus parvus, Lignincola laevis and Verruculina enalia, were grown on wood singly or mixed combinations and different effects noted. For example, sporulation of A. parvus was markedly reduced by L. laevis, while it enhanced ascocarp formation in V. enalia. Tam et al. (2003) carried out field experiments by the pre-inoculuation of lignicolous marine fungi into wood test blocks and their immersion in the sea are required to determine interactions in the colonization of woody substrates. Future studies on fungal species consortia or co-occurrence in mangrove fungi should include statistical analyses, in vitro studies, molecular analyses including metagenomics to have a better understanding on the co-occurrence patterns of mangrove fungi. Many factors may influence the fungal assemblages or co-occurrence including the host examined and its chemical composition, availability of moisture, salinity, and others (Jones 2000). An example of antagonistic activity by a mangrove is shown by Pyrenographa xylographoides on the prop roots of Rhizophora sp. (Figure 3a). Earlier studies suggest that the fungicolous species from mangroves are very rare (Sun et al. 2019). Li et al. (2018) introduced a new genus Marinophialophora typified by M. garethjonesii which is associated with Halocyphina villosa on mangrove wood of Avicennia marina and Bruguiera parviflora.
Mangrove fungi have been shown to produce a wide range of secondary metabolites: Xyloketals from mangrove fungus Xylaria sp. (Lin et al. 2001), enalin A and B from Verruculina enalia (Lin et al. 2002a), eniatin G from the mangrove fungus Halosarpheia sp. (Lin et al. 2002b), and Aigialomycins and related polyketide metabolites from Aigialus parvus (Isaka et al. 2009). Mangrove endophytes have also yielded a wide range of bioactive natural products from various chemical classes such as alkaloids, chromones, coumarins, polyketides peptides and terpenes etc. and this subject has been reviewed by few authors (Debbab et al. 2013; Xu 2015; Deshmukh et al. 2018). Deshmukh et al. (2020) listed 106 novel compounds and their anti- intfective acitivity from mangrove endophytic fungi. For example, antibacterial compound pestalotiopisorin B was extracted from endophytic fungus Pestalotiopsis sp., isolated from Rhizophora stylosa (Xu et al. 2019). Deshmukh et al. (2018) reviewed novel anticancer compounds reported from mangrove endophytic fungi. A cytotoxic compound beauvericin as obtained from Fusarium sp., isolated from the bark of Kandelia candel from mangroves of China (Tao et al. 2015).
Worldwide studies of mangrove fungi
In a comparison on the geographical distribution of mangrove fungi, it is pertinent to point out that mangrove plants vary in diversity at each location, with differences in salinity from estuarine brackish water, to sea water and locations with a salinity of 0.5% to 3.8% (e.g. Red Sea, Ulken 1986; El-Sharouny et al. 1998). Consideration must be given to tidal amplitude extant at mangroves under study. In some locations there is little tidal amplitude (e.g. Florida) while at others it is extensive (e.g. Morib, Malaysia).
Around 54 ‘typical mangrove’ plant species and 60 ‘mangroves associates’ occur in the new and old world (Tomlinson 1986). Mangrove plant species diversity at various geographical locations varies and is particularly more diverse in the Pacific Ocean and less common in subtropical countries (Tomlinson 1986; Spalding et al. 2010). Mangroves in Asia have greater species diversity with 30 or more tree species and this will undoubtedly support a wider range of fungal taxa (Spalding et al. 2010). Figures 3 and 4 illustrate typical substrates that have been surveyed for mangrove fungi.
It is 16 years since Schmit and Shearer (2003, 2004) documented the world distribution of mangrove fungi and therefore it is opportune to reassess their observations. For example, a number of country monographs on mangrove fungi have been published: Iraq (Al-Saadoon 2006), Malaysia (Alias and Jones 2009), Taiwan (Pang et al. 2011), Thailand (Sangtiean et al. 2014; Suetrong et al. 2016) and India (Raveendran and Manimohan 2007). Many new geographical studies, including Saudi Arabia (Abdel-Wahab et al. 2014), Guam and Bahamas (Jones and Abdel-Wahab 2005), Everglades, Florida, USA (Fell et al. 2004; Jones and Puglisi 2006) and Australia (Fryar et al. 2019) have been investigated for their mangrove fungi.
Mangrove Basidiomycota are least frequently collected when compared to Ascomycota (Jones et al. 2015). Three phylogenetic lineages of the Basidiomycota have been recognized within the homobasidiomycetes, representing three to four independent transitions from fresh water and terrestrial to marine habitats (Hibbett and Binder 2001; Maekawa et al. 2005a). The mangrove Basidiomycota are represented in two marine clades: The Nia clades includes Calathella mangrovei, Halocyphina villosa and Nia vibrissa (Hibbett and Binder 2001; Binder et al. 2006). More recently, Abdel-Wahab et al. (2019b) in introducing the taxon Nia lenicarpa from Red Sea mangroves in Saudi Arabia, deomstrated that these basidiomycetes group in the family Niaceae (Jülich 1981, Læssøe et al. 2016). The second clade comprises Haloaleurodiscus mangrovei reported from dead trunks and branches of Sonneratia alba in mangrove forests of Japan (Maekawa et al. 2005a, b). Jones et al. (2019) listed 22 filamentous marine basidiomycetes in 17 genera and 80 marine basidiomycete yeasts in 39 genera.
Most of the studies on mangrove fungi have been focused on the filamentous fungi, while the Chytridiomycota and Mucoromycota are poorly studied in mangroves (Jones et al. 2019). Most of the studies on Chytridiomycota in mangroves have been carried out by Ulken (1972, 1978, 1981, 1983). Paludomyces mangrovei was isolated and described from mud collected from mangrove swamps (Ulken 1978). Ulken (1978) studied the growth of the four P. mangrovei isolates in the presence of several physical factors and found that they can tolerate different saline conditions. Fungi present in the water column and sediments, chytrids and yeasts were not included in the papers by Schmidt and Shearer (2003, 2004) and this is addressed in the current study.
Taxonomic classification of mangrove fungi
The aim of the present study is to produce a checklist of mangrove fungi known from different geographic locations, and on different substrates: attached wood, intertidal wood and driftwood, fruits, leaves, seagrasses, ferns, palms as well as those to be found in the water column and sediments (Figs. 3, 4). All taxonomic groups have been included in this review: Ascomycota, Basidiomycota, Chytridiomycota, and Mucoromycota. Fungi isolated as endophytes and mangrove yeasts are covered in this compilation.
The data on mangrove fungi occurring on various mangrove sources were collected from the published literature available through the years 1950 to 2020, and their geographical distribution and hosts were reviewed to provide an accurate check list. This checklist includes different groups of marine fungi reported from various mangrove substrata. Mangrove endophytic fungi isolated from different mangrove plant tissues, roots, stems and leaves in contact with sea water and mangrove fungi isolated from mangrove water and soil or sediments are also considered in this study. Where the asexual/sexual morph connections have been established, we have included the currently accepted name (Réblová et al. 2016).
Table 1 comprises the mangrove fungi reported from various mangroves spread across the world. The taxa recorded so far from different mangroves sources have been arranged alphabetically in four sections: Ascomycota, Basidiomycota, Chytridiomycota and Mucoromycota. Besides, different columns representing details of habitat, fungal nature, substrate, host, and distribution, we have also included adjacent to each species the relevant publication reference. Taxa and their authorities have been established by examining the websites of www.marinefungi.org, MycoBank and Index Fungorum. Their classification follows the current study of Jones et al. (2015, 2019) and for hierarchical placement of each mangrove fungal species into different fungal families is based on recent publications such as Wijayawardene et al. (2017, 2018, 2020), Refined families of Sordariomycetes (Maharachchikumbura et al. 2016; Hyde et al. 2020) and Refined families of Dothideomycetes (Hyde et al. 2013; Hongsanan et al. 2020a, b). All the mangrove fungal taxa from mangroves identified up to species level are listed in Table 1 and those identified up to genus level exempted. The checklist also includes details such as familial placement, habitat, nature of fungi, substrate and host distribution, as well as relevant references. Taxa in bold are those introduced since 2015. The results of the data have been shown in graphs, pie charts, tables to examine and discuss the diversity of mangrove fungi among the different taxonomic groups. Bar chart was generated using GraphPad Prism 5.03 software for Windows, www.graphpad.com. The heat map was generated using the Tableau Desktop 2019.2.
Major Phyla
Schmit and Shearer (2003) recorded 625 mangrove fungi, but only 287 were considered to be found in the intertidal/submerged zone by Lee et al. (2017). Currently we list 850 species in 413 genera which belong to 161 families in 68 orders and 17 classes (Table 1), with many higher order groups recorded for the first time in the Ascomycota, Basidiomycota, Chytridiomycota and Mucoromycota. Mangrove yeasts were not included by Schmit and Shearer (2003, 2004), but we list 142 species in 55 genera that belong to 23 families in 11 orders and 6 classes. These were mainly isolated from the Everglades, USA, various mangroves in Thailand, and China and from other parts of the world (Limtong et al. 2010; Statzell-Tallman et al. 2010; Am-In et al. 2011; Fell et al. 2011; El-Samawaty et al. 2020).
Ascomycota
This is the dominant group of fungi from mangroves accounting for 90.94% (773 species) of the taxa listed (Fig. 5) and this is also the case for marine fungi in general (Jones et al. 2019). Most of the ascomycetes are reported from intertidal or submerged zones. The ascomycetes are widely distributed across the three ocean basins and dominant in contrast to other groups of mangrove fungi. The results of the collected data showed that main classes, orders, families and genera belong to this phylum. Among ascomycetes the major classes include Sordariomycetes, Dothideomycetes, Saccharomycetes and Eurotiomycetes. Major orders were Pleosporales, Microascales, Saccharomycetales, Eurotiales, Hypocreales and Xylariales.
Basidiomycota
Few mangrove fungi are basidiomycetes (6.82%) (58 species) and this also reflects the number of known marine fungi (Jones et al. 2019).
Blastocladiomycota
Only one species has been reported so far from this phylum with lowest number 0.12% (Fig. 5) of reported mangrove fungi.
Chytridiomycota
The Chytridiomycota are the most primitive true marine fungi often referred to as “the basal fungi”. They are characterized by uniflagellate zoospores that necessitate water for dispersal and account for only 0.59% (5 species) (Fig. 5) of mangrove fungi.
Mucoromycota
This group accounts only for 1.53% (13 species) of the total mangrove taxa listed in Fig. 5.
Major Classes
Mangrove fungi can be referred to 17 different classes (Fig. 6). The class with the largest number of species is the Sordariomycetes accommodating 39.65% of the mangrove fungi. The Dothideomycetes are the second largest class with 23.65% of the species followed by Saccharomycetes (12%), Eurotiomycetes (10.8%) and others. Ascomycetes with least mangrove species include: Orbiliomycetes (1.5%), Leotiomycetes (1.06%), Arthroniomycetes (0.1%) and Schizosaccharomycetes (0.1%). Some 2% of the mangrove fungi were not assigned to any of the above classes (Jones et al. 2019).
The Basidiomycota are fewer in number (6.8%) and have representative taxa in the classes, Tremellomycetes (2.1%), Agaricomycetes (2.2%), Microbotryomycetes (1.2%), Cystobasidiomycetes (0.9%) and Ustilaginomycetes (0.4%).
The Chytridiomycota includes only the Chytridiomycetes (0.4%) and Blastocladiomycetes (0.1%) in the Blastocladiomycota with a low diversity. Two classes are represented in the Mucoromycota: Mucoromycetes (1.5%) and Rhizophydiomycetes (0.2%) also with low diversity. This may be attributed to the substrates collected and the methods required for their enumeration. The Eurotiomycetes includes the most speciose genera Aspergillus and Penicillium with relatively higher diversity recorded from mangrove soils or sediments.
Major Orders
Mangrove fungi are diverse and distributed among 68 different fungal orders with Pleosporales being the largest order accommodating 133 species followed by Saccharomycetales (102), Microascales (101), Eurotiales (87), Hypocreales (60) and Xylariales (54) (Fig. 7).
Major Families
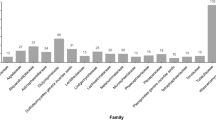
Mangrove fungi recorded on different mangrove substrata were distributed across161 families. The Halosphaeriaceae (94) is the largest family accommodating the highest number of taxa followed by Aspergillaceae (72), Debaryomycetaceae (52) and Pleosporaceae (24) (Fig. 8).
Most speciose genera among different classes of mangrove fungi
The most speciose genera from each class of mangrove fungi are listed in Table 2. Among the different classes of mangrove fungi, Aspergillus is the most speciose genus accommodating 53 species followed by Aniptodera, Arthrobotrys, Candida, Corollospora, Penicillium and Trichoderma. The genera Aspergillus and Penicillium are often referred to as marine derived fungi which are frequently isolated as endophytes and from mangrove soils and the water column.
Nature of mangrove fungi
Most fungi recorded from mangroves are saprobic with very few reports of parasitic or endophytic mangrove fungi in contact with sea water (Table 3).
Distribution patterns of mangrove fungi by habitat
Most of the fungi reported from mangrove forests belong to the intertidal parts of mangrove substrata. Very few mangrove fungi are reported from totally submerged mangrove substrata (Table 4).
Distribution of mangrove fungi by oceans
Geographically a larger number of mangrove fungi were recorded from the Pacific Ocean (553) which is known as the largest ocean, followed by Indian (408) and Atlantic Oceans (259) (Fig. 9). Among the 850 mangrove fungi recorded from the three different oceans, 302 mangrove fungi are exclusively reported from Pacific, 206 from Indian and 81 from Atlantic Oceans, with 109 common to Atlantic, Indian and Pacific Oceans (Fig. 9). Ten mangrove fungi overlapped between Atlantic and Indian Oceans. Likewise, 83 mangrove fungi were common to both Indian and Pacific Oceans. While 59 mangrove fungi were found to be common between Atlantic and Pacific Oceans (Fig. 9). Among the countries with reports of mangrove fungi, India accommodates the highest number of mangrove fungi (339) followed by Thailand (303), Malaysia (171), Florida (134) and Brunei (134).
A total of 259 mangrove fungi were recovered from the Atlantic Ocean mangroves, of which 81 fungi are restricted to Atlantic Ocean, with Florida state (USA) accounting for 134 species followed by Brazil (73) and Cuba (60). Mangrove studies in the following countries yielded fewer taxa: Belize (48), Bahamas (45) and East Mexico (40) (Fig. 10).
The Indian Ocean accounted for 408 mangrove fungi with 206 fungi are specific to the Indian Ocean. Indian Ocean mangrove forests include those of India and Bangladesh and is the largest in the world (Fig. 11). About 83% of the mangrove fungi recorded for the Indian Ocean were reported from India (339 species). Fewer mangrove fungi were reported from Seychelles (68), Mauritius (49) and Egypt (43). India, with a vast coastline covering west and east coasts is covered by extensive mangroves in various states and most of the studies were conducted on decaying mangrove woody substrata and leaf litter.
The highest number of mangrove fungi is documented for the Pacific Ocean (553) and can be attributed to the intense number of studies carried out in Thailand and Malaysia. Numbers of fungi listed for the major countries are: Thailand (303), Malaysia (171), China (150), Brunei (134), and Hawaii (107) (Fig. 12).
The Pacific Ocean supports the greatest number and diversity of mangrove fungi with 553 species while the recorded number for the Atlantic Ocean is 259 taxa which mirrors the findings of Schmit and Shearer (2003). Although a wider range of substrates have been sampled (sediment/mud, water) and new locations surveyed since 2003 (Bahamas, Florida, and temperate mangroves of Egypt, Saudi Arabia) with an increase in the number of species listed, the total remains lower than for the Pacific and Indian Oceans. Intensive surveys conducted in Brunei, India and Thailand may well account for the greater number of fungi documented for theses geographical locations. Various reasons have been advanced to account for the differences observed including: sampling bias, some of the fungi described from the Pacific Ocean have only recently been described and reported only once, while the Atlantic Ocean has fewer host plants (Tomlinson 1986; Schmit and Shearer 2004).
Eighty-one fungi listed in Table 1 appear to be unique to the Atlantic Ocean, many only recently described and with no corresponding studies in the Indian/Pacific Oceans e.g. the yeasts Kwoniella mangrovensis, Papiliotrema mangalensis, Rhodotorula evergladensis, Sakaguchia cladiensis and others. Sixty-nine of those listed are unique to the Pacific Ocean and include: Fulvifomes siamensis, Haloaleurodiscus mangrovei, Lasiodiplodia avicenniarum, Lautospora simillima and Naganishia albida to mention but a few. Recently described fungi from the Indian Ocean include Phaeoseptum manglicola, Peroneutypa mangrovei, Pontoporeia mangrovei, Pseudoastrosphaeriellopsis kaveriana and others. Further studies are required to determine if these are unique to the different ocean basins. Clearly, the greater diversity of mangrove trees in the Indian and Pacific Oceans may account for a rich mangrove fungal occurrence, as well they have been surveyed extensively for mangrove fungi.
Distribution patterns of mangrove fungi by substrate types
Figure 13 lists the substrates from which mangrove fungi have been reported and it shows that dead and decomposing wood supports the greatest diversity with 395 species, while fungi isolated from soils and sediments numbered 193. Mangrove palms were rich in fungi (95 species) the majority being Ascomycota and yielded a wide range of new taxa. Yeasts isolated from mangrove water column yielded 96 species, with senescent and decaying leaves of various plants totalled 81 taxa. Thirty-eight endophytes were isolated from living leaves of seagrasses and the roots of various tree species. Substrates supporting lower numbers of fungi include seedlings (21), fruit (5), bark (3), foam (4), and dead grasses (3) while 43 mangrove fungi were isolated from various animal hosts such as corals, molluscs, endoskeleton of Sebia sp., and feathers (Amend et al. 2012).
Considerable strides have been made in documenting the occurrence of marine yeasts in the Everglades, USA and mangroves in Thailand (Am-In et al. 2011; Fell et al. 2011), and fungi isolated from mangrove sediments (Garg 1983). Other new substrates explored for mangrove fungi are endophytes of seagrasses, and roots of mangrove trees (Sakayaroj et al. 2012). Most of the taxa listed (Table 1) are saprobic (803) on various substrates with a greatest number from woody tissue. This is hardly surprising as this is the most abundant material found in mangroves, and it is easy to document the fungi present. Ligninolytic fungi possess powerful enzymes and play a major role in the decomposition of woody tissue, in particular in mangroves (Sridhar 2012; Velmurugan and Lee 2012). Their activity leads to breakdown of complex lignocellulose and fragmentation of the substrate (Pointing et al. 1998, 1999; Bucher et al. 2004; Sridhar 2012; Jones et al. 2019).
Taxonomically the Ascomycota dominate the list of fungi found on mangrove substrates, although a number of Basidiomycota are also quite common: Calathella mangrovei, Halocyphina villosa and Nia vibrissa, with Haloaleurodiscus mangrovei less common (Alias et al. 2010; Maekawa et al. 2005a). Three bracket forming Fulvifomes basidiomycete species (F. halophilus, F. siamensis and F. xylocarpicola) have been shown to be aggressive in causing butt rot of the mangrove tree Xylocarpus granatum (Fig. 14) (Sakayaroj et al. 2012; Hattori et al. 2014). A significant addition to mangrove basidiomycetes has been the isolation of yeasts (39 species) from the water column e.g. Kwoniella mangrovei, Occultifur externus, Papiliotrema laurentii and Pseudozyma hubeiensis.
a Seagrass and algae amongst pneumatophores of Avicennia and Sonneratia spp., b Juncus kraussii in the intertidal zone of an Australian mangrove, c, e leaf litter, seagrass and Avicennia fruits, d Corollospora maritima colonizing mangrove sediments, f Fragmented leaf litter at Morib mangrove, Malaysia. Gareth’s photos except d which belongs to Deva
Other woody substrates found in mangroves are the prop roots and pneumatophores of trees and these are very often colonized by specific fungi. Okeanomyces cucullatus and Mycosphaerella pneumatophorae are often found colonizing the pneumatophores of Sonneratia alba and Avicennia species, respectively. Prop roots of Rhizophora apiculata are also colonised by fungi such as Halocyphina villosa, Halorosellinia oceanica, Halojullela avicenniae and Pyrenographa xylographoides (Alias and Jones 2000b) (Fig. 3a).
Over the past 10 years many new taxa have been described from mangrove wood and studied intensively at the molecular level with the new genera and species: Acuminatispora palmarum (Zhang et al. 2018), Amarenographium solium (Hodhod et al. 2012), Amphisphaeria mangrovei (Phookamsak et al. 2019), Annabella (Fryar et al. 2019), Bacusphaeria (Abdel-Wahab et al. 2017), Diatrypasimilis australiensis (Chalkley et al. 2010), Dyfrolomyces tiomanensis (Pang et al. 2013), Farasanispora (Li et al. 2016), Halocryptosphaeria, Halotestudina and Halodiatrype (Dayarathne et al. 2016, 2020a, b), Moleospora (Abdel-Wahab et al. 2010), Kallichroma asperum and K. ellipsoideum (Abdel-Wahab et al. 2016), Nia lenicarpa (Abdel-Wahab et al. 2019b), Phaeoseptum manglicola (Dayarathne et al. 2020b), Pseudoastrosphaeriellopsis (Phookamsak et al. 2019), Raghukumaria (Jones et al. 2019b), Saagaromyces mangrovei (Liu et al. 2015), Striatiguttula (Zhang et al. 2019), Tirisporella (Jones et al. 1996), Thyridariella, Vittaliana (Devadatha et al. 2018a, 2019) and many others, see www.marinefungi.org. All fungi introduced since 2015 are in bold in Table 1 and a selection are illustrated in Figs. 17, 18, 19 and 20. This surge in the discovery of new Ascomycota can be attributed to the intensive studies on mangrove fungi in India, Saudi Arabia and Thailand (Ariyawansa et al. 2015; Liu et al. 2015; Li et al. 2016; Abdel-Wahab et al. 2016, 2017, 2019a, b; Devadatha et al. 2017, 2018a, b, 2019; Dayarathne et al. 2019a, b, 2020a, b; Zhang et al. 2019).
Fungi found from Nypa fruticans and Phoenix paludosa in Thai mangroves. a, b Acuminatispora palmarum (found from both hosts). c–e Longicorpus striataspora (found from both hosts). f, g Kirschsteiniothelia phoenicis. h, i Melanographium phoenicis. j, k Oxydothis phoenicis. l, m Salsuginea phoenicis. n, o Savoryella nypae. p, r Striatiguttula nypae. s, t Striatiguttula phoenicis. u, v Tirisporella beccariana (seems only found on the submerged petiole of Nypa fruticans). w, x Vaginatispora palmae (found from immersed rachides of N. fruticans). y, z Yunnanomyces phoenicis. Substrates: h, i, j, k were found from fallen rachides and petioles; others were found from rachides or petioles that immersed in mangrove mud and water. Scale bars: j = 1 mm, c, u = 500 μm, l, n, w = 200 μm, a, f, h, p, s, y = 100 μm, q = 50 μm, i, k, m, v, w = 20 μm, b, d, e, g, o, r, t, x, z = 10 μm
Mangrove Dothideomycetes found in Muthupet mangroves, India. a, b Thyridariella mangrovei. c, d Thyridariella mahakoshae. e, f Phaeoseptum manglicola (found from both A. marina and S. monoica). g, h Phaeoseptum carolshearerianum (found from A. marina) i, j Deniquelata vittalii (found from S. monoica). k, l Verruconis mangrovei (found from both Excoecaria agallocha and Aegiceras corniculatum). m, n Raghukumaria keshaphalae. (found from Ae. corniculatum) Substrates: a, c, e, g, i, k, m found from dead and decaying mangrove wood and stems. Scale bars: a, c, e, g, i, m = 100 μm, b, d, f, h, j, k, l, n = 10 μm
Mangrove Dothideomycetes found in Muthupet, Parangipettai and Pondicherry mangroves of India. a, b Vittaliana mangrovei (found from A. marina). c, d Pseudoastrosphaeriellopsis kaveriana (found from both A. marina and S. monoica). e, f Vaginatispora microarmatispora. (found from Aegiceras corniculatum) g, h Pontoporeia mangrovei (found from both A. marina and S. monoica) i, j Neodevriesia manglicola. k, l Morosphaeria muthupetensis (found from Rhizophora mucronata). m, n Nigrograna samueliana (found from A. marina) Substrates: a, c, e, g, i, k, m found from dead and decaying mangrove wood and stems. Scale bars: a, c, e, g, i, k, m = 100 μm, b, d, f, h, j, l, n = 10 μm
Mangrove Sordariomycetes found in Muthupet and Pondicherry mangroves of India. a, b Amphisphaeria mangrovei. c, d Lanspora cylindrospora (found from Suaeda monoica). e, f Cryptosphaeria avicenniae. g, h Fusicolla bharatavarshae (found from Avicennia marina) i, j Zopfiella indica (found from intertidal mangrove wood). k, l Peroneutypa polysporae (found from Suaeda monoica). m, n Hypoxylon teeravasati (found from both A. marina and S. monoica) o, p Peroneutypa mangrovei (found from A. marina) q–t P. indica (found from S. monoica). Substrates: a, c, e, g, i, k, m, o, q were found from dead and decaying mangrove wood and stems. Scale bars: m, q = 200 μm, a, c, e, g, i, k, o, q = 100 μm, b, f, h, j, n, s = 10 μm, d, l, p, t = 5 μm
Mangrove fungi reported from mangrove soils and sediments total 193, the second largest group of taxa after those on woody substrates. Most of the fungi reported from soil or sediments comprises asexual fungi e.g. Acremonium species, Acrophialophora fusispora, Penicillium vermiculatum, and Scopulariopsis silvatica (Lee and Baker 1973; Garg 1983). However, soil fungi in mangroves are understudied and further investigations are warranted. As these fungi have been isolated on various media by sampling mangrove sediments, it is difficult to determine if they are active in the mangrove ecosystem, or just present as spores. Figure 15 illustrates different stages in substrate deposition and decomposition and its fragmentaion prior to exportation to the open ocean.
Many mangrove trees (e.g. Rhizophora harrisonii) have never been investigated for mangrove fungi and likewise a plethora of saltmarsh plants that form part of the mangrove ecosystem (Sainty et al. 2012). Alva et al. (2002) and Sakayaroj et al. (2010) investigated endophytic fungi of seagrasses in the Philippines and Thailand, but we know little on those in other countries and continents. Sainty et al. (2012) lists no fewer than 20 seagrasses for Australia, but fungi growing as saprobes or endophytes are documented for only the Philippines, Thailand and USA, with no data for Australia (Alva et al. 2002; Sakayaroj et al. 2012). Other mangrove substrata rarely surveyed are mangrove seaweeds with some 23 species listed for Australian mangroves (Sainty et al. 2012) and others in Malaysia and Thailand (Fig. 15). Mangroves are also rich in vegetation other than the classic trees but few have been examined for fungi e.g. Acanthus ilicifolius (Sadaba et al. 1995), Suaeda maritima and Salicornia species (Furtado et al. 2019). Recently, fungi on Suaeda monoica and Phoenix paludosa have been surveyed for mangrove fungi and many new species have been described (Devadatha et al. 2018a, b; Zhang et al. 2019) (Figs. 17, 18, 19, 20). Many low and high salt marsh plants also form part of the mangrove ecosystem such as Juncus kraussii in Australia (Fig. 15) and remain to be surveyed for fungi (Sainty et al. 2012).
One of the major factors in determing the frequency of occurrence of mangrove fungi is substrate preference. Sarma and Vittal (2000) investigated diversity of mangrove fungi on different substrata: Rhizophora apiculata and Avicennia species (Avicennia officinalis, A. marina) from Godavari and Krishna river deltaic mangroves, East coast of India. Rhizophora apiculata prop roots yielded the highest number of fungi (61 species from 2524 samples) when compared to wood (24 species from 192 samples) and seedlings (21 species from 273 samples). The lower number of fungi recorded on seedlings and wood of R. apiculata may be attributed to the availability of these substrata. Ravikumar and Vittal (1996) also reported higher species numbers on prop roots of R. apiculata from Pichavaram mangroves, Tamil Nadu, East coast of India. Alias and Jones (2009) opined that the amount of substratum available for colonization is the overriding factor in determining fungal diversity. Fungi recorded with higher percentage occurrence on R. apiculata wood were Verruculina enalia, Sclerococcum haliotrephum, Hysterium sp., Rimora mangrovei and Epicoccum purpurascens. On prop roots, Verrucullina enalia, Rhizophila marina, Hydea pygmea, Halodiatrype mangrovei and Sclerococcum haliotrephum were the fungi recorded with a high percentage occurrence. On seedlings, Verrucullina enalia, Phomopsis mangrovei, Dyfrolomyces rhizophorae and Rimora mangrovei had highest percentage occurrence. In the case of Avicennia species three substrata were studied viz., wood, roots and pneumatophores. Of these substrates, wood (61 species from 1775 samples) had highest diversity when compared to roots (17 species from 118 samples) or pneumatophores (14 species from 111 samples). Fungi with highest percentage occurrence on wood were Halocryptosphaeria bathurstensis, Verruculina enalia, Rimora mangrovei, Halocyphina villosa, Lulworthia sp.; on roots Verruculina enalia, Lulworthia sp., Rimora mangrovei, Hypoxylon sp., Halorosellinia oceanica and Halocyphina villosa; on pneumatophores, Verruculina enalia, Camarosporium roumeguerii, Lulworthia sp., Leptosphaeria australiensis, and Bathyascus avicenniae. Thus, it may be concluded that fungi colonizing the host plant may show a preference for a particular part of a mangrove tree as in this case or for Rhizophora species as mentioned above (Ravikumar and Vittal 1996; Sarma and Vittal 2000).
There is little evidence of strict host specificity in mangrove fungi as most species have been collected and reported on multiple hosts. Loilong et al. (2012) list 77 fungi growing on the brackish water palm Nypa fruticans of which 31 are host specific and not reported from other mangrove plants. These fungi include: Anthostomella nypicola, Arecophila nypae, Carinispora nypae, Helicascus nypae, Tirisporella beccariana, Plectophomella nypae and Pleurophomopsis nypae (Loilong et al. 2012; Jones et al. 2019).
Richness of mangrove fungi among different mangrove plant hosts
Several mangrove trees and associates, salt marsh plants inhabiting mangroves, distributed across the different countries of the world have been examined for mangrove fungi. Among them Avicennia marina accommodated highest number of taxa (142), followed by Rhizophora mangle (125), R. apiculata (109) (Fig. 16). Nypa fruticans (91) hosts highest taxa among the mangrove palms (Fig. 16).
Mangrove yeasts
Ninety-six yeasts have been isolated from water samples taken in mangroves, primarily in the Everglades, USA (Statzell-Tallman et al. 2010; Fell et al. 2011), and Thailand (Limtong et al. 2010; Am-In et al. 2011). These studies considerably enhance our knowledge of aquatic yeasts and their potential role in the mangrove ecosystem. Many of these taxa were new to science e.g. Candida laemsonensis, Kluyveromyces siamensis, Kwoniella mangrovensis and Saturnispora mangrovi (Statzell-Tallman et al. 2008; Am-In et al. 2011; El-Samawaty et al. 2019). Some yeasts were isolated at multiple times in the mangrove section of the Everglades e.g. Candida thimueangensis and Kwoniella mangroviensis, while others were restricted to the freshwater sector e.g. Kregervanrija fluxuum and Sporobolomyces beijingensis (Fell et al. 2011). Although a wide range of yeasts have been isolated from mangroves and other marine locations, their role remains speculative. The study by Fell et al. (2011) indicates what little is known about aquatic yeast ecology and highlights the need for further studies to determine the role of this unique group of fungi play in mangrove ecosystems.
Mangrove fungi on palms
Another substrate that supports a rich diversity of fungi are mangrove palms: Phoenix paludosa and Oncosperma tigillarium found in the upper reaches of mangroves, and Nypa fruticans common in estuaries and shallow lagoons in the Indian and Pacific Oceans (Tomlinson 1986). A total of 95 mangrove fungi have been recorded on the fronds, petioles and fruits of palms (Table 1) with Loilong et al. (2012) listing 77 on the brackish water palm Nypa fruticans. Many of the fungi colonizing N. fruticans are unique to this host, circa 31, e.g. Anthostomella nypae, Fasciatispora nypae, Helicascus nypae and Pleurophomopsis nypae (Hyde et al. 1999; Hyde and Alias 2000; Loilong et al. 2012). Some of the fungi on Nypa fruitcans are restricted to the petioles: Bacusphaeria nypae, Manglicola guatemaelensis and Tirisporella baccariana (Jones et al. 1996; Suetrong et al. 2009; Abdel-Wahab et al. 2017). Two new genera introduced growing on submerged rachis and petioles of palms are Acuminatispora (A. palmarum) on both Nypa fruticans and Phoenix paludosa (Zhang et al. 2018) and Striatiguttula (S. nypae on N. fruticans; S. phoenicis on both N. fruticans and Ph. paludosa) (Zhang et al. 2019). A few fungi have also been recovered from Nypa fruits: Anthostomella spp., Fasciatispora spp., and Vaginatispora nypae) (Jayasiri et al. 2019; Zhang unpublished data).
Mangrove endophytes
A wide range of endophytic fungi have been isolated from mangrove plants, but most are from aerial terrestrial parts and are not included in this review (Pang et al. 2008; Chareprasert et al. 2010; Sakayaroj et al. 2012; Chi et al. 2019; Rashmi et al. 2019). Only a few studies have been devoted to submerged parts of mangrove trees (roots) and mangrove seagrasses (Ananda and Sridhar 2002; Maria and Sridhar 2003; Sakayaroj et al. 2010; Supaphon et al. 2017). Of the 38 endophytes listed in Table 1, most are species of Cladosporium, Colletotrichum, Glomerella, Fusarium, Mycosphaerella, Phyllosticta, Phoma and Sporormiella, and the obligate mangrove fungi e.g. Cumulospora marina, Hydea pygmea, Lulwoana uniseptata, Sammeyersia grandispora and Trichocladium alopallonellum (Ananda and Sridhar 2002; Maria and Sridhar 2003). Endophytes may play a key role in the early stages of mangrove leaf decomposition, becoming saprobes on senescence of leaf material (Hyde and Soytong 2008). Mangrove endophytes have also been shown to be a rich source of secondary metabolites (Rukachaisirikul et al. 2011).
Fungi on fallen mangrove leaves
The earliest studies on the breakdown of leaves in mangroves was by Fell and Masters (1973) and Fell et al. (1975). They found that the attached but senescent leaves harbor a number of parasitic and saprobic terrestrial fungi. They reported that during the first week of submergence straminipilous organisms (Labyrinthulomycetes) were prevalent, and a few other primary invaders appeared, mostly asexual morphs. Within the second and third week the first obligate mangrove fungi, Lulworthia sp. and Halenospora varia (as Zalerion varium), were observed, while at the end of this period most of the straminipilous organisms have disappeared. Raghukumar et al. (1994, 1995) have studied mangrove litter degradation by fungi by using litterbags (both in the field and laboratory). Halophytophthora species and thraustrochytrids were found to colonize the leaves initially followed by a few asexual morphs e.g. Acremonium sp., Aspergillus spp., Cladosporium herbarum, Cirrenalia basiminuta, Fusarium moniliforme and Penicillium spp.
Other studies listed fungi isolated from senescent leaves, mostly asexual morphs with taxa such as Pestalotiopsis spp., Zygosporium spp., Aspergillus spp., Cephalosporium spp., Cladosporium oxysporum, Penicillium spp., (Kuthubutheen 1984; Ananda and Sridhar 2004). Hyde and Sarma (2006) documented fungi occurring on the mangrove palm Nypa fruticans and listed 25 taxa including Aniptodera chesapeakensis, A. mangrovei, Astrosphaeriella nypae, A. striatispora, Helicascus nypae, Helicorhoidon nypicola, Lignincola laevis, Linocarpon appendiculatum, L. bipolaris, L. longisporum, L. nypae, Marinosphaera mangrovei, Oxydothis nypae and Trichocladium nypae.
Decomposers and shreders of mangrove leaves include straminipilous organisms, crabs and filamentous mangrove fungi (Newell 1976; Leano et al. 1998; Fan et al. 2002). Three stages can be discerned in the decomposition of senescent leaves once they fall into the water.
- Stage 1:
-
Leaching of minerals, phenolics/tannins and early colonization by fungi (Cundell et al. 1979). Bremer (1995) reported labyrinthulids and thraustochytrids colonising exposed leaves of Sonneratia within 24 h in mangrove waters. Early fungi are those that may have been present as endophytic fungi and other filamentous fungi, initially typical terrestrial genera: Aspergillus, Cladosporium and Phoma species (Nakagiri et al. 1989; Ananda et al. 2008)
- Stage 2:
-
This is the most active phase in terms of increase in recruitment of fungi such as the asexual morphs: Hydea pygmea, Periconia prolifera and Trichcladium alopallonellum and the ascomycete Lulworthia sp. (Nakagiri et al. 1989; Ananda et al. 2008; Sridhar et al. 2012). Nakagiri et al. (1997) report the ascomycete Lanceispora amphibia on fallen leaves of Bruguiera gymmorhiza, while Maldonado-Ramírez and Torres-Pratts (2005) report the basidiomycete Clathrus c.f. crispus on decomposing leaves of Rhizophora mangle in Puerto Rico
- Stage 3:
-
This stage is characterized by fragmentation of leaf material, loss in weight and a decrease in C/N ratio (Raghukumar 2017). Numerous studies have examined the degradation of leaf material in mangrove habitats (Fell and Masters 1973; Fell et al. 1975; Newell 1973, 1976; Cundell et al. 1979; Steinke et al. 1990; Raghukumar et al. 1995), while others have focused on the rate and biochemical changes in mangrove leaf decomposition (Fell et al. 1980; Misra et al. 1984; Narayanasamy and Kathiresan 2007)
Mangrove fungal diversity on animal substrata
Mangrove fungi play a vital role in the decomposition of dead animals and animal parts (Hyde et al. 1998). Few mangrove fungi live in commensalistic association with mangrove animals or as saprobes on animal substrates. Araujo et al. (1995) reported 322 yeast cultures on marine invertebrates of which 37 are identified from molluscs and crabs from Brazilian mangroves. Ananda and Sridhar (2001) recorded Aspergillus spp., Corollospora intermedia, Epicoccum nigrum and Scolecobasidium spp as the most frequent mangrove fungi from molluscans, cuttle fish endoskeletons, crab exoskeletons and bird feathers collected from mangroves of Karnataka, India. Bird feathers accommodated the highest number (14) of mangrove fungi among the different animal substrata examined (Ananda and Sridhar 2001).
Calcareous shells produced by marine animals such as molluscs, barnacles and corals become colonized by endolithic fungi (Kohlmeyer and Kohlmeyer 1979; Golubic et al. 2005; Raghukumar 2008). Kohlmeyer and Volkmann-Kohlmeyer (1991, 1992) recorded 23 marine fungi on calcareous materials. Ten mangrove fungi were reported from calcareous shells of molluscans from Karnataka coast, India (Ananda and Sridhar 2001). Arenariomyces parvulus and Corollospora intermedia are the dominant mangrove fungi found on the calcareous shells of molluscans.
Many studies reported the occurrence of fungi as pathogens and saprobes on marine animals from marine environment (Ananda et al. 1998; Kohlmeyer and Volkmann-Kohlmeyer 2003; Gleason et al. 2011; Raghukumar 2017; Godinho et al. 2019). However, studies on fungi occurring on animal substrata from mangroves are very rare (Araujo et al. 1995; Ananda and Sridhar 2001). Hence, further studies are required to document the mangrove fungal diversity on different animal substrata from mangroves (Jones 2011; Jones et al. 2019).
Common mangrove fungi
Hyde and Jones (1988), Sarma and Hyde (2001), Maria and Sridhar (2003) and others, have attempted to draw up lists of common mangrove fungi, but this is difficult as sampling procedures are not comparable as discussed in the introduction. Alias and Jones (2009) list the ten most common mangrove fungi based on an analysis of nine surveys from different parts of the tropics: Dictyosporium pelagicum, Halocyphina villosa, Halorosellinia oceanica, Halosarpheia marina, Hydea pygmea, Kallichroma tethys, Leptopshaeria australiensis, Lignincola laevis, Sammeyersia grandispora, Sclerococcum haliotrephum and Verruculina enalia. The most frequently reported mangrove fungi in this study are listed in Table 5, with Anntenospora quadricornuta and Sclerococcum haliotrephum most frequently cited.
The frequency of occurrence of mangrove fungi varies greatly between different studies, typically only a few fungi will dominate a given area, while the others will be rarely encountered (Cooke and Rayner 1984). Generally, surveys of mangrove fungi have been grouped as ‘most common’ or ‘very frequent’ followed by ‘frequent’, ‘infrequent’ and ‘rare’. Hyde (1986) used a formula to calculate the percentage occurrence of mangrove fungi as follows: Percentage occurrence = Number of occurrences of a particular fungus/Total number of samples supporting sporulating fungi × 100. This was subsequently revised by Hyde and Jones (1988) as Percentage occurrence = Number of occurrences of a particular fungus/Total number of samples examined × 100. This modified formula has been used by other workers (Leong et al. 1991; Alias et al. 1995). Antennospora quadricornuta, Halocryptosphaeria bathurstensis, Halocyphina villosa, Halorosellinia oceanica, Halosarpheia marina, Kallichroma tethys, Leptosphaeria australiensis, Lulworthia sp., Rimora mangrovei, Rhizophila marina, Sammeyersia grandispora, Savoryella longispora, Sclerococcum haliotrephum and Verruculina enalia are the very frequently encountered fungi in the Indian Ocean mangroves in addition to several frequently recorded species (Sarma and Hyde 2001). In the case of Pacific Ocean, Caryosporella rhizophorae, Halocyphina villosa, Halosarpheia marina, Hydea pygmea, Lignincola laevis, Linocarpon appendiculatum, Lophiostoma acrostichi, Lulworthia sp., Morosphareria velatospora, Phomopsis sp., Sammeyersia grandispora, Sclerococcum haliotrephum, Torpedospora radiata and Trichocladium linderii were reported to be very frequently recorded species (Sarma and Hyde 2001). It can be found from the above list that there is an overlap of very frequently recorded species between the Indian and Pacific Oceans (Sarma and Hyde 2001). Very meagre information is available from Atlantic Ocean. Jones and Abdel-Wahab (2005) reported the following mangrove fungi as ‘core group’ from Bahamas Islands, Atlantic Ocean: Anthostomella sp., Kallichroma tethys, Leptosphaeria australiensis, Sammeyersia grandispora and Verruculina enalia.
Molecular diversity of mangrove fungi
Circa 77.4% of the mangrove fungi listed in Table 1 are supported by molecular data and this compares with that for pelagic marine fungi (49%) (Hassett et al. 2019). This has implications when inventorying fungal populations employing metagenomics and determining their functional role in the ecosystem (Arfi et al. 2012a, b; Vanegas et al. 2019). A number of studies have looked at the molecular diversity of the fungal mangrove community using 454 pyrosequencing in the region of the mangrove trees Avicennia marina and Rhizophora stylosa (Arfi et al. 2012a, b) and Avicennia germinans (Vanegas et al. 2019). Arfi et al. (2012a) recorded 209,544 reads from exposed and submerged parts of A. marina and R. stylosa and showed that the Ascomycota dominated (82%) while Basidiomycota were rare (3%), thus supporting the observations of more traditional surveys (Jones et al. 2019). The most abundant taxonomic classes in the four microhabitats studied were Dothideomycetes, Lecanoromycetes and Sordariomycetes, and surprisingly a low Eurotiomycetes diversity (Arfi et al. 2012a). Vanegas et al. (2019) noted 854,662 reads with 680 OTU’s and 46 genera in the rhizosphere of A. germninans. Saprotrophs dominated all salinity levels (high 23.2% salt, median 14.6% salt, low 2.8% salt) in the mangroves with such genera as Amorosia, Aspergillus, Penicillium Talaromyces and Trichoderma. At high salinity, Aspergillus, Cystofilobasidium, Podosphaera Saitozyma and Trichoderma species were most abundant; at low salinity Amorosia, Aspergillus, Phaeoacremonium, Talaromyces and Trichoderma species were dominant while at median salinity Aspergillus, Cystobasdium, Penicillium and Trichoderma species were most abundant.
Simões et al. (2015) also undertook a metagenomic study of soil and rhizosphere associated fungi in an Avicennia marina mangrove in the Red Sea and noted that the Ascomycota were dominant (76–85% of all reads), the Basidiomycota with 14–24% reads and minor occurrence of taxa belonging to Blastocladiomycota, Chytridiomycota, Glomeromycota and Neocallimastigomycota. Within the Ascomycota, dominant classes included members of the Eurotiomycetes and Saccharomycetes, with Aspergillus and Schizosaccharomyces dominating in the samples examined in the study. Several taxa in the mycobiome were not previously reported as mangrove-associated fungi: Ajellomyces capsulatus, Blastocladiella emersoni, Neocallimastix, Orpinomyces, Piromyces, or listed in www.marinefungi.org. Clearly further studies are required to explore these undocumented fungi.
Conclusions
This extensive search of the literature on mangrove fungi yielded 850 taxa, which greatly enhances our knowledge of their world distribution and documents species not previously included in the reviews of Schmit and Shearer (2003, 2004). Taxonomical groups that are poorly represented in mangroves are the Chytridiomycota (5 species) and Mucoromycota (13) with no change from those listed by Schmit and Shearer (2003). This can only be attributed to lack of interest by marine mycologists to study these groups. It is vital to survey mangroves for these organisms as chytrids are often associated with infections of phytoplankton (Scholz et al. 2016; Hassett et al. 2019). Baiting mangrove swampy muddy soils, mud flats, volcanic muds with keratin and pollen may yield additional isolates and taxonomic diversity. Greater effort to examine plankton samples for chytrids may help to better understand their ecology in mangrove habitats.
Mangrove fungi have been widely surveyed as the results of this review shows (Fig. 21), but still many mangrove forests remain unexplored, especially those in Africa, Australia, New Zealand and South America. The mangrove forests of Bangladesh, China, Indonesia, Myanmar, Pakistan and Sri Lanka, are also understudied for mangrove fungi. Hence, more fungal surveys in search of a better understanding of their ecology needs to be conducted throughout the world.
Field (1995) opines that climate change will significantly affect the ecology of mangroves and their distribution with the major factor’s temperature, carbon dioxide concentration, salinity of seawater and sea level rise. The constant pollution of mangroves with rubbish and plastics will surely also impact on the mycota of coastal waters (Fig. 22).
The checklist presented in this study significantly extends earlier lists of mangrove fungi (Schmit and Shearer 2003; Sridhar et al. 2012). This is due to the inclusion of yeasts and lower mangrove fungi whereas earlier lists have excluded them. This study comprises 850 mangrove fungi that are exclusive to mangrove environments excluding the terrestrial fungi on mangroves.
Metagenomic analyses of mangrove fungi from mangrove soil, water and litter is a target topic that requires undertaking so as to enhance our knowledge of fungal interactions in the mangroves of America and Asia. Many mangrove plants have been extensively studied, e.g. Avicennia, Nypa and Rhizophora species, while others have been neglected: Aegiceras, Excoecaria and Sonneratia species. The most neglected substrates are mangrove seagrasses and saltmarsh plants that extend into estuarine mangroves and phytoplankton and seaweeds. For example: Australian mangroves commonly have 22 seaweeds, 20 seagrasses. Clearly, studies of mangrove fungi and their role in marine ecosystems remain a challenge for present day mycologists, ecologists and physiolgists.
References
Abdel-Wahab MA (2005) Diversity of marine fungi from Egyptian Red Sea mangroves. Bot Mar 48:348–355
Abdel-Wahab MA, Pang KL, Nagahama T, Abdel-Aziz FA, Jones EBG (2010) Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulospora with the description of eight new genera and four new species. Mycol Prog 9:537–558
Abdel-Wahab MA, Hodhod MS, Bakhali AH, Jones EBG (2014) Marine fungi of Saudi Arabia. Bot Mar 57:323–335
Abdel-Wahab MA, Bahkali AH, Jones EBG, Elgorbani A, Abdel-Aziz FA, Hodhodi MS, Al-Hebshi MO (2016) Two new species of Kallichroma (Bionecteriaceae, Hypocreales) from mangroves of Saudi Arabia. Phytotaxa 260:66–74
Abdel-Wahab MA, Dayarathne MC, Suetrong S, Guo SY, Alias SA, Bahkali AH, Nagahama T, Elgorban AM, Abdel-Aziz FA, Hodhod MS, Al-Hebshi MO, Hyde KD, Nor NA, Pang KL, Jones E (2017) New saprobic marine fungi and a new combination. Bot Mar 60:469–488
Abdel-Wahab MA, Jones EBG, Bahkali AH, El-Gorban AM (2019a) Marine fungi from Red Sea mangroves in Saudi Arabia with Fulvocentrum rubrum sp. nov. (Torpedosporales, Ascomycota). Nova Hedwigia 108:365–377
Abdel-Wahab MA, Jones EBG, Bahkali AH, El-Gorban AM (2019b) Nia lenicarpa sp. nov. (Niaceae, Agaricales) from Red Sea mangroves in Saudi Arabia with comments on Nia vibrissa. Phytotaxa 406:157–168
Aleem AA (1980) Distribution and ecology of marine fungi in Sierra Leone (tropical West Africa). Bot Mar 23:679–688
Alias SA, Jones EBG (2000a) Colonization of mangrove wood by marine fungi at Kuala Selangor mangrove stand, Malaysia. In: Aquatic Mycology Across the Millennium. (eds KD Hyde, WH Ho, SB. Pointing). Fungal Divers 5:9–21
Alias SA, Jones EBG (2000b) Vertical distribution of marine fungi on Rhizophora apiculata at Morib mangrove, Selangor, Malaysia. Mycoscience 41:431–436
Alias SA, Jones EBG (2009) Fungi from mangroves of Malaysia. Inst Ocean Earth Sci Uni Malaya 54:1–109
Alias SA, Jones EBG, Kuthubutheen AJ (1995) Frequency of occurrence of fungi on wood in Malaysian mangroves. Hydrobiologia 295:97–106
Alias SA, Zianuddin N, Jones EBG (2010) Biodiversity of marine fungi in Malaysian mangroves. Bot Mar 53:545–554
Al-Saadoon AH (2006) A new arenicolous species of Corollospora from Iraq. Marsh Bull 2:134–139
Alva P, McKenzie EHC, Pointing SB, Pena-Muralla R, Hyde KD (2002) Do sea grasses harbour endophytes? Fungal Divers Res Ser 7:167–178
Amend AS, Barshis DJ, Oliver TA (2012) Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J 6:1291–1301
Amend A, Burgaud G, Cunliffe M, Edgcom VP, Ettinger CL, Gutiérrez MH, Heitman J, Hom EFY, Ianiri G, Jones AC, Kagami M, Picard KT, Quandt CA, Raghukumar S, Riquelme M, Stajich J, Vargas-Muñiz J, Walker AK, Yarden O, Gladfelterp AS (2019) Fungi in the marine environment: open questions and unsolved problems. Ecol Evol Sci 10:e01189–18
Am-In S, Limtong S, Yongmanichai W, Jindamorakot S (2011) Candida andamanensis sp. nov., Candida laemsonensis sp. nov. and Candida ranongensis sp. nov., anamorphic yeast species isolated from estuarine waters in a Thai mangrove forest. Int J Syst Evol Microbiol 61:454–461
Ananda K, Sridhar KR (2001) Mycoflora on dead animal materials of mangrove habitats of Karnataka Coast, India. Sri Lanka. J Aqua Sci 6:85–93
Ananda K, Sridhar KR (2002) Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can J Microbiol 48:871–878
Ananda K, Sridhar KR (2004) Diversity of filamentous fungi on decomposing leaf and woody litter of mangrove forests of southwest coast of India. Curr Sci 87:1431–1437
Ananda K, Prasannarai K, Sridhar KR (1998) Occurrence of higher marine fungi on marine animal substrates of some beaches along the west coast of India. Indian J Mar Sci 27:233–236
Ananda K, Sridhar KR, Raviraja NS, Barlocher (2008) Breakdown of fresh and dried Rhizophora mucronata leaves in a mangrove of Southwest India. Wet Ecol Manage 16:1–9
Araujo FV, Soares CAG, Hagler AN (1995) Ascomycetous yeast communities of marine invertebrates in a Southeast Brazilian mangrove ecosystem. Antonie Van Leeuwenhoek 68:91–99
Arfi Y, Buee M, Marchand C, Levasseur A, Record E (2012a) Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees Avicennia marina and Rhizophora stylosa. FEMS Microbiol Immunol 79:433–444
Arfi Y, Marchand C, Wartel M, Record E (2012b) Fungal diversity in anoxic-sulfidic sediments in a mangrove soil. Fungal Ecol 5:282–285
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lucking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal Forno M, Dollhofer V, Fliegerova K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytovuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Nuankaew S, Parnmen S, de Azevedo Santiago ALCM, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Wen TC, Yuan HS, Zhou LW, Reblova M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH (2015) Fungal diversity notes 111–252 taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:1–248
Besitulo AD, Samra VV, Hyde KD (2002) Mangrove fungi from Siargao Island, Philippines. In: Hyde KD (ed) Fungi in marine environments. Fungal Diversity Research Series 7. Fungal Divers Press, Hong Kong, pp 267–283
Binder M, Hibbett DS, Wang Z, Farnham WF (2006) Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycetes pathogen of a subtidal Rhodophyte. Am J Bot 93:547–556
Bremer G (1995) Lower marine fugi (Labyrinthulomycetes) and the decay of mangrove leaf. Hydrobiologia 295:89–95
Bucher VVC, Hyde KD, Pointing SB, Reddy CA (2004) Production of wood decay enzymes, mass loss and lignin solubilization in wood by marine ascomycetes and their anamorphs. Fungal Divers 15:1–14
Byrne PJ, Jones EBG (1974) Lignicolous marine fungi. Veroff Inst Meeresf 5:301–320
Byrne P, Jones EBG (1975) Effect of salinity on spore germination of terrestrial and marine fungi. Trans Bri mycol Soc 64:497–503
Chalkley DB, Suh SO, Volkmann-Kohlmeyer B, Kohlmeyer J, Zhou JJ (2010) Diatrypasimilis australiensis, a novel xylarialean fungus from mangrove. Mycologia 102:432
Chareprasert S, Piapukiew J, Whalley AJS, Sihanonth P (2010) Endophytic fungi from mangrove plant species of Thailand: their antimicrobial and anticancer potentials. Bot Mar 53:555–564
Chen W, Shearer CA, Crane JL (1999) Phylogeny of Ophioceras spp. based on morphological and molecular data. Mycologia 91:84–94
Chi WC, Chen WL, He CC, Guo SY, Cha HJ, Tsang LM, Ho TW, Pang KL (2019) A highly diverse fungal community associated with leaves of the mangrove plant Acanthus ilicifolius var xiamenensis revealed by isolation and metabarcoding analyses. Peer J. https://doi.org/10.7717/peerj.7293
Chinnaraj S (1993) Manglicolous fungi from Atollos Maldives. Indian Ocean Ind J Mar Sci 22:14–142
Cooke RC, Rayner ADM (1984) Ecology of saprophytic fungi. Longman, London
Cribb AB, Cribb JW (1955) Marine fungi from Queensland. University of Queensland Press, Brisbane
Cundell AM, Brown MS, Stanford R, Mitchell R (1979) Microbial degradation of Rhizophora mangle leaves immersed in the sea. East Coast Mar Sci 9:281–286
Dayarathne MC, Phookamsak R, Hyde KD, Manawasinghe IS, To-Anun C, Jones EBG (2016) Halodiatrype, a novel diatrypaceous genus from mangroves with H. salinicola and H. avicenniae spp. nov. Mycosphere 4:363–454
Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Wei D, Devadatha B, Yang Jing, Ekanayake H, De Silva W, Samra VV, Al-Sadi AM, Khongphinitbunjong K, Hyde KD, Zhao RL (2019a) Phylogenetic revision of Savoryellaceae and evidence for its ranking as a subclass. Front Microbiol 10:84
Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Al-Sadi AM, Hyde KD, Chomnunti P (2019b) Sexual morph of Phaeoacremonium aureum from Rhizophora mucronata collected in southern Thailand. Phytotaxa 387:21–39
Dayarathne MC, Devadatha B, Wanasinghe DN, Abeywickrama P, Jones EBG, Chomnunti P, Sarma VV, Liu JK, Hyde KD (2020a) Modern taxonomic approaches to identifiying Diatrypaceous fungi from marine habitats, a novel genus Halocryptovalsa and expansion of Halodiatrype. Cryptogamie Mycologie 41:21–67
Dayarathne MC, Jones EBG, Maharachchikumbura SSN, Devadatha B, Sarma VV, Khongphinitbunjong K, Chomnunti P, Hyde KD (2020b) Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11:1–188
Debbab A, Aly AH, Proksch P (2013) Mangrove derived fungal endophytes – a chemical and biological perception. Fungal Divers 61:1–27
Deshmukh SK, Gupta MK, Prakash V, Reddy MS (2018) Mangrove-associated fungi: a novel source of potential anticancer compounds. J Fungi 4:101
Deshmukh SK, Agrawal S, Prakash V, Gupta MK, Reddy MS (2020) Anti-infectives from mangrove endophytic fungi. S Afr J Bot 16:1–27
Devadatha B, Sarma VV (2018) Pontoporeia mangrovei sp. nov, a new marine fungus from an Indian mangrove along with a new geographical and host record of Falciformispora lignatilis. Curr Res Environ Appl Mycol 8:238–246
Devadatha B, Sarma VV, Wanasinghe DN, Hyde KD, Jones EBG (2017) Introducing the new Indian mangrove species, Vaginatispora microarmatispora (Lophiostomataceae) based on morphology and multigene phylogenetic analysis. Phytotaxa 329:139–149
Devadatha B, Sarma VV, Jeewon R, Wanasinghe DN et al (2018a) Thyridareilla a novel marine fungal genus from India: morphological characterization and phylogeny inferred from multigene DNA sequence analyses. Mycol Prog. https://doi.org/10.1007/s11557-018-1387-4
Devadatha B, Sarma VV, Ariyawansa HA, Jones EBG (2018b) Deniquelata vittalii sp. nov., a novel Indian saprobic marine fungus on Suaeda monoica and two new records of marine fungi from Muthupet mangroves, East coast of India. Mycosphere 9:565–582
Devadatha B, Mehta N, Wanasinghe DN, Baghela A, Sarma VV (2019) Vittaliana mangrovei, gen. nov., sp. nov. (Phaeosphaeriaceae) from mangroves near Pondicherry (India), based on morphology and multigene phylogeny. Cryptogam Mycol 40:117–132
El-Samawaty AMA, Boekhout T, Yassin MA, Abdel-Wahab MA (2019) Saturnispora mangrovi f.a., sp. nov. from Syhat mangrove, Saudi Arabia. Int J Syst Evol Microbiol 70:977–981
El-Sharouny HM, Raheem AM, Abdel-Wahab MA (1998) Manglicolous fungi of the Red Sea in Upper Egypt. Mycol Res 153:81–96
Fan KW, Vrijmoed LLP, Jones EBG (2002) Physiological studies of subtropical mangrove Thraustochytrids. Bot Mar 45:50–57
FAO (2007) The world's mangroves 1980–2005. FAO forestry paper no. 153. Rome, Forest Resources Division, FAO, pp 77
Fell JW, Masters IM (1973) Fungi associated with the degradation of mangrove (Rhizophora mangle L.) leaves south Florida. In: Stevenson HL, Colwell RR (eds) Estuarine microbial ecology. University of South Carolina Press, Columbia
Fell JW, Masters IM (1995) Phycomycete (Phytophthora spp. nov. and Pythium sp. nov.) associated with degrading mangrove leaves. Can J Bot 53:2908–2922
Fell JW, Cefalu RC, Master IM, Tallman AS (1975) Microbial activities in the mangrove (Rhizophora mangle) leaf detrital system. In: Walsh G, Snedaker S, Teas H (eds) Proc Int Symp Biol Managmt Mangroves. University of Florida, Gainesville, pp 661–679
Fell JW, Masters IM, Newell SY (1980) Laboratory model of’the potential role of fungi (Phytophthora spp.) in the decomposition of red mangrove (Rhizophora mangle) leaf litter. In: Tenore KR, Coull BC (eds) Marine Benthic dynamics. University of South Carolina Press, Columbia, pp 359–363
Fell JW, Statzall-Tallman A, Kurtzman CP (2004) Lachancea meyersii sp. nov., an ascosporogenous yeast from mangrove regions in the Bahama Islands. Stud Mycol 50:359–363
Fell JW, Statzell-Tallman S, Scorzetti G, Gutiérrez MH (2011) Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Anton Leeuw 99:533–549
Field CD (1995) Impact of expected climate change on mangroves. Hydrobiologia 295:75–81
Frisvad JC (2015) Fungal chemotaxonomy. In: Zeilinger S, Martín J-F, García-Estrada C (eds) Biosynthesis and molecular genetics of fungal secondary metabolites, vol 2. Springer, New York, pp 103–121
Frisvad JC, Andersen B, Thrane U (2008) The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res 112:231–240
Fryar SC, Booth W, Davies J, Hodgkiss IJ, Hyde KD (2004) Distribution of fungi on wood in the Tuton River, Brunei. Fungal Divers 17:17–38
Fryar SC, Haelewaters D, Catcheside DEA (2019) Annabella australiensis gen. and sp. nov. (Helotiales, Cordieritidaceae) from South Australian mangroves. Mycol Prog 18:973–981
Furtado BU, Gołebiewski M, Skorupa M, Hulisz P, Hrynkiewicz K (2019) Bacterial and fungal endophytic microbiomes of Salicornia europaea. Appl Environ Microbiol 85:e00305–e00319
Garg GK (1983) Vertical distribution of fungi in Sunderban mangrove mud. Indian J Mar Sci 12:48–51
Gleason FH, Küpper FC, Amon JP, Picard K et al (2011) Zoosporic fungi in marine ecosystems: a review. Mar Freshw Res 62:383–393
Godinho VM, de Paula MT, Silva DA, Paresque K, Martins AP, Colepicolo P, Rosa CA, Rosa LH (2019) Diversity and distribution of hidden cultivable fungi associated with marine animals of Antarctica. Fungal Biol 123(7):507–516
Golubic S, Radtke G, Le Campion-Alsumard T (2005) Endolithic fungi in marine ecosystems. Trends Microbiol 13:229–235
Hassett BT, Vonnahme TR, Peng XF, Jones EBG, Heuzé C (2019) Global diversity and geography of the planktonic marine fungi. Bot Mar 63:121–139
Hattori T, Sakayaroj J, Jones EBG, Suetrong S, Preedanon S, Klaysuban A (2014) Three species of Fulviformes (Basidiomycota, Hymenochaetales) associated with rots on mangrove tree Xylocarpus granatum in Thailand. Mycoscience 55:344–354
Hawksworth DL, Eriksson OE (1986) The names of accepted orders of Ascomycetes. Syst Ascom 5:175–184
Hibbett DS, Binder M (2001) Evolution of marine mushrooms. Biol Bull 201:319–322
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Day Y-C, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside IE, Koljalg U, Kurtzman CP, Larsson K-H, Lichtwardt R, Longcore J, Miądlikowska J, Miller A, Moncalvo J-M, Mozley-Standtridge S, Oberwinkler F, Parmasto E, Reeb W, Rogers J-D, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao Y-J, Zhang N (2007) A higher- level phylogenetic classification of the Fungi. Mycol Res 111:509–547
Hodhod MS, Abdel-Wahab MA, Bahkali AH, Hyde KD (2012) Amarenographium solium sp. nov. from Yanbu mangroves in the Kingdom of Saudi Arabia. Cryptogam Mycol 33:285–295
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC et al (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Pem D, Bhat JD, Liu N, Tennakoon DS, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana TKW, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-Gonzáles R, Aptroot A, Kashiwadani H, Harishchandra D, Aluthmuhandiram JVS, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Samarakoon MC, Suetrong S, Chaiwan N, Dayarathne MC, Jing Y, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020a) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11(1):1553–2107
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Lücking R, Boonmee S, Bhat JD, Liu N, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana TKW, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-Gonzáles R, Aptroot A, Kashiwadani H, Harishchandra D, Aluthmuhandiram JVS, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Samarakoon MC, Suetrong S, Chaiwan N, Dayarathne MC, Jing Y, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020b) Refined families of Dothideomycetes: orders and families incertae sedis in Dothideomycetes. Fungal Divers 105:17–318
Hyde KD (1986) Frequency of occurrence of lignicolous marine fungi in the tropics. In: Moss ST (ed) The biology of marine fungi. Cambridge Univeristy Press, Cambridge, pp 311–322
Hyde KD (1988) Studies on the tropical marine fungi of Brunei. Bot J Linn Soc 98:135–151
Hyde KD (1990a) Intertidal fungi from warm temperate mangroves of Australia, including Tunicatispora australiensis, gen. et sp. nov. Aust Syst Bot 3:711–718
Hyde KD (1990b) A study of the vertical zonation of intertidal fungi on Rhizophora apiculata at Kampong Kapok mangrove, Brunei. Aquatic Bot 36:255–262
Hyde KD (1991) Fungal colonization of Rhizophora apiculata and Xylocarpus granatum poles in Kampong Kapok mangrove, Brunei. Sydowia 43:31–38
Hyde KD, Alias SA (2000) Biodiversity and distribution of fungi associated with decomposing Nypa fruticans. Biol Cons 9:393–402
Hyde KD, Jones EBG (1986) Marine fungi from Seychelles. IV. Cucullospora mangrovei gen. et sp. nov. from dead mangrove. Bot Mar 29:491–495
Hyde KD, Jones EBG (1988) Marine mangrove fungi. Mar Ecol 9:15–38
Hyde KD, Jones EBG (1989) Marine fungi from Seychelles. VIII. Rhizophila marina, a new ascomycete from mangrove prop roots. Mycotaxon 34:527–533
Hyde KD, Lee SY (1995) Ecology of mangrove fungi and their role in nutrient cycling: what gaps occur in our knowledge? Hydrobiologia 295:107–118
Hyde KD, Sarma VV (2006) Biodiversity and ecological observations on filamentous fungi of mangrove palm Nypa fruticans Wurumb (Liliopsida-Arecales) along the Tutong River, Brunei. Indian J Mar Sci 35:297–307
Hyde KD, Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33:163–173
Hyde KD, Chalermpongse A, Boonthavikoon T (1990) Ecology of intertidal fungi at Ranong mangrove, Thailand. Trans Mycol Soc Jpn 31:17–27
Hyde KD, Jones EBG, Leaño E, Pointing SB, Poonyth AD, Vrijmoed LLP (1998) Role of fungi in marine ecosystems. Biodivers Conserv 7:1147–1161
Hyde KD, Goh TK, Lu BS, Alias SA (1999) Eleven new intertidal fungi from Nypa fruticans. Mycol Res 103:1409–1422
Hyde KD, Jones EBG, Ariyawansa H, Liu JK, Binder M, Jayawardene N, Boehm E, Boonmee S, Chomnunti P, Dai DQ, Doilom M, Lücking R, Monkai J, Nelsen MP, Phookamsak R, Shearer CA, Wu ZH, Diederich P, Lawrey JD, Suetrong S, Tanaka K, Gueidan C, Knudsen K, Muggia L, Braun U, Yacharoen S, Wikee S, Li YM, Aptroot A, Bezerra JL, Raja HA, Alias SA, Bhat JD, Chukeatirote E, Dissanayake A, Doveri F, Crous PW, De Hoog S, Jayawardena R, Pang KL, Zhang Y, Hirayama K, Miller A (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat DJ, Jones EBG, Bundhun D, Chen YJ, Bao DF, Boonmee S, Calabon MS, Chaiwan N, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J, Zeng XY, Zhang SN, Xiang MM (2020) Refined families of Sordariomycetes. Mycosphere 11:305–1059
Isaka M, Yngchum A, Intamas S, Kocharin K, Jones EBG, Kongsaree P, Prabpai S (2009) Aigialomycins and related polyketide metabolites from the mangrove fungus Aigialus parvus BCC 5311. Tetrahedron 65:4396–4403
Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat DJ, Wanasinghe DN, Liu JK, Lu YZ, Kang JC, Xu JC, Karunarathna SC (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10(1):1–186
Jones EBG (1963) Marine fungi 2. Ascomycetes and Deuteromycetes from submerged wood and drift Spartina. Trans Br Mycol Soc 46:135–144
Jones EBG (2000) Marine fungi: some factors influencing biodiversity. Fungal Divers 4:53–73
Jones EBG (2011) Are there more marine fungi to be described? Bot Mar 54:343–354
Jones EBG, Abdel-Wahab MA (2005) Marine fungi from the Bahamas Islands. Bot Mar 48:356–364
Jones EBG, Hyde KD (1988) Methods for the study of marine fungi from the mangroves. In: Agate AD, Subramanian CV, Vannucci M (eds) Mangrove microbiology. Role of microorganisms in nutrient cycling of mangrove soils and waters. UNDP/UNESCO, New Delhi, pp 9–27
Jones EBG, Oliver AC (1964) Occurrence of aquatic hyphomycetes on wood submerged in fresh and brackish water. Trans Br mycol Soc 47:45–48
Jones EBG, Puglisi MP (2006) Marine fungi from Florida. Florida Sci 69:157–164
Jones EBG, Hyde KD, Read SJ, Moss ST, Alias SA (1996) Tirisporella gen. nov., an ascomycete from the mangrove palm Nypa fruticans. Can J Bot 74:1487–1495
Jones EBG, Sakayaroj J, Sueterong S, Somrithipol A, Pang KL (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–187
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Jones EBG, Devadatha B, Abdel-Wahab MA, Dayarathne MC, Zhang SN, Hyde KD, Liu JK, Bahkali AH, Samra VV, Zhang H, Wang MM, Liu F, Cai L (2019a) New marine fungal genera and species. Bot Mar 63:155–181
Jones EBG, Pang KL, Abdel-Wahab MA, Scholz B, Hyde KD, Boekhout T, Ebel R, Rateb ME, Henderson L, Sakayaroj J, Suetrong S, Dayarathne MC, Kumar V, Raghukumar S, Sridhar KR, Bahkali AH, Gleason F, Norphanphoun C (2019b) An online resource for marine fungi. Fungal Divers 96:347–433
Jülich W (1981) Higher taxa of Basidiomycetes. Bibliotheca Mycologica 85:1–485
Kohlmeyer J (1969) Marine fungi from Hawaii including the new genus Helicascus. Can J Bot 47:1469–1487
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology: the higher fungi. Academic Press, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1989) Hawaiian marine fungi, including two new genera of Ascomycotina. Mycol Res 92:410–421
Kohlmeyer J, Volkmann-Kohlmeyer B (1991) Marine fungi from Queensland, Australia. Aust J Mar Freshw Res 42:91–99
Kohlmeyer J, Volkmann-Kohlmeyer B (1992) Two Ascomycotina from coral reefs in the Caribbean and Australia. Cryptogam Bot 2:367–374
Kohlmeyer J, Volkmann-Kohlmeyer B (2003) Marine ascomycetes from algae and animal hosts. Bot Mar 46:285–306
Kong RYC, Chan PC, Mitchell JI, Vrijmoed LLP, Jones EBG (2000) Relationships of Halosarpheia, Lignincola and Nais inferred from partial 18S rDNA. Mycol Res 104:35–43
Kuthubutheen AJ (1984) Leaf surface fungi associated with Avicennia alba and Rhizophora mucronata in Malaysia. In: Proceedingd of the Asian symposium on mangrove environment - research and management, Kuala Lumpur pp 153–171
Læssøe T, Davey ML, Petersen JH (2016) A new species of Maireina on Filipendula ulmaria. Karstenia 56:39–46
Leańo EM, Vrijmoed LLP, Jones EBG (1998) Physiological studies on Halophytophthora vesicular (Straminipilous Fungi) isolated from fallen mangroves leaves from Mai Po, Hong Kong. Bot Mar 41:411–419
Lee BKH, Baker GE (1973) Fungi associated with the roots of the red mangrove. Mycologia 656:894–906
Lee SY, Jones EBG, Diele K, Castellanos-Galindo GA, Nordhaus I (2017) Biodiversity. In: Rivera-Monry Lee (ed) Mangrove ecosystems: a global biogeographic perspective. Springer, New York, pp 55–84
Leong WF, Tan TK, Jones EBG (1988) Lignicolous marine fungi of Singapore. Can J Bot 66:2167–2170
Leong WF, Tan TK, Jones EBG (1991) Fungal colonization of submerged Bruguiera cylindrica and Rhizophora apiculata wood. Bot Mar 34:69–76
Li GJ, Hyde KD, Zhao RN, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, Crop ED, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Goes-Neto A, Gorczak M, Haitjema GH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jeronimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lucking R, Lumbsch HT, Lumyong S, Leano EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspe O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasak J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Yang SD, Hu Y, Zhang JF, Zhao J, Zhou LW, Persoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78:1–237
Li J, Jeewon R, Phookamsak R, Bhat DJ, Mapook A, Chukeatirote E, Hyde KD, Lumyong S, Mckenzie EH (2018) Marinophialophora garethjonesii gen. et sp. nov.: a new hyphomycete associated with Halocyphina from marine habitats in Thailand. Phytotaxa 345(1):1–2
Limtong S, Kaewwichian R, Am-In S, Boonmark C, Jindamorakot S, Yongmanitchai W, Srisuk N, Kawasaki H, Nakase T (2010) Three anamorphic yeast species Candida sanitii sp. nov., Candida sekii sp. nov., and Candida suwanaritii, three novel yeasts in the Saturispora clade isolated in Thailand. FEMS Yeast Res 10:114–122
Lin YC, Wu XY, Fenf SA, Jiang GC, Luo JH, Zhou SN, Vrijmoed LLP, Jones EBG, Krohn K, Steingrover K, Zsila F (2001) Five unique compounds: Xyloketals from mangrove fungus Xylaria sp. from the South China Sea. J Organic Chem 66:6252–6256
Lin YC, Wu X, Deng Z, Wang J, Zhou S, Vrijmoed LLP, Jones EBG (2002a) The metabolites of the mangrove fungus Verruculina enalia No 2606 from a salt lake in the Bahamas. Phytochem 59:469–471
Lin YC, Wang J, Wu XY, Zhou SN, Vrijmoed LLP, Jones EBG (2002b) A novel compound eniatin G from the mangrove fungus Halosarpheia sp. ( #732) from the South China Sea. Aust J Chem 55:225–227
Liu JK, Hyde KD, Gareth Jones EB, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčik S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu JK, Hyde KD, Jeewon R, Phillips AJ, Maharachchikumbura SS, Ryberg M, Liu ZY, Zhao Q (2017) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84:75–99
Logesh AR, Kalaiselvam M, Upreti DK, Nayaka S, Kathiresan K (2013) Mangroves – An abode for unique lichens Coastal ecosystems of India, special publication. Annamalai University, Parangipettai, pp 39–44
Loilong A, Salayaroj J, Ringjindamain Choeyklin R, Jones EBG (2012) Biodiversity of fungi on the palm Nypa fruticans. In: Jones EBG, Pang KL (eds) Marine and fungal-like organisms. De Gruyter, Germany, pp 273–290
Maekawa N, Suhara H, Kinjo K, Kondo R, Hoshi Y (2005) Haloaleurodiscus mangrovei gen. sp. nov. (Basidiomycota) from mangrove forests in Japan. Mycol Res 109:825–832
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat J, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang Q, Xiao Y, Dsouza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali A, Boonmee S, Boonyuen N, Cheewangkoon R, Disanayake AJ, Kang J, Li QR, Liu JK, Liu ZY, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 72:199–301
Maldonado-Ramírez SL, Torres-Pratts H (2005) First report of Clathrus cf crispus (Basidiomycota: Clathraceae) occurring on decomposing leaves of Rhizophora mangle in Puerto Rico. Caribbean J Sci 41:357–359
Manimohan P, Amritha M, Sairabanu NK (2011) A comparison of diversity of marine fungi on three co-habiting mangrove plants. Mycosphere 2:533–538
Maria GL, Sridhar KR (2003) Endophytic fungal assemblage of two halophytes from west coast mangrove habitats, India. Czech Mycol 55:241–251
Meyers SP, Reynolds ES (1958) A wood incubation method for the study of lignicolous marine fungi. Bull Mar Sci Gulf Caribbean 8:342–347
Misra S, Vijaykumar Y, Amitabha G, Dutta J (1984) Microbial decomposition of Aviccenia officinalis leaf litter in a mangrove forest biome: biochemical changes during decomposition. In: Krshnamurthy V, Untawale AH (eds) Marine plants. Seaweed Research Utilization Association, Madras, pp 257–262
Nakagiri A, Tokumasu S, Araki H, Koreeda S, Tubaki K (1989) Succession of fungi in decomposing mangrove leaves in Japan. In: Hattori T, Ishida Y, Maruyama Y, Morita RY, Uchida A (eds) Recent advances in microbial ecology (proceeding of the 5th international symposium on microbial ecology). Japan Scientific Societies Press, Tokyo, pp 297–301
Nakagiri A, Okane I, Ito T, Katumoto K (1997) Lanceispora amphibia gen. et sp. nov., a new amphisphaericeous ascomycete inhabiting senescent and fallen leaves of mangrove. Mycoscience 38:207–213
Narayanasamy R, Kathiresan K (2007) Microbial flora associated with submerged leaf litter in India. Rev Biol Tropl 55:34–44
Nayak SS, Gonsalves V, Nazareth S (2011) Isolation and salt tolerance of halophilic fungi from mangroves and solar salterns - India. Indian J Geo-Mar Sci 41:164–172
Newell SY (1973) Succession and role of fungi in the degradation of red mangrove seedlings. In: Stevenson LH, Colwell RR (eds) Estuarine microbial ecology. Univ. of South Carolina Press, Columbia, pp 467–480
Newell SY (1976) mangrove fungi: the succession in mycoflora of red mangrove (Rhizophoara mangle L.) seedlings. In: Jones EBG (ed) Recent advances in aquatic mycology. Elsevier, London, pp 51–91
Overy DP, Rämä T, Oosterhuis R, Walker A, Pang KL (2019) The neglected marine fungi sensu stricto and their isolation for natural products discovery. Marine Drugs 17:42
Pang KL, Vrijmoed LLP, Goh TK, Plaingam N, Jones EBG (2008) Fungal endophytes associated with Kandelia candel (Rhizophoraceae) in Mai Po Nature Reserve, Hong Kong. Bot Mar 51:171–178
Pang KL, Jheng JS, Jones EBG (2011) Marine mangrove fungi of Taiwan. National Taiwan Ocean University, Chilung, pp 1–131
Pang KL, Hyde KD, Alias SA, Suetrong S, Guo SY, Idid R, Jones EBG (2013) Dyfrolomycetaceae, a new family in the Dothideomycetes, Ascomycota. Cryptogam Mycol 34:223–232
Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Jones EBG, Maharachchikumbura SSN, Raspé O, Karunarathna SC, Wanasinghe DN, Hongsanan S, Doilom M, Tennakoon DS, Machado AR, Firmino AL, Ghosh A, Karunarathna A, Mešić A, Dutta AK, Thongbai B, Devadatha B, Norphanphoun C, Senwanna C, Wei DP, Pem D, Ackah FK, Wang GN, Jiang HB, Madrid H, Lee HB, Goonasekara ID, Manawasinghe IS, Kušan I, Cano J, Gené J, Li JF, Das K, Acharya K, Raj KNA, Latha KPD, Chethana KWT, He MQ, Dueñas M, Jadan M, Martín MP, Samarakoon MC, Dayarathne MC, Raza M, Park MS, Telleria MT, Chaiwan N, Matočec N, de Silva NI, Pereira OL, Singh PN, Manimohan P, Uniyal P, Shang QJ, Bhatt RP, Perera RH, Alvarenga RLM, Nogal-Prata S, Singh SK, Vadthanarat S, Oh SY, Huang SK, Rana S, Konta S, Paloi S, Jayasiri SC, Jeon SJ, Mehmood T, Gibertoni TB, Nguyen TTT, Singh U, Thiyagaraja V, Sarma VV, Dong W, Yu XD, Lu YZ, Lim YW, Chen Y, Tkalčec Z, Zhang ZF, Luo ZL, Daranagama DA, Thambugala KM, Tibpromma S, Camporesi E, Bulgakov TS, Dissanayake AJ, Senanayake IC, Dai DQ, Tang LZ, Khan S, Zhang H, Promputtha I, Cai L, Chomnunti P, Zhao RL, Lumyong S, Boonmee S, Wen TC, Mortimer PE, Xu JC (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers 95:1–273
Pointing SB, Vrijmoed LLP, Jones EBG (1998) A qualitative assessment of lignocellulose degrading enzyme activity in marine fungi. Bot Mar 41:293–298
Pointing SB, Buswell JA, Jones EBG, Vrijmoed LLP (1999) Extracellular cellulolytic enzyme profiles of five lignicolous mangrove fungi. Mycol Res 103:696–700
Poon MOK, Hyde KD (1998) Biodiversity of intertidal estuarine fungi on Phragmites at Mai Po marshes, Hong Kong. Bot Mar 41:141–155
Poonyth AD, Hyde KD, Peerally A (2001) Colonization of Bruguiera gymnorrhiza and Rhizophora mucronata wood by marine fungi. Bot Mar 44:75–80
Pouska V, Svoboda M, Leps J (2013) Co-occurrence patterns of wood-decaying fungi on Picea abies log: does Fomitopsis pinicola influence the other species? Polish J Ecol 61:119–134
Raghukumar C (2008) Marine fungal biotechnology: an ecological perspective. Fungal Divers 31:19–35
Raghukumar S (2017) Fungi in coastaland oceanic marine ecosytems. Springer, New York, pp 1–364
Raghukumar S, Sharma S, Raghukumar C, Sathe-Pathak V (1994) Thraustochytrid and fungal component of marine detritus. IV. Laboratory studies on decomposition of leaves of them mangrove Rhizophora apiculata Blume. J Exp Mar Biol Ecol 183:113–131
Raghukumar S, Sathe-Pathak V, Sharma S, Raghukumar C (1995) Thraustochytrid and fungal component of marine detritus. III. Field studies on the decomposition of leaves of the mangrove Rhizophora apiculata Blume. Aquat Microbiol Ecol 9:117–125
Rai JN, Tewari JP, Mukerji KG (1969) Mycoflora of mangrove mud. Mycopathol Mycol Appl 38:17–31
Raja AR, Upreti DK, Kalaiselvam M, Nayaka S, Kandasamy K (2012) Lichen flora of Pichavaram and Muthupet mangroves (Southeast Coast of India). Mycosphere 3:884–888
Rashmi M, Kushveer JS, Sarma VV (2019) A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10:798–1079
Raveendran R, Manimohan P (2007) Marine fungi of Kerala, A preliminary floristic and ecological study. Malabar Natural History Society (MNHS), 17/548, Indira Gandhi Link Road, Calicut - 673 004, Kerala, India
Ravikumar DR, Vittal BPR (1996) Fungal diversity on decomposing biomass of mangrove plant Rhizophora in Pichavaram estuary, east coast of India. Indian J Mar Sci 25:142–144
Réblová M, Miller AN, Rossman AY, Seifert KS, Crous PW, Hawksworth DL, Abdel-Wahab MA, Cannon PF, Daranagama DA, De Beer ZW, Huang SK, Hyde KD, Jayawardena R, Jaklitsch W, Jones EBG, Ju YM, Judith C, Maharachchikumbura SSN, Pang KL, Petrini LE, Raja HA, Romero AI, Shearer CA, Senanayake IC, Voglmayr H, Weir BS, Wijayawarden NN (2016) Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7:131–153
Richards TA, Jones MD, Leonard G, Bass D (2012) Marine fungi: their ecology and molecular diversity. Annu Rev Mar Sci 4:495–522
Rukachaisirikul V, Rodglin A, Phongpaichit S, Buatong J, Sakayaroj J (2011) alpha Pyrone and seiricuprolide derivatives from the mangrove-derived fungi Pestalotiopsis spp. PSU-MA92 and PSU-MA 119. Phytochem Lett 5:13–17
Sadaba RB, Vrijmoed LLP, Jones EBG, Hodkiss IJ (1995) Observations on vertical distribution of fungi associated with standing senescent Acanthus ilicifolius stems at Mai Po mangrove, Hong Kong. Hydrobiology 295:119–126
Sainty GR, Hosking J, Carr G, Adan P (eds) (2012) Estuary plants and what’s happening to them in south-east Australia. Sainty and Associates Pty Ltd., Australia
Sakayaro J, Preedanon S, Phongpaichit S, Buatong J, Chaowalit P, Rukachaisirikul A (2012) Diversity of endophytic and marine-derived fungi associated with marine plants and animals. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. De Gryter, Berlin, pp 291–328
Sakayaroj J, Pang KL, Phongpaichi S, Jones EBG (2005) A phylogenetic study of the genus Haligena (Halosphaeriales, Ascomycota). Mycologia 97:804–811
Sakayaroj J, Preedanon S, Supaphon O, Jones EBG, Phongpaichit S (2010) Phylogenetic diversity of endophyte assemblages associated with tropical seagrass Enhalus acoroides from Thailand. Fungal Divers 41:27–45
Sakayaroj J, Preedanon S, Suetrong S, Klaysuban A et al (2012) Molecular characterization of basidiomycetes associated with the decayed mangrove tree Xylocarpus granatum in Thailand. Fungal Divers 56:145–156
Sangtiean T, Suetrong S, Sakayaroj J, Unakul P, Preedanon S, Klaysuban A (2014) Species diversity of manglicolous marine fungi at Trat Province. The Agricultural Co-operative Federation of Thailand Limited, Bangkok, pp 1–86
Sarma VV, Hyde KD (2001) A review of frequently occurring fungi in mangroves. Fungal Divers 8:1–34
Sarma VV, Hyde KD (2018) Fungal species consortia on Nypa fruticans at Brunei. Stud Fungi 3:19–26
Sarma VV, Raghukumar S (2013) Manglicolous fungi from Chorao mangroves, Goa, West coast of India: observations on fungal species consortia. Kavaka 41:18–22
Sarma VV, Vittal BPR (1998–1999) Ecological studies on mangrove fungi from east coast of India. Observations on seasonal occurrence. Kavaka: 26 & 27:105–120
Sarma VV, Vittal BPR (2000) Biodiversity of mangrove fungi on different substrata of Rhizophora apiculata and Avicennia spp. from Godavari and Krishna deltan, east cost of India. In: Hyde, K.D., Ho, W.H. and Pointing, S.B. (eds.) Aquatic Mycology across the Millennium. Fungal Divers 5:23–41
Sarma VV, Vittal BPR (2002) A preliminary study on vertical distribution of manglicolous fungi on prop roots of Rhizophora apiculata Blume at Krishna delta, east coast of India. Kavaka 30:21–29
Sarma VV, Vittal BPR (2017) Seasonal occurrence of marine ascomycetes and anamorphic marine fungi in mangroves of Godavari and Krishna deltas, East coast of India. Kavaka 48:61–69
Sarma VV, Newell SY, Hyde KD (2001) Koorchaloma spartinicola sp. nov., a new marine sporodochial fungus from Spartina alterniflora. Bot Mar 44:321–326
Schmit JP, Shearer CA (2003) A checklist of mangrove occurring fungi, their geographical distribution and known host plants. Mycotaxon 85:432–477
Schmit JP, Shearer CA (2004) Geographic and host distribution of lignicolous mangrove micro-fungi. Bot Mar 47:496–500
Scholz B, Kupper FC, Vyverman W, Karsten U (2016) Effects of eukaryotic pathogens (Chytridiomycota and Oomycota) on marine microphytobenthic diatom community compositions in the Soltho¨rn tidal flat (southern North Sea, Germany). Eur J Phycol 5:253–269
Shearer CA (1972) Fungi of the Chesapeake Bay and its tributaries. III. The distribution of wood-inhabiting ascomycetes and fungi imperfecti of the Patuxent river. Am J Bot 59:961–969
Simões MF, Antunes A, Ottoni CA, Amini MS, Alam I, Alzubaidy H, Mokhtar NA, Archer JAC, Bajic VB (2015) Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the Red Sea—a metagenomic approach. Genomics Proteomics Bioinform 13:310–320
Spalding M, Kainuma M, Collins L (2010) Word atlas of mangroves. Earthscan, London
Spatafora JW, Blackwell M (1994) The polyphyletic origins of ophiostomatoid fungi. Mycol Res 98:1–9
Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J (1998) Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). Am J Bot 85:1569–1998
Sridhar KR (2012) Decomposition of materials in the sea. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin, pp 475–500
Sridhar KR, Alias SA, Pang KL (2012) Mangrove fungi. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. Walter de Gruyter GmbH & Co. KG, Berlin, pp 253–271
Statzell-Tallman A, Belloch C, Fell JW (2008) Kwoniella mangroviensis gen. nov., sp. nov. (Tremellales, Basidiomycota), a teleomorphic yeast from mangrove habitats in the Florida Everglades and Bahamas. FEMS Yeast Res 8:103–113
Statzell-Tallman A, Scorzetti G, Fell JW (2010) Candida spencermartinsiae sp. nov., Candida taylorii sp. nov. and Pseudozyma abaconensis sp. nov., novel yeasts from mangrove and coral reef ecosystems. Inter J Syst Evol Microb 60:1978–1984
Steinke TD, Barnabas AD, Somaru R (1990) Structural changes and associated microbial activity accompanying decomposition of mangrove leaves in Mgeni Estuary. S Afr J Bot 56:39–48
Stevens FL (1920) New or noteworthy Porto Rican fungi. Bot Gaz 70:399–402
Stevens GN (1979) Distribution and related ecology of macrolichens on mangroves on the east Australian coast. Lichenologist 11:293–305
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkman-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Suetrong S, Sakayaroj J, Unakul P, Somrithipol S, Preedanon S, Gundool W, Klaysuban A, Sangtiean T, Promchu W (2016) Species diversity of manglicolous marine fungi at Nakhon Si Thammarat, Phetchaburi and Prachuap Khiri Khan Provinces. The Agricultural Co-operative Federation of Thailand Limited, Bangkok
Sun JZ, Liu XZ, McKenzie EHC, Jeewon R, Liu JK, Zhang XL, Zhao Q, Hyde KD (2019) Fungicolous fungi: terminology, diversity, distribution, evolution and species checklist. Fungal Divers 95:1–94
Supaphon P, Phongpaichit S, Sakayaroj J, Rukachaisirikul V, Kobmoo N, Spatafora JW (2017) Phylogenetic community structure of fungal endophytes in seagrass species. Bot Mar 60:489–502
Tam WY, Pang KL, Jones EBG (2003) Ordinal placement of selected marine Dothideomycetes inferred from small subunit ribosomal DNA sequence analysis. Bot Mar 46:487–494
Tan TK, Leong WF, Jones EBG (1989a) Succession of fungi on wood of Avicennia alba and A. lanata in Singapore. Can J Bot 67:2686–2691
Tan TK, Leong WF, Mouzouras R, Jones EBG (1989b) Occurrence of fungi on mangrove wood and its decomposition. In: Hattori T, Ishida Y, Maruyama Y, Morita RY, Uchida A (eds) Recent advances in microbial ecology. Japan Scientific Societies Press, Tokyo, pp 307–310
Tan TK, Teng CL, Jones EBG (1995) Substrate type and microbial interactiona as factors affecting ascocarp formation by mangrove fungi. Hydrobiologia 195:127–134
Tang AMC, Jeewon R, Hyde KD (2007) Phylogenetic utility of protein (RPB2, b-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematic of Sordariomycetes (Ascomycota, Fungi). Antonie Van Leeuwenhoek 91:327–349
Tao YW, Lin YC, She ZG, Lin MT, Chen PX, Zhang JY (2015) Anticancer activity and mechanism investigation of beauvericin isolated from secondary metabolites of the mangrove endophytic fungi. Anti-Cancer Agents Med Chem 15(2):258–266
Tomlinson TB (1986) The botany of mangroves. Cambridge University Press, Cambridge, p 413
Tsui CKM, Hyde KD (2004) Biodiversity of fungi on submerged wood in a stream and estuaries in the Tai Ho Bay, Hong Kong. Fungal Divers 15:171–186
Ulken A (1972) Physiological studies on a phycomyceten from a mangrove at Cananeia, Sao Paulo, Brazil. Veroff. Inst. Meeresforsch. Bremerhaven 13:217–230
Ulken A (1978) Growth experiments with different isolates of Phlyctochytrium mangrovis (Phycomycetes). Veröffentlichen Institut Meeresforschung Bremerhaven 17:21–31
Ulken A (1981) The phycomycetes flora of mangrove swamps in the South Pasific. Verroff. Inst. Meeresfoasch. Bremerh 19:45–59
Ulken A (1983) Distribution of Phycomycetes in mangrove swamps with brackish waters and wates of high salinity, Chapter 8. In: Teas HJ (ed) Biology and ecology of mangroves tasks for vegetations science, vol 8. Springer, Amsterdam, pp 111–116
Ulken A (1986) Estimation of Thraustochytrid propagules in two mangrove swamps. Bot Mar 29:85–89
Vanegas J, Munoz-García A, Pérez-Parra KA, Figueroa-Galvis I, Mestanza O, Polanía J (2019) Effect of salinity on fungal diversity in the rhizosphere of the halophyte Avicennia germinans from a semi-arid mangrove. Fungal Ecol 42:1–9
Velmurugan N, Lee YS (2012) Enzymes from marine fungi: current research and future prospects. In: Jones EBG, Pang KL (eds) Marine fungi and fungal-like organisms. De Gryter, Berlin, pp 441–474
Vrijmoed LLP, Hyde KD, Jones EBG (1994a) Observations on mangrove fungi from Macau and Hong Kong, with the description of two new ascomycetes: Diaporthe salsuginosa and Aniptodera haispora. Mycol Res 98:699–704
Vrijmoed LLP, Jones EBG, Hyde KD (1994b) Observations on subtropical mangrove fungi in the Pearl River Estuary. Acta Scientiarum Naturalium Universitalis Sunyatseni 33:78–85
Vrijmoed LLP, Jones EBG, Alias SA (1996) Preliminary observations on marine and mangrove fungi from Hainan island in Southern China. Asian J Trop Biol 2:31–38
Weber RWS (2006) On the ecology of fungal consortia of spring sap-flows. Mycologist 20:140–143
Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lucking RL, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch T, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castaneda-Ruiz RF, Bahkali AH, Pang KL, Tanaka K, Dai DQ, Sakayaroj J, Hujslova M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Caceres MES, Perez-Ortega S, Fiuza PO, Monteiro JS, Vasilyev LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel-Wahab MA, Takamatsu S, Bensch K, de Silva NI, De Kesel A, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera RH, Tang LZ, Phukhamsakda C, Hernandez-Restrepo M, Ma X, Tibpromma S, Gusmao LFP, Weerahewa D (2017) Notes for genera: Ascomycota. Fungal Divers 86:1–594
Wijayawardene NN, Hyde KD, Lumbsch T, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018) Outline of Ascomycota—2017. Fungal Divers 88:167–263
Wijayawardene NN, Hyde KD, Al-Ani LKT, Tedersoo L, Haelewaters D, Rajeshkumar KC, Zhao RL, Aptroot A, Leontyev DV, Saxena RK, Tokarev YS, Dai DQ, Letcher PM, Stephnson SL, Ertz D, Lumbsch HT, Kukwa M, Issi IV, Madrid H, Phillips AJL, Slebmann L, Pfliegler WP, Horváth E, Bensch K, Kirk PM, Kolaříková K, Raja HA, Radek R, Papp V, Dima V, Ma J, Malosso E, Takamatsu S, Rambold G, Gannibal PB, Triebel D, Gautam AK, Avasthi S, Suetrong S, Timdal E, Fryar SC, Delgado G, Réblová M, Doilom M, Dolatabadi S, Pawłowska JZ, Humber RA, Kodsueb R, Sánchez-Castro I, Goto BT, Silva DKA, de Souza FA, Oehl F, da Silva GA, Silva IR, Błaszkowski J, Jobim K, Maia LC, Barbosa FR, Fiuza PO, Divakar PK, Shenoy BD, Castañeda-Ruiz RF, Somrithipol S, Lateef AA, Karunarathna SC, Tibpromma S, Mortimer PE, Wanasinghe DN, Phookamsak R, Xu J, Wang Y, Tian F, Alvarado P, Li DW, Kušan I, Matočec N, Mešić A, Tkalčec Z, Maharachchikumbura SSN, Papizadeh M, Heredia G, Wartchow F, Bakhshi M, Boehm E, Youssef N, Hustad VP, Lawrey JD, Santiago ALCMA, Bezerra JDP, Souza-Motta CM, Firmino AL, Tian Q, Houbraken J, Hongsanan S, Tanaka K, Dissanayake AJ, Monteiro JS, Grossart HP, Suija A, Weerakoon G, Etayo J, Tsurykau A, Vázquez V, Mungai P, Damm U, Li QR, Zhang H, Boonmee S, Lu YZ, Becerra AG, Kendrick B, Brearley FQ, Motiejūnaitė J, Sharma B, Khare R, Gaikwad S, Wijesundara DSA, Tang LZ, He MQ, Flakus A, Rodriguez-Flakus P, Zhurbenko MP, McKenzie EHC, Stadler M, Bhat DJ, Liu JK, Raza M, Jeewon R, Nassonova ES, Prieto M, Jayalal RGU, Erdoğdu M, Yurkov A, Schnittler M, Shchepin ON, Novozhilov YK, Silva-Filho AGS, Gentekaki E, Liu P, Cavender JC, Kang Y, Mohammad S, Zhang LF, Xu RF, Li YM, Dayarathne MC, Ekanayaka AH, Wen TC, Deng CY, Pereira OL, Navathe S, Hawksworth DL, Fan XL, Dissanayaka LS, Kuhnert E, Grossart HP, Thines M (2020) Outline of Fungi and fungus-like taxa. Mycosphere 11:1060–1456
Xu J (2015) Bioactive natural products derived from mangrove-associated microbes. RSC Adv 5(2):841–892
Xu Z, Wu X, Feng Li G, Xu Z (2019) Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat Prod Res 34(7):1002–1007
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1077–1088
Zhang SN, Hyde KD, Jones EBG, Cheewangkoon R, Liu JK (2018) Acuminatispora palmarum gen. et sp. nov. from mangrove habitats. Mycol Prog 17:1–16
Zhang SN, Hyde KD, Jones EBG, Jeewon J, Cheewangkoon R, Li JK (2019) Striatiguttulaceae, a new pleosporalean family to accommodate Longicorpus and Striatiguttula gen. nov. from palms. MycoKeys 49:99–129
Acknowledgements
This research was supported under the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Kingdom of Saudi Arabia. Gareth Jones thanks Kamarudin Isa for help with the fieldwork in Malaysia, the British Council for logistical and financial support for early mangrove studies in Hong Kong, Malaysia and Singapore and his students who have worked on mangrove fungi. Ka-Lai Pang thanks the Ministry of Science and Technology for financial support (MOST 105-2621-B-019-002-). V.V. Sarma thanks the Ministry of Earth Sciences, Govt. of India for funding a project (MOES/36/OO1S/Extra/40/2014/PC-IV dt.14.1.2015). K.D Hyde is grateful to the Thailand Research Fund (TRF) Grant No. RSA5980068 entitled Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans; National Research Council of Thailand (NRCT) for a Grant entitled Diseases of mangrove trees and maintenance of good forestry practice (Grant Number: 60201000201). Jariya Sakayaroj thanks the Institute of Research and Innovation, School of Science and Walailak University for facilities and financial support (Grant No. WU62234). M.S. Calabon is grateful to the Mushroom Research Foundation and the Department of Science and Technology - Science Education Institute (Philippines).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Devadatha, B., Jones, E.B.G., Pang, K.L. et al. Occurrence and geographical distribution of mangrove fungi. Fungal Diversity 106, 137–227 (2021). https://doi.org/10.1007/s13225-020-00468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-020-00468-0