Abstract
There have been considerable advances in the understanding of species concepts in the genus Colletotrichum. This has lead to the need to carry out fresh surveys of Colletotrichum species associated with important hosts. Colletotrichum species are associated with Citrus plants as saprobes, important pre-harvest and post-harvest pathogens, as well as endophytes. In this study, a total of 312 Colletotrichum strains were isolated from leaves, shoots and fruits of cultivated Citrus and Fortunella species with or without disease symptoms across the main citrus production areas in China. The morphology of all strains were studied and multilocus (ACT, TUB2, CAL, GAPDH, GS, ITS) phylogeny established. Strains were from four important species complexes of Colletotrichum, namely C. gloeosporioides species complex, C. boninense species complex, C. acutatum species complex and a final group including C. truncatum, which was rare on Citrus species. The species belonging to the C. gloeosporioides species complex comprised C. gloeosporioides and C. fructicola, the C. boninense complex comprised C. karstii and a new species C. citricola and the C. acutatum complex included a new species, C. citri. The ability of strains to cause anthracnose on citrus fruits was tested by inoculation and strains of Colletotrichum gloeosporioides, C. fructicola and C. truncatum were pathogenic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colletotrichum is among the most economically important genera of plant pathogenic fungi worldwide (Sutton 1992; Cai et al. 2009; Phoulivong 2011). Many species of Colletotrichum cause diseases of a wide range of important crops commonly known as anthracnose (Sutton 1992; Hyde et al. 2009a). In addition, many Colletotrichum species are latent plant pathogens, species essentially being endophytes, epiphytes or saprobes, switching to a pathogenic lifestyle when host plants are stressed or in postharvest storage (Hyde et al. 2009b).
The history of the naming of Colletotrichum species has recently been reviewed in several important papers (Cannon et al. 2008; Hyde et al. 2009a; Weir et al. 2012) and recent protocols for the identification of new species were outlined in Cai et al. (2009). Following adoption of these polyphasic protocols for studying the genus Colletotrichum, especially the use of multi-gene phylogenetic analysis, the classification and species concepts in Colletotrichum changed significantly (Cai et al. 2009; Cannon et al. 2012; Damm et al. 2012a, b; Weir et al. 2012). A systematic study of nearly all acknowledged species, revealed at least nine clades in this genus (Cannon et al. 2012); many species previously known as a single species proved to be polyphyletic taxa (Cannon et al. 2012). Colletotrichum gloeosporioides (Cannon et al. 2008; Phoulivong et al. 2010b; Weir et al. 2012), C. acutatum (Marcelino et al. 2008; Shivas and Yu 2009; Damm et al. 2012a), C. boninense (Moriwaki et al. 2003; Yang et al. 2009; Damm et al. 2012b) and C. truncatum (Damm et al. 2009; Cannon et al. 2012) are important species complexes and well resolved among all the nine clades. Further studies in these species complexes; such as the newly published species, C. murrayae (Peng et al. 2012) and C. viniferum (Peng et al. 2013) in the C. gloeosporioides species complex improve our knowledge concerning Colletotrichum systematics.
The citrus industry is one of the most important fruit industries worldwide and therefore the study and knowledge of pathogens of this crop is very important (Wang et al. 2012). Three important diseases of Citrus caused by Colletotrichum species worldwide are anthracnose, postbloom fruit drop and key lime anthracnose (Timmer et al. 2000; Lima et al. 2011; McGovern et al. 2012). Anthracnose was previously thought to be caused by C. gloeosporioides (Sutton 1980) only, but recent work has shown that at least thirteen species of Colletotrichum are associated with Citrus in China (Peng et al. 2012) and other countries (Damm et al. 2012a, b; Weir et al. 2012). Postbloom fruit drop was thought to be caused by C. acutatum, but C. gloeosporioides was also found to be the causal agent of postbloom fruit drop in Brazil (Lima et al. 2011) and Bermuda (McGovern et al. 2012). Key lime anthracnose was reported to be caused by “C. acutatum” but the taxon was different from C. acutatum species causing postbloom fruit drop (Peres et al. 2008; MacKenzie et al. 2009) and now recognized as C. limetticola (R.E. Clausen) Damm, P.F. Cannon and Crous (Damm et al. 2012a). With the recent changes in understanding of species concepts in Colletotrichum, new surveys are required to identify the species causing Citrus diseases (Ko Ko et al. 2011b).
Colletotrichum diseases of Citrus in China had not been well researched, and thus C. gloeosporioides is generally recognized as one of the most important pathogens in all cultivated Rutaceae all over China (Peng et al. 2012). Colletotrichum gloeosporioides is reported to cause young shoot and leaf blight, leaf spot, stem-end wither followed by premature fruit drop and postharvest fruits anthracnose (Chinese Research Institute of Pomology and Citrus 1994). However, previous identifications were based on techniques available at the time with identification being based on examination of symptoms, and morphology of conidia produced on the infected tissues (Sutton 1992). Peng et al. (2012) however, showed that seven Colletotrichum species caused anthracnose of citrus leaves, while C. acutatum, which is the causal agent of postbloom fruit drop (Timmer et al. 1994) of many cultivated citrus species has not been reported in China.
The objective of the present study was to characterize Colletotrichum species associated with cultivated Citrus and Fortunella species in the major citrus growing areas in China. Accurate data on Colletotrichum species causing disease of citrus in China will allow quarantine officials, plant breeders and plant health practitioners to make informed decisions concerning import and export, epidemiology, research, and control and management of citrus diseases.
Material and methods
Isolation and culture
Material of both asymptomatic and diseased citrus organs were collected from the major Citrus growing areas in China, including Zhejiang, Jiangxi, Guangdong, Guangxi, Yunnan, Fujian and Shaanxi provinces. Isolation of Colletotrichum species from the diseased tissues followed the methods outlined by Cai et al. (2009). Symptomless and diseased tissues without sporulation were cut into small pieces and surface sterilized by dipping in 1 % sodium hypochlorite for 1 min, 70 % ethanol for 1 min and washed three times in sterile water and dried on sterilized filter paper. All pieces were placed on PDA plates and incubated until mycelium grew out. If conidial mass formed, the conidia were harvested and suspended in sterile water. Isolations were made directly from single conidium in the case of pathogens or saprobes were produced on the samples as detailed in Chomnunti et al. (2011). All isolates were grown at 25 °C on PDA for further study. Selected strains used for further study are listed in Table 1.
Morphological study
Isolates were transferred from the actively growing edge of 4 day old colony by cutting small squares of agar with mycelium, plating on fresh PDA plates and incubating at 25 °C in 12/12 h fluorescent light and the dark. The growth rate was recorded by measuring the diameter of the colonies until day 5, and the mean growth rate was calculated per day. The colonies characters were also recorded. All experiments were preformed in triplicate. After 7 days, the shape, colour and size of 30 conidia and conidiophores was recorded (Eclipse 80i, Nikon, Japan). Appressoria was induced using a slide culture technique (Johnston and Jones 1997; Cai et al. 2009) and after 7 days, the shape, colour and size of 30 appressoria were recorded (Eclipse 80i, Nikon, Japan).
DNA extraction, PCR amplification and sequencing
Isolates were grown for 4 days and surface mycelia were removed using a sterile scarpel blade. The collected mycelia were placed in centrifuge tubes and the genomic DNA was extracted using a Biospin Fungus Genomic DNA Extraction Kit (Bio-Flux, Bioer Technology Co., China) (Wang et al. 2012). Final DNA was suspended in 1× TE buffer and stored at −20 °C. PCR amplification was carried out in S1000TM Thermal Cycler (Bio-Rad Laboratories, Germany). The primers used in this study and their references are listed in Table 2. The protocols for amplification followed previous studies (Su et al. 2011). The amplicons were verified by staining with gelview (BioTeke Corporation, Beijing) and separated on 1 % agarose electrophoresis gel. Predicted fragments were collected and purified. The products were recombined into pMD18-T vector (TaKaRa Biotech. Co., Dalian, China), and transferred to E. coli (TG1) for proliferation. A single clone colony was harvested and the amplified product was sequenced. Sequences were edited by Lasergene 7 (DNAstar, USA).
Phylogenetic analysis
Multi-locus sequences were prepared for phylogenetic analysis, the accession numbers of newly submitted and cited sequences from GenBank are listed in Online Resource 1. Sequences were aligned using Clustal X (Larkin et al. 2007), the aligned file was analyzed using the criterion of Maximum Parsimony (MP) in PAUP* 4b10 (Swofford 2003). Trees were produced using a heuristic search option with random sequences addition at 1000. Maxtree number was unlimited and all trees were saved. Descriptive tree statistics were recorded, including tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI). The examination and evaluation of trees followed the methods reported in Yang et al. (2009) and Su et al. (2011). Trees are shown in Figtree (Rambaut and Drummond 2010).
Pathogenicity tests
One representative strain from each species except C. karstii and C. citricola was selected for pathogenicity testing. Citrus fruits (Ponkan, Citrus reticulata Blanco) were transported to the laboratory directly from an orchard in Quzhou, Zhejiang Province, China. Non-wounded fruits were selected and surface-sterilized by immersing the fruits in 1 % sodium hypochlorite for 5 min, then washed completely in tap water and then dried in a fume hood. Conidial suspensions (104 conidia per ml) were prepared by growing the strains on PDA for 7 days and diluting the conidial masses with sterile water. A drop of 2.5-μl conidial suspension was added to a wound created by puncturing of the cortex with five sterile needles to about 0.5 mm depth. The fruits inoculated with sterile water were prepared as a negative control. The inoculated fruits were laid on the plastic tray, and the trays were covered with the plastic wrap to maintain the moisture, and incubated at 25 °C with 12/12 h fluorescent light and darkness. On each fruit, 1 to 4 sites (Fig. 5n) were inoculated, and the site was regarded as infected when the necrotic spot was significantly larger than that of negative control. At least ten fruits were inoculated for each strain, the trial was carried out in triplicate. The incidence of infection was calculated by the formula [Incidence (%) = (infected sites or fruits/inoculated sites or fruits) × 100 %] at 12-day post inoculation. The significant difference was analyzed with a LSD test by SAS 9.1 TS level 1 M3 (Inc. 2002–2004) software.
Results
Collection of Colletotrichum species
In total, 312 Colletotrichum strains were isolated from citrus tissues from the main citrus growing regions in China; 212 strains (68 %) were isolated from diseased tissues, 70 strains (22.4 %) from asymptomatic tissues and 30 strains (9.6 %) were saprobes. Based on morphology on PDA, most strains produced conidia similar to C. gloeosporioides (Fig. 5a-d). Seven strains produced the “Glomerella” sexual stage (Fig. 5e-n). Eleven strains produced conidia with a hilum or scar at the base (Fig. 6g, h); this is typical of the C. boninense species complex (Damm et al. 2012b). Four strains produced curved conidia (Fig. 7c), three strains produced fusiform conidia (Fig. 8b) which is typical for many species in the C. acutatum species complex (Damm et al. 2012a). Based on these morphological features, representative strains were selected for phylogenetic analysis and further taxonomic study.
Phylogenetic analysis
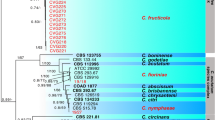
We selected 38 strains for molecular analysis which included 24 strains from the C. gloeosporioides complex, nine strains from the C. boninense complex, two strains with curved conidia and three strains from C. acutatum species complex. Figure 1 is the phylogram calculated to identify the strains in the C. gloeosporioides species complex. The Kahawae and Musae clades could be distinguished, similar to the finding of Weir et al. (2012). Eighteen strains could be confidently identified as C. gloeosporioides as they clustered together with the ex-epitype strain CBS 953.97 with 100% bootstrap support. Six other strains clustered with C. fructicola strains (including the ex-type culture: ICMP 18581) with 99% bootstrap support in the Musae clade. Figure 2 was calculated to identify the strains placed in the C. boninense species complex. Six strains clustered with two representative strains (including the ex-type culture: CORCG6) of C. karstii with robust support (93%), while three strains (ZJUC34, ZJUC35, ZJUC36) appear in a distinct clade and could not be assigned to any currently known species. Two strains producing curved conidia clustered with C. truncatum (including the ex-type type: CBS 151.35) strains with 100% bootstrap support (Fig. 3). Figure 4 was calculated to identify three strains producing elliptical or fusiform conidia. These strains clustered in a distinct clade (100% bootstrap support) and were closest to C. nymphaeae strains (87% bootstrap support).
One of 1000 phylograms of the Colletotrichum gloeosporioides species complex based on maximum parsimony analysis with combined actin (ACT), β-tubulin (TUB2), calmodulin (CAL), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), glutamine synthetase (GS) and internal transcribed spacer (ITS) sequences. Tree Length = 1942, CI = 0.730, RI = 0.865, RC = 0.631, HI = 0.270. C. boninense and C. hippeastri are selected as the outgroup, ex-type and ex-epitype cultures are emphasized in bold
One of 11 phylograms of the Colletotrichum boninense species complex based on maximum parsimony analysis with combined actin (ACT), β-tubulin (TUB2), calmodulin (CAL), chitin synthase 1 (CHS-1), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and internal transcribed spacer (ITS) sequences. Tree Length = 1218, CI = 0.750, RI = 0.877, RC = 0.658, HI = 0.250. C. gloeosporioides strain CBS 953.97 is selected as the outgroup, ex-type or ex-epitype cultures are emphasized in bold
One of 1000 phylograms of the Colletotrichum truncatum species complex based on maximum parsimony analysis with combined actin (ACT), β-tubulin (TUB2), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and internal transcribed spacer (ITS) sequences. Tree Length = 2817, CI = 0.580, RI = 0.863, RC = 0.501, HI = 0.420. C. lindemuthianum was selected as the taxon, ex-type and ex-epitype cultures are emphasized in bold
One of 12 phylograms of the Colletotrichum acutatum species complex from maximum parsimony analysis based on actin (ACT), β-tubulin (TUB2), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and internal transcribed spacer (ITS) sequences. Tree Length = 853, CI = 0.769, RI = 0.844, RC = 0.649, HI = 0.231. C. gloeosporioides strain CBS 953.97 was selected as the outgroup, ex-type or ex-epitype culture are emphasized in bold
Taxonomy
Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. Fig. 5a-d
Colletotrichum gloeosporioides: a, Conidia of ZJUC15; b, Conidia of ZJUC10; c, Conidia of ZJUC18; d, Conidia of ZJUC5. Colletotrichum fructicola (ZJUC22): e and f, Ascocarps. g, Asci. h, Conidia. i, Conidia (from ZJUC24). j and k, Appressoria. l, Colonies on PDA agar medium above. m, Colonies on PDA agar medium below. n, Induced symptoms on fruit (Citrus reticulata). Scale: E = 20 μm, F = 50 μm, others = 10 μm
Of the 312 strains isolated from Citrus sinensis, C. unchiu, C. grandis, C. reticulata and other Citrus species, 287 strains (92 %) were identified as C. gloeosporioides sensu stricto. These strains were isolated as pathogens (202 strains), endophytes (58 strains) and saprobes (27 strains). The conidia of the C. gloeosporioides species varied, strain ZJUC15 was generally shorter, being 11.3–14.7 × 5.3–6.4 μm (\( \overline{x} = 12.6 \times 5.8\,\upmu \mathrm{m} \), Fig. 5a); ten strains produced conidia with two guttules (Fig. 5b), ZJUC17 and ZJUC18 were somewhat fusiform (Fig. 5c), and ZJUC5 and ZJUC19 unusually large (Fig. 5d).
Colletotrichum fructicola Prihastuti, L. Cai & K. D. Hyde Fig. 5e-n
Colonies growing about 7 mm in diameter each day at 25°C, olive to grey with white edge, stroma or sclerotia readily formed on PDA in 20 days. Ascomata 50–116 μm abundant, brown, globose, with a neck, and mostly sterile. Asci 34–70.3 × 7.6–11 μm (\( \overline{x} = 51 \times 8.8\,\mu \mathrm{m} \)), unitunicate and clavate to cymbiform. Ascospores 11–17.4 × 2.7–4.7 μm (\( \overline{x} = 14.4 \times 3.6\,\upmu \mathrm{m} \)), one-celled, hyaline, guttulate, fusiform to slightly curved and with rounded ends. Conidiomata rarely observed. Conidia 12.9–18.9 × 4.5–6.2 μm (\( \overline{x} = 15.5 \times 5.4\,\upmu \mathrm{m} \)), in brick-red masses, hyaline, cylindrical, with rounded ends. Appressoria 8.2–16.4 × 4–8.8 μm (\( \overline{\mathrm{x}} = 12 \times 5.6\,\upmu \mathrm{m} \)), formed from mycelia, brown to dark brown, oval to clavate, sometimes with one end pointed, ovoid or irregular.
Habitat: endophytes from Citrus reticulata cv. nanfengmiju and Fortunella margarita (Lour. ) Swingle.
Notes: Compared to the original description of C. fructicola (Prihastuti et al. 2009), where conidia were 3–4.3 μm wide, strain ZJUC22 was 4.5–6.2 μm wide, strain ZJUC26 was 4.4–5.6 μm wide and strain ZJUC24 was 4.2–5.8 μm wide; thus all strains in this study were wider.
Colletotrichum karstii Y.L. Yang, Z.Y. Liu, K.D. Hyde & L. Cai
Six strains of this species were isolated, three from anthracnose spots on leaves, the other three from asymptomatic tissues of Citrus.
Colletotrichum citricola F. Huang, L. Cai, K.D. Hyde & H.Y. Li, sp. nov. Fig. 6
Mycobank: MB 803017
Etymology: in reference to its occurrence on Citrus = citricola
Holotype: Chenggu, Shaanxi province, China, Saprobes on leaf of Citrus unshiu, May 2012, F. Huang (ZJUC34H , holotype), culture ex-type, ZJUC34=CBS134228=CGMCC3.15227; Chenggu, Shaanxi province, China, Saprobes on leaf of Citrus unshiu, May 2012, F. Huang, ZJUD35=CBS134229, ZJUC36=CBS134230.
When grown on PDA, colonies orange, mycelia loose. Stroma observed in about 1 week. Ascomata orange to brown, globose to near globose. Asci 61 × 13 μm, unitunicate, clavate. Ascospores 12.8–18.4 × 5.3–6.7 μm, (\( \overline{\mathrm{x}} = 15.8 \times 6.1\,\upmu \mathrm{m} \)), one-celled, hyaline, slightly curved to cylindrical, rounded at the ends. Conidiomata barely observed, conidia formed in orange masses, conidiophores hyaline, branched. Conidiogenous cells 14.9–31.8 × 2.8–4.3 μm (\( \overline{\mathrm{x}} = 20.6 \times 3.6\,\upmu \mathrm{m} \)), hyaline, tapered at the apex. Conidia 13.7–16.1 × 5.9–6.9 μm, (\( \overline{\mathrm{x}} = 15.1 \times 6.4\,\upmu \mathrm{m} \)), cylindrical, hyaline, rounded at two ends, often with one end wider, sometimes with a hilum-like base. Appressoria 5.8–10.9 μm diam. (\( \overline{\mathrm{x}} = 8.2\,\upmu \mathrm{m} \)), brown, roundish.
Habitat: Saprobes on Citrus unchiu Hort. ex Tanaka.
Known distribution: Chenggu, Shaanxi Province, China.
Notes: This species belongs to the C. boninense species complex (Fig. 2) and clustered with C. phyllanthi with relative low support (67%). All three strains form a distinct clade with 100% bootstrap support indicating they represent a distinct species. Therefore, C. citricola is introduced to accommodate this new species. Colletotrichum phyllanthi differs from C. citricola by its narrower conidia at 3–5 μm (Pai 1970) versus 5.9–6.9 μm in C. citricola.
Colletotrichum truncatum (Schwein.) Andrus & W.D. Moore Fig. 7
Colonies growing 10–13 mm diameter each day at 25 °C, after 8 days, flat, grey at the center from above, with obvious olive to green margin; in reverse grey to black with radial mycelia growth. Stroma formed and masses of saffron conidia observed. Conidia 23.1–29.6 × 2.6–3.9 μm (\( \overline{\mathrm{x}} = 26.3 \times 3.4\,\upmu \mathrm{m} \)), hyaline, aseptate, smooth walled, slightly curved at the centre with hook-like ends. Setae on leaf spot, 171 × 4.3 μm, brown, tapered to the apex.
Colletotrichum citri F. Huang, L. Cai, K.D. Hyde & H.Y. Li, sp. nov. Fig. 8
Mycobank: MB 803017
Etymology: from Citrus in reference to the occurrence on this host.
Holotype: Ruili, Yunnan province, China, on Anthracnose of a shoot of Citrus aurantifolia, August 2008, G. Q. Chen (ZJUC41H, holotype), culture ex-type, ZJUC41=CBS134233=CGMCC3.15228; Ruili, Yunnan province, China, on Anthracnose shoot of Citrus aurantifolia, August 2008, G. Q. Chen, ZJUC42=CBS134234 and ZJUC43=CBS134235.
Colonies growing 6–8 mm in diameter each day at 25 °C with 12/12 alteration between fluorescent light and dark. After 8 days, the colony was grey and flat from above, and grey to black from below. When inoculated on PDA, stromata produced in the surface of culture, setae absent, light red masses of conidia formed. Conidia 9.5–13.7 × 3.3–4.4 μm (\( \overline{\mathrm{x}} = 12 \times 3.9\,\upmu \mathrm{m} \)), hyaline, smooth, acute to near rounded at two ends. Appressoria 8 × 4.5 μm diam, produced from mycelia, light brown to brown, roundish to ellipsoidal. Sexual state not observed.
Habitat: ZJUC41, ZJUC42 and ZJUC43 were isolated from the shoots of Key Lime (Citrus aurantifolia), causing black withered symptoms.
Known distribution: Ruili, Yunnan Province, China.
Notes: This species is similar to the three species clarified by Shivas and Yu (2009), when the author relied on the differences of interspecies on colonial characteristics and phylogenetic analysis. This species can be differed with C. nymphaeae (Johnson et al. 1997) and C. limetticola (Damm et al. 2012a) by conidia shape and size, while C. citri conidia was shorter and narrower on average.
Pathogenicity testing
The result of pathogenicity testing is shown in Table 3. Colletotrichum gloeosporioides representative (strain ZJUC17) was virulent on most experimental fruits with a mean infection incidence of 93%. Colletotrichum fructicola (strain ZJUC22) (Fig. 5n) infected experimental fruits with a lower mean infection incidence (90%) but this was not significantly different to ZJUC17. Colletotrichum truncatum (strain ZJUC37) was also pathogenic to Citrus fruit with a mean infection incidence of 83% on experimental fruits (Fig. 7f). Colletotrichum citri strain ZJUC41 showed no virulence to Citrus fruits, but it could infect the petal by inoculation (data not shown). Colletotrichum citri is not common on Citrus as only three strains were obtained in this study.
Discussion
Previous studies on Colletotrichum species causing Citrus disease used morphological and single gene based identifications and thus taxa would have been identified to species complexes rather than individual species. A typical study to identify the diseases of Citrus caused by Colletotrichum species using multi-locus data was that of Peng et al. (2012) who isolated seven species of Colletotrichum from diseased Citrus leaves in Guizhou and Yunnan provinces in China.
In this study we surveyed a much wider area of China, and sampled more Citrus varieties and tissues and obtained more Colletotrichum strains. Based on multi-locus data we found that Colletotrichum gloeosporioides in the C. gloeosporioides species complex was a predominant species. It infects the main cultivated Citrus species in China, including Citrus sinensis, C. unchiu, C grandis, C. reticulata and C. limon. Colletotrichum fructicola also in the C. gloeosporioides species complex was only obtained from symptomless tissues. However, pathogenicity tests showed that both species can cause disease of Citrus fruits, indicating they could switch their lifestyle from endophyte to pathogen when the host is wounded or mature. Among the two species differentiated in the C. boninense species complex in this study, C. karstii was previously found to be an important pathogen on Orchidaceae hosts (Yang et al. 2011), and has also been isolated from Citrus plants in South Africa, New Zealand (Damm et al. 2012b) and China (Peng et al. 2012). Peng et al. (2012) showed that this species cause Citrus leaf anthracnose. Colletotrichum citricola is a new species isolated from leaf anthracnose in the northwestern Citrus cultivation areas of China. Colletotrichum truncatum may also be a potential pathogen of Citrus species as shown in the pathogenicity tests. This species occurs in herbaceous plants (Damm et al. 2009), but this is the first report on Citrus spp.. Colletotrichum citri, a new species with fusiform conidia in the C. acutatum species complex (Damm et al. 2012a), was closest to C. nymphaeae (Van der Aa 1978).
Colletotrichum comprises species that can infect several host genera, such as C. siamense (Prihastuti et al. 2009; Yang et al. 2009; Wikee et al. 2011). On the other hand, a single host can harbor several species of Colletotrichum (Peng et al. 2012; Peng et al. 2013). Some species of Colletotrichum, however, appear to be specific to a single host species or genus (Prihastuti et al. 2010; Liu et al. 2011; Su et al. 2011)
Colletotrichum gloeosporioides was previously thought to be a common pathogen of numerous crops and most tropical fruits (Sutton 1992; Cannon et al. 2008). However, epitypification of this species by Cannon et al. (2008) has allowed for accurate identification of the taxon using analysis of multi-locus molecular data. Subsequent studies by Phoulivong et al. (2010a) have shown that C. gloeosporioides is not the common tropical pathogen as once believed (Phoulivong et al. 2010a). Since multi-locus analysis of Colletotrichum disease showed this species had been common on Citrus where it causes anthracnose, but rare on most other hosts (Prihastuti et al. 2009; Yang et al. 2009; Wikee et al. 2011; Weir et al. 2012; Peng et al. 2013).
In this study we have determined the species of Colletotrichum that cause disease of Citrus in the main growing areas of China. The most important causal agent is C. gloeosporioides. C. acutatum has not been found in China, probably because we did not survey in flowering season, and the collected strains were from the infected petals. In this study pathogenicity testing was only carried out on mature Citrus fruits by wound inoculation. The virulence and potential threat of these species to cause disease of leaves, shoots and petals needs to be clarified (Ko Ko et al. 2011a; Ko Ko et al. 2011b).
References
Cai L, Hyde KD, Taylor PWJ, Weir B, Waller J, Abang MM, Zhang JZ, Yang YL, Phoulivong S, Liu ZY (2009) A polyphasic approach for studying Colletotrichum. Fungal Diversity 39:183–204
Cannon PF, Buddie AG, Bridge PD (2008) The typification of Colletotrichum gloeosporioides. Mycotaxon 104:189–204
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum-current status and future directions. Stud Mycol 73:181–213
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Chinese Research Institute of Pomology and Citrus (1994) The records of Chinese fruit trees’ diseases and pests (second edition) (in Chinese). China agriculture press
Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EBG, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH (2011) Capnodiaceae. Fungal Diversity 1–32
Crouch JA, Beirn LA, Cortese LM, Bonos SA, Clarke BB (2009a) Anthracnose disease of switchgrass caused by the novel fungal species Colletotrichum navitas. Mycol Res 113(12):1411–1421
Crouch JA, Clarke BB, Hillman BI (2006) Unraveling evolutionary relationships among the divergent lineages of Colletotrichum causing anthracnose disease in turfgrass and corn. Phytopathology 96(1):46–60
Crouch JA, Clarke BB, White JF Jr, Hillman BI (2009b) Systematic analysis of the falcate-spored graminicolous Colletotrichum and a description of six new species from warm-season grasses. Mycologia 101(5):717–732
Crouch JA, Tomaso-Peterson M (2012) Anthracnose disease of centipede grass turf caused by Colletotrichum eremochloae, a new fungal species closely related to Colletotrichum sublineola. Mycologia 104(5):1085–1096
Damm U, Cannon PF, Woudenberg JHC, Crous PW (2012a) The Colletotrichum acutatum species complex. Stud Mycol 73:37–113
Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, Tan YP, Shivas RG, Crous PW (2012b) The Colletotrichum boninense species complex. Stud Mycol 73:1–36
Damm U, Woudenberg J, Cannon P, Crous P (2009) Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39:45–87
Du M, Schardl CL, Nuckles EM, Vaillancourt LJ (2005) Using mating-type gene sequences for improved phylogenetic resolution of Collectotrichum species complexes. Mycologia 97(3):641–658
Farr DF, Aime MC, Rossman AY, Palm ME (2006) Species of Colletotrichum on Agavaceae. Mycological Research 110(12):1395–1408
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61(4):1323–1330
Hyde KD, Cai L, Cannon PF, Crouch JA, Crous PW, Damm U, Goodwin PH, Chen H, Johnston PR, Jones EBG (2009a) Colletotrichum-names in current use. Fungal Diversity 39:147
Hyde KD, Cai L, McKenzie EHC, Yang YL, Zhang JZ, Prihastuti H (2009b) Colletotrichum: a catalogue of confusion. Fungal Diversity 39:1–17
Inc. SI (2002–2004) SAS 9.1.3 Help and documentation. Cary, NC: SAS Institute Inc
Johnson DA, Carris LM, Rogers JD (1997) Morphological and molecular characterization of Colletotrichum nymphaeae and C. nupharicola sp. nov. on water-lilies (Nymphaea and Nuphar). Mycol Res 101(6):641–649
Johnston PR, Jones D (1997) Relationships among Colletotrichum isolates from fruit-rots assessed using rDNA sequences. Mycologia :420–430
Ko Ko TW, McKenzie EHC, Bahkali AH, To-anun C, Chukeatirote E, Promputtha I, Abd-Elsalam KA, Soytong K, Wulandari NF, Sanoamuang N (2011a) The need for re-inventory of Thai phytopathogens. Chiang Mai Journal of Science 38:625–638
Ko Ko TW, Stephenson SL, Bahkali AH, Hyde KD (2011b) From morphology to molecular biology: can we use sequence data to identify fungal endophytes? Fungal Diversity 50(1):113–120
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Lima WG, Spósito MB, Amorim L, Gonçalves FP, de Filho PAM (2011) Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. Eur J Plant Pathol 131(1):157–165
Liu F, Hyde KD, Cai L (2011) Neotypification of Colletotrichum coccodes, the causal agent of potato black dot disease and tomato anthracnose. Mycology 2(4):248–254
MacKenzie SJ, Peres NA, Barquero MP, Arauz LF, Timmer LW (2009) Host range and genetic relatedness of Colletotrichum acutatum isolates from fruit crops and leatherleaf fern in Florida. Phytopathology 99(5):620–631
Marcelino J, Giordano R, Gouli S, Gouli V, Parker BL, Skinner M, TeBeest D, Cesnik R (2008) Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100(3):353–374
McGovern RJ, Seijo TE, Hendricks K, Roberts PD (2012) New report of Colletotrichum gloeosporioides causing postbloom fruit drop on citrus in Bermuda. Canadian Journal of Plant Pathology 34(2):187–194
Moriwaki J, Sato T, Tsukiboshi T (2003) Morphological and molecular characterization of Colletotrichum boninense sp. nov. from Japan. Mycoscience 44(1):47–53
Noireung P, Phoulivong S, Liu F, Cai L, Mckenzie EH, Chukeatirote E, Jones E, Bahkali AH, Hyde KD (2012) Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogam Mycol 33(3):347–362
O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E (2000) A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41(1):61–78
Pai HS (1970) Life cycle of Colletotrichum heveae inciting spot anthracnose of phyllanthus acidus. Mycopathologia 42(1):65–72
Peng LJ, Sun T, Yang YL, Cai L, Hyde KD, Bahkali AH, Liu ZY (2013) Colletotrichum species on grape in Guizhou and Yunnan provinces, China. Mycoscience 54(1):29–41
Peng LJ, Yang YL, Hyde KD, Bahkali AH, Liu ZY (2012) Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam Mycol 33(3):267–283
Peres N, MacKenzie S, Peever T, Timmer L (2008) Postbloom fruit drop of citrus and key lime anthracnose are caused by distinct phylogenetic lineages of Colletotrichum acutatum. Phytopathology 98(3):345–352
Phoulivong S (2011) Colletotrichum, naming, control, resistance, biocontrol of weeds and current challenges. Current Research in Environmental Applied Mycology 1(1):53–73
Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, Chukeatirote E, Hyde KD (2010a) Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Diversity 44(1):33–43
Phoulivong S, Cai L, Parinn N, Chen H, Abd-Elsalam KA, Chukeatirote E, Hyde KD (2010b) A new species of Colletotrichum from Cordyline fruticosa and Eugenia javanica causing anthracnose disease. Mycotaxon 114(1):247–257
Prihastuti H, Cai L, Chen H, McKenzie EHC, Hyde KD (2009) Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity 39:89–109
Prihastuti H, Cai L, Crouch JA, Phoulivong S, Moslem MA, McKenzie EHC, Hyde KD (2010) Neotypification of Colletotrichum falcatum, the causative agent of red-rot disease in sugarcane. Sydowia 62:283–293
Rambaut A, Drummond A (2010) FigTree v1. 3.1. Institute of evolutionary biology, University of Edinburgh
Rojas EI, Rehner SA, Samuels GJ, Van Bael SA, Herre EA, Cannon P, Chen R, Pang J, Wang R, Zhang Y (2010) Colletotrichum gloeosporioides sl associated with Theobroma cacao and other plants in Panamá: multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 102(6):1318–1338
Shivas R, Yu Y (2009) A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Diversity 39:111
Stephenson SA, Green JR, Manners JM, Maclean DJ (1997) Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis. Current Genetics 31(5):447–454
Su YY, Noireung P, Liu F, Hyde KD, Moslem MA, Bahkali AH, Abd-Elsalam KA, Cai L (2011) Epitypification of Colletotrichum musae, the causative agent of banana anthracnose. Mycoscience :1–7
Sutton BC (1980) The Coelomycetes, Fungi Imperfecti with acervuli, pycnidia and stromata. Commonwealth Mycological Institute, Kew
Sutton BC (1992) The genus Glomerella and its anamorph Colletotrichum. In: Bailey JA, Jeger MJ (eds) Colletotrichum: Biology, pathology and control. CAB international, Wallingford, pp 1–26
Swofford DL (2003) PAUP*: Phylogenetic analysis using parsimony, version 4.0 b10
Templeton MD, Rikkerink EH, Solon SL, Crowhurst RN (1992) Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 122(1):225–230
Timmer LW, Agostini JP, Zitko S, Zulfiqar M (1994) Postbloom fruit drop, an increasingly prevalent disease of citrus in the Americas. Plant Dis 78(4):329–334
Timmer LW, Garnsey SM, Graham JH (2000) Compendium of Citrus Diseases(secong edition). The American Phytopathological Society 28–29:44–45
Van der Aa HA (1978) A leaf spot disease of Nymphaea alba in the Netherlands. Eur J Plant Pathol 84(3):109–115
Wang XH, Chen GQ, Huang F, Zhang JZ, Hyde KD, Li HY (2012) Phyllosticta species associated with citrus diseases in China. Fungal Diversity 52(1):209–224
Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols A guide to methods and applications 315–322
Wikee S, Cai L, Pairin N, McKenzie EHC, Su YY, Chukeatirote E, Thi HN, Bahkali AH, Moslem MA, Abdelsalam K, Hyde KD (2011) Colletotrichum species from Jasmine (Jasminum sambac). Fungal Diversity 46(1):171–182
Yang YL, Cai L, Yu ZN, Liu ZY, Hyde K (2011) Colletotrichum species on Orchidaceae in southwest China. Cryptogam Mycol 32(3):229–253
Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN, McKenzie EHC (2009) Colletotrichum anthracnose of Amaryllidaceae. Fungal Diversity 39:123–146
Acknowledgment
This work was supported by the China Agriculture Research System (CARS-27). L. Cai acknowledges grants KSCX2-YW-Z-1026 ⁄ NSFC31070020. Fang Liu is thanked for technical advices in the phylogenetic analyses. K.D. Hyde thanks the National Research Council of Thailand, Colletotrichum grant number 54201020003 for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 67.3 kb)
Rights and permissions
About this article
Cite this article
Huang, F., Chen, G.Q., Hou, X. et al. Colletotrichum species associated with cultivated citrus in China. Fungal Diversity 61, 61–74 (2013). https://doi.org/10.1007/s13225-013-0232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-013-0232-y