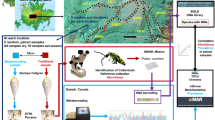
Abstract
Understanding the role of ectomycorrhizal fungi in plant communities is hampered by a lack of knowledge about fungal diversity. DNA barcoding of the ectomycorrhizal fungal genus Cortinarius was used to compare fungal diversity in soil from four plant communities: (i) Nothofagus forest (where Cortinarius is common and diverse), (ii) Kunzea forest (where Cortinarius is present but with low diversity), (iii) a Pinus radiata plantation (Cortinarius is not thought to be present) and (iv) a sub-Antarctic island (where known ectomycorrhizal hosts are absent). PCR primers specific for the ITS region of Cortinarius species were developed. Specificity was tested in vitro and in silico against DNA from basidiocarps of Cortinarius and non-Cortinarius species. The primers were tested for their ability to amplify Cortinarius DNA in soil from forests of the three ectomycorrhizal forest communities and a range of soils from the ectomycorrhiza-free subantarctic Campbell Island. High diversity of Cortinarius was associated with soil of all three ectomycorrhizal communities, despite Cortinarius being previously unrecorded from Pinus. Soil from all three communities share some ectomycorrhizal fungi (including fungi shared between native and exotic hosts), having implications for community succession, introduction of exotic fungi and biodiversity assessment. No Cortinarius was detected from Campbell Island samples. The validated molecular protocol assessed species diversity in a rapid and cost effective way. Baseline biodiversity assessment based on DNA barcoding is more effective at detecting diversity than traditional methods, but requires careful consideration of the difference between ectomycorrhizal fungal diversity in soil versus root-tips.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ectomycorrhizal fungi are essential for the growth and survival of many important forest tree species (Smith and Read 2008) but the subterranean, cryptic lifestyle of the fungi presents unique challenges for research (Horton and Bruns 2001; Anderson and Cairney 2004). The assessment of ectomycorrhizal fungal diversity is a prerequisite to understanding the functional significance of these complex communities (Jonsson et al. 2001; Leake 2001; Baxter and Dighton 2005). Molecular methods recruited to the study of ectomycorrhizal fungi include DNA fingerprinting/profiling by terminal restriction fragment length polymorphism (TRFLP) analysis (Dickie and FitzJohn 2007) or denaturing gradient gel electrophoresis (DGGE) analysis (Anderson et al. 2003; Smit et al. 2003), and DNA sequence analysis/barcoding by conventional (e.g. Edwards and Zak 2010) or high-throughput (e.g. Tedersoo et al. 2008) methods. Amplification of total soil DNA using specific primers cannot target ectomycorrhizal fungi, as the ectomycorrhizal trait has been independently derived many times, both in the Ascomycota (Tedersoo et al. 2006) and Basidiomycota (Horton and Bruns 2001). The use of hyphal ingrowth bags does allow enrichment of ectomycorrhizal fungi in soil DNA, but does not exclude saprotrophic fungi completely (Bastias et al. 2006; Wallander et al. 2010).
Phylogenetic analyses of environmental clones are revealing a great number of previously unknown fungal phylotypes with no close sequence matches on open resources such as GenBank (Taylor et al. 2000; Ryberg et al. 2008). Giving species names to environmental sequences can be difficult, and confounded by the problem that there is no guarantee all named sequences on open resources are identified correctly (Bridge et al. 2003). Environmental DNA derived from soil can only be analyzed with one gene at a time, making resolution of phylogenies poor; this creates further difficulty when discovering described and new species from environmental clone sequences. To circumvent the issue of identifying clone sequences to species levels, authors use the terms operational taxonomic units (OTUs) or molecular operational taxonomic units (MOTUs) when referring to clone sequences grouped together on the basis of percentage similarity or phylogenetic analysis (Anderson et al. 2003; O’Brien et al. 2005; Li and Godzik 2006; Porras-Alfaro et al. 2007; Buée et al. 2009). Integrating taxonomic diversity based on sequences from named sporocarp collections with molecular diversity from soil or ectomycorrhizal root tips has the potential to unify these different approaches for studying ectomycorrhizal communities.
The use of taxon-specific primers is commonly practiced in molecular amplification of soil fungal communities, with fungal specific (Gardes and Bruns 1993), basidiomycete and ascomycete specific (Gardes and Bruns 1993; Tedersoo et al. 2008) and a variety of genus-specific (Tedersoo et al. 2008) primers currently in use. Genus-specific primers reduce complexity in environmental samples, facilitating a more direct focus on taxa of interest. This would be valuable for common, ecologically important but difficult to culture genera such as Cortinarius (Pers.) Gray and Russula Pers. (Brundrett et al. 1996; Cairney and Chambers 1999). The use of specific primers would be less expensive on a small scale than next generation sequencing and is more immediate than using hyphal in-growth bags. Environmental DNA can be screened with genus-specific primers and diversity measured by the analysis of DNA fingerprinting/profiling or MOTUs as defined by cluster analysis.
The fungal genus Cortinarius is one of the larger genera of agaricoid basidiomycetes (Peintner et al. 2001; Danks et al. 2010) and the largest genus of ectomycorrhizal fungi (Høiland and Holst-Jensen 2000; Kirk et al. 2001). With ~2000 named species, Cortinarius represents over a third of all named ectomycorrhizal fungi (Høiland and Holst-Jensen 2000; Kirk et al. 2001). The generic concept is expanding with a number of sequestrate genera (e.g., Thaxterogaster, Protoglossum and some Hymenogaster) and agaricoid genera (e.g., Cuphocybe, Dermocybe and Rozites) shown to be taxonomic synonyms of Cortinarius (Peintner et al. 2001, 2002a, b, 2004; Garnica et al. 2005). Cortinarius is widely distributed in both hemispheres, throughout mainly temperate to subarctic-alpine climates (Peintner et al. 2001, 2004; Danks et al. 2010), although some species are described from the tropics (Peintner et al. 2003). The large number of species in the genus, combined with the wide range of ecologically and economically important host species (Smith and Read 2008) makes Cortinarius a valuable target genus for further study.
This paper describes the development and testing of a PCR-based protocol to amplify Cortinarius DNA in environmental samples using genus-specific primers. To determine primer specificity, the primers were tested on DNA extracted from basidiocarps of ectomycorrhizal species from within and outwith the genus Cortinarius. To determine the effectiveness of the protocol, we attempted to detect Cortinarius, and determine its diversity, in environmental DNA extracted from soil collected in three temperate forest types where ectomycorrhizal hosts are present, and on a sub-Antarctic island where no ectomycorrhizal hosts are found.
Materials and methods
Primer design
Genus-specific primers were developed with the program primer-blast from NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) using the internal transcribed spacer (ITS) region of the nuclear ribosomal RNA cistron as the target region (Fig. 1). Primers were designed against the template Cortinarius rotundisporus NZ8501 (GenBank AF389127) and their specificity was checked against the nr database, with an optimum primer melting temperature of 60 °C and minimum amplified sequence length of 200 base pairs. The specificity check returned 20 matches from the nr database that would be amplified by the primer pair, and we selected primers that returned only Cortinarius sequences in this list. Six primer pairs were used for preliminary screening against herbarium collections. The primer pair “cort4” (5′CGTGATGAGTTGCTGCTGGTTCT3′) and “cort5” (5′GGGCCAGCAAAACCCCCACAT3′) (Fig. 1) were found to amplify Cortinarius and were selected for further testing.
Testing with sporocarp DNA
To test the specificity of the cort4/cort5 primer pair, the primers were tested on genomic DNA extracted from the herbarium sporocarps of 20 Cortinarius species (including two with names only in Dermocybe) and twenty species from other ectomycorrhizal genera (Table 1). Genomic DNA was extracted using either the DNeasy Plant Mini Kit (QIAGEN) or the Genomic DNA Mini Kit (Plant) (Geneaid) following the manufacturer’s protocols. Concentration of DNA was quantified using a spectrophotometer (Nanodrop ND-1000) and adjusted to 0.5 ng/μl. To ensure the DNA extraction had been successful, all forty specimens were amplified using the universal fungal primer pair ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990).
All PCR reactions were carried out in a volume of 20 μl, and used 17 μl of 1.1× ReddyMix PCR Master Mix (Thermo Scientific) 10 pmol of each primer and 0.5 ng of template. Negative controls used sterile purified water instead of the template. All reactions were conducted in a Mastercycler gradient PCR machine (Eppendorf AG, Germany). The protocol for amplification using the cort4/cort5 primer pair was: 95 °C for 5 min, followed by 30 cycles of: 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; then a final elongation step of 72 °C for 10 min. The PCR conditions for amplification using ITS1F/ITS4 were: 95 °C for 5 min, followed by 35 cycles of: 94 °C for 45 s, 45 °C for 45 s, and 72 °C for 45 s; then a final elongation step of 10 min at 72 °C. Gel electrophoresis was used to determine the success of amplification; the DNA was observed by UV transillumination and images captured using a Gel Logic Image System (Kodak, USA). The ITS region was sequenced using the ITS1F/ITS4 amplification products. All sequencing used BigDye® Terminator Version 3.1 (Applied Biosystems Inc., Scoresby, Victoria, Australia) on an ABI3730 DNA Analyser (Applied Biosystems Inc.) at the Genetic Analysis Service, Department of Anatomy and Structural Biology, University of Otago, Dunedin, New Zealand.
Sequences were assembled using geneious pro v5.3 (Drummond et al. 2010) and the cort4/cort5 primer attachment sites were located in an alignment of all sequences amplified. Sequences were aligned in the program geneious pro v5.3 with the mafft (Katoh et al. 2002) plugin using the L-INS-i algorithm, scoring matrix 200PAM/k = 2 and a gap open penalty of 1.53; obvious misalignments (due to poor alignment of short and long sequences) were then corrected manually. The alignment is available on TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S12824). Sequences were tested with the program amplify 3 version 3.1.4 (Engels 2005 ), which uses sequence data to test primer compatibility and ability to amplify in silico. The sporocarp sequences from amplification with the primer pair ITS1F/ITS4 were analysed with amplify 3, along with the primer sequences for cort4 and cort5. All sequences were analysed with the PCR option and default settings to test for the ability to be amplified by the pair. For the species Cortinarius indotatus (OTA60293), multiple intragenomic, polymorphic indels prevented successful reading of a direct sequencing reaction, and a clean sequence was not obtained. Therefore, a sequence of this species retrieved from GenBank (C. indotatus (as D. indotata) GU222322) was used to locate the primer attachment sites and for amplify 3 analysis.
The Cortinarius sequence amplicons obtained from in vitro amplification with primers ITS1F/ITS4 and in silico amplification with primers cort4/cort5 were used in two separate clustering analyses to compare MOTU formations of the entire ITS region against the region amplified by the specific primers. Full-length ITS and trimmed cort4/cort5 sequences were uploaded to the online program CD-HIT Suite: Biological Sequence Clustering and Comparison (http://weizhong-lab.ucsd.edu/cdhit_suite/cgi-bin/index.cgi?cmd=cd-hit-est). Both analyses used the identity cutoff threshold of 0.975 as described below for “Detection range of Cortinarius ”.
Testing on soil DNA
To determine if the cort4/cort5 primer pair could amplify Cortinarius DNA from soil, the primer pair was tested on DNA extracted from soil collected in forests of known ectomycorrhizal hosts and soil collected from a sub-Antarctic island, where no ectomycorrhizal hosts were present.
For the ectomycorrhizal host sites, an approximately 214 ha forest block privately owned by A and J Knight at Tuapeka West, South Otago, New Zealand was selected. The block contains a planted stand of Pinus radiata D. Don at its highest eastern end, descending through several stands of Kunzea ericoides (A.Rich.) Joy Thomps., to a mixed stand of Nothofagus menziesii (Hook.f) Oerst. and N. solandri var. cliffortioides (Hook.f) Poole. All of these species are known to form ectomycorrhizal associations (Chu-Chou and Grace 1988; McKenzie et al. 2000; Orlovich and Cairney 2004; McKenzie et al. 2006). During March 2010, two sites were chosen within each of three forest types, with 10 samples collected per site: Pinus radiata (Samples 1–20), Kunzea ericoides (Samples 21–40) and Nothofagus menziesii and N. solandri var. cliffortioides (Samples 41–60). Samples were collected within a 10 × 10 m plot using a random sampling plan. Each site was at least 50 m away from the edge of the stand and from the other site within the same forest type. For each sample, a 50 ml soil core was taken using a clean and sterile 50 ml polypropylene centrifuge tube. The tubes were capped to prevent cross contamination. Samples were homogenized by grinding briefly with a clean, sterile mortar and pestle; large visible roots (>2 mm thick) were removed by hand. 0.25 g of each soil sample (including root fragments <2 mm thick) was weighed, placed in a collection tube (MoBio PowerBead Tubes, MoBio Laboratories, Inc; Solana Beach, CA) and stored at 4 °C until extraction.
For the non-ectomycorrhizal sites, New Zealand’s southernmost sub-Antarctic territory, Campbell Island, 660 km south of Bluff, New Zealand was selected. Two soil samples were collected approximately 5–10 m apart from each of five locations around the island in November 2007. The sites cover a range of vegetation types, all expected to be comprised of non-ECM hosts, including Poa litorosa Cheesman, Bulbinella and Chionochloa grassland (Samples 1 and 1a, S5231.72 E16908.24 206 m.a.s.l.), mixed Chionochloa and Dracophyllum (Samples 3 and 3a 160 m.a.s.l.), Dracophyllum shrubland (Samples 6 and 6a, S5233.08 E16908.45, 8.5 m.a.s.l.), amongst Pleurophyllum speciosum (Samples 8 and 8a, S5232.91 E16906.65 191 m.a.s.l.), and a P. litorosa and Dracophyllum heath (Samples 9 and 9a, S5235.63 E16908.67 23 m.a.s.l.). The ten soil samples were collected from the top 5–10 cm of soil using individual, new, clean, wooden ice-cream sticks. The approximate weight of each sample was 0.1–0.25 g. Soil was added directly to the MoBio PowerBead tubes and stored at 4 °C until extraction.
Soil DNA extraction
Extractions were done using the PowerSoil DNA Isolation Kit (MoBio) following the manufacturer’s protocol, except at step 5 where samples were placed in a bead beater (Retsch Mixer Mill MM301) at maximum frequency (30.0 cycles/s) for 2 min. DNA was stored at −20 °C.
Isolation of Cortinarius clones from soil samples
The cort4/cort5 primer pair was tested on the DNA extracted from soil samples using the same PCR protocol used for the sporocarp amplifications. The samples were also amplified using the fungal-specific primers ITS1F and ITS4 as a positive control, as described above. The successfully amplified cort4/cort5 PCR products from the soil samples were ligated into plasmid vector pCR2.1, transformed into competent E. coli (strain: Top10), selecting 10 clones for each sample using the TA Cloning Kit (Invitrogen). The clones were screened with the restriction enzymes AluI (Roche), HaeIII (Roche) and HinfI (Roche) and the unique RFLP types for each site were then sequenced with the primers M13f and M13r, as described previously.
Detection range of Cortinarius
The program cd-hit-est (Li and Godzik 2006) was used to define molecular operational taxonomic units (MOTUs) from the soil clone sequences. Previous studies have used a range of cut-offs (90–97 %) when conducting cluster analysis of ITS sequence data as a proxy for species boundaries between fungal genera (O’Brian et al. 2005; Higgins et al. 2007). The sequence data were analysed using a range of percentage identity thresholds (90–99.9 %) to determine a suitable cut-off point for the maximum number of MOTUs. cd-hit-est cluster analysis used a greedy incremental clustering algorithm. If the similarity with a representative is equal to or greater than the set threshold (90–99.9 %), the sequence is grouped into that cluster (Li and Godzik 2006). Similarities below the threshold create new clusters. The representative for each MOTU was blasted using sequence search megablast against the GenBank nucleotide (nr) database within geneious pro version 5.3, recording the top five blast hits as ordered by the bit-score.
To place the representative sequence from each MOTU into a systematic framework for the genus Cortinarius, the alignment of Peintner et al. (2001) was aligned with the MOTU representative sequences and all sequences found on GenBank using the keyword search ‘Cortinarius, New Zealand, Internal’ to produce a phylogeny of the genus. The data set comprised of 244 in-group taxa and three out-group taxa from the related genus Hebeloma (Peintner et al. 2001).
Sequences were aligned as described previously for basidiocarp sequences; resulting alignments were then manually edited. A Bayesian phylogeny was created using MrBayes version 3.2.1 (Ronquist and Huelsenbeck 2003) with a randomly selected starting tree, run for 50 million generations with every 500th tree stored. The burn-in value was equal to 25 % of the sampled generations (25,000). The 18S and 28S genes were excluded from the Bayesian analysis.
Cortinarius diversity literature survey
The host-associations of Cortinarius in New Zealand were recorded from the following sources: Chu-Chou and Grace 1988, 1990; McKenzie et al. 2000, 2006; Segedin and Pennycook 2001; Soop 2001, 2002, 2005, 2012; Orlovich and Oliver 2002; Orlovich and Cairney 2004; Gasparini and Soop 2008; Walbert et al. 2010a, b.
Results
Primer development and testing
The developed primer pair cort4 and cort5 amplify the majority of ITS1, all of the 5.8S gene and the 5′ end of ITS2 (Fig. 1). All species used for the primer test were successfully amplified with the universal fungal primer pair ITS1F/ITS4 (Fig. 2). The cort4/cort5 primer pair successfully amplified 19 of the 20 Cortinarius test specimens and failed to amplify 18 out of 20 species from other genera (Fig. 2). The primer pair was unable to amplify positive test species Cortinarius subcalyptrosporus and did amplify negative test species Inocybe calamistratoides and Tricholoma elegans (Fig. 2).
Gel image of amplification products from sporocarp samples amplified using primer pairs ITS1F/ITS4 and cort4/cort5. Individual lanes are labeled for primer pairs ITS1F/ITS4 (F) and cort4/cort5 (C). Samples 1–20: Cortinarius/Dermocybe species; samples 21–40: non-Cortinarius species. −ve: negative controls; 1: Cortinarius alboroseus; 2: C. austrovenetus; 3. C. caryotis; 4: C. chalybaeus; 5: C. collybianus; 6: C. cycneus; 7: C. dulciolens; 8: C. magellanicus; 9: C. mariae; 10: C. sp. aff. peraurantiacus; 11: C. sp. OTA60292; 12: C. sp. aff. perelegans; 13: C. porphyroideus; 14: C. sp. aff. taylorianus; 15: C. subcalyptrosporus; 16: C. sp. OTA61427; 17: C. sp. aff. xenosma; 18: Dermocybe cardinalis; 19: C. indotatus; 20: D. kula; 21: Austropaxillus nothofagi; 22: Descolea recedens; 23: D. phlebophora; 24: Gallacea eburnea; 25: Hebeloma sacchariolens; 26: Inocybe calamistratoides; 27: I. leptospermi; 28: Laccaria fibrillosa; 29: L. glabripes; 30: Lactarius hauroko ined.; 31: Leotia lubrica; 32: Rossbeevera pachyderma; 33: Russula tawai; 34: R. pseudoareolata; 35: R. atroviridis; 36: R. umerensis; 37: R. roseopileata; 38: R. tricholomopsis; 39: Tricholoma elegans; 40. T. viridiolivaceum. 1 kb plus ladder is indicated with ‘M’
An alignment of the sequences obtained from amplification of the complete ITS region in all test species was used to show the attachment sites of the cort4/cort5 primer pair (Fig. 3). Those test species that amplified in vitro had good base pairing at the 3′ end of both genus specific primers, whereas overall those that did not amplify in vitro had poor base pairing at the 3′ end of one or both primers (Fig. 2 and Fig. 3). All species amplified in vitro using the PCR protocol were also analysed using the program amplify3. The species Hebeloma sacchariolens, Laccaria glabripes and Tricholoma viridolivaceum did not amplify in vitro (Fig. 2), however, binding sites cort5 were found with the program amplify3 (Fig. 3). Overall, the Cortinarius species tested displayed good matches to the cort4 and cort5 primers at the 3′ ends of both primers. Both Cortinarius subcalyptrosporus and C. collybianus displayed poorer matches to the primer sequences at the 3′ end of cort5, but C. subcalyptrosporus also did not match well at the 3′ end of cort4 and was similarly not amplified in silico (Fig. 3). In the two non-target species that did amplify in vitro and in silico, Inocybe calamistratoides and Tricholoma elegans, attachment sites for both primers cort4 and cort5 show few mismatches at the 3′ end (Fig. 3).
Alignment of the cort4/cort5 primer sequences with each species used to test for primer specificity. Expected amplification of each species in silico with ampliy3X, is indicated as positive (+) or negative (−) attachment for each primer, and amplification success in vitro as analysed by gel electrophoresis is indicated as positive (+) or negative (−). 1 Cortinarius indotatus OTA60293 was used for in vitro amplification
The clustering analysis determined 19 unique MOTUs from the 20 full-length ITS Cortinarius sequences and 18 unique MOTUs from the 20 trimmed cort4/cort5 sequences (Online Resource 1). These results show that using a 97.5 % identity cutoff is 94.7 % accurate at determining species diversity using the full-length ITS region, and that the narrower cort4/cort5 amplicon detects 89.5 % of known species diversity in the pool tested here. These results are to some degree dependent on the relatedness of the species in the sample pool, so these percentages are not likely to be directly transferrable to other studies.
Isolation of Cortinarius clones from soil samples
Fungal DNA was successfully amplified from all soil samples using the universal primer pair ITS1F/ITS4 (Fig. 4). The primer pair cort4/cort5 successfully amplify DNA from soil samples collected beneath the ectomycorrhizal hosts K. ericoides, Nothofagus spp. and P. radiata (Fig. 4a). The cort4/cort5 primer pair did not amplify any of the soil DNA samples collected from Campbell Island (Fig. 4b). A total of 145 soil-clones were sequenced from the cort4/cort5 primer pair amplifications of soil samples collected from the ectomycorrhizal host forests. 53 soil-clones were amplified from the Nothofagus spp. stand, 57 from the K. ericoides stand and 36 from the P. radiata stand (Online Resource 2).
Gel image of soil samples testing for amplification of fungal DNA (ITS1F/ITS4) and Cortinarius DNA (cort4/cort5). Individual lanes are labeled for primer pairs ITS1F/ITS4 (F) and cort4/cort5 (C). a. Amplification products from representative soil samples chosen from the Tuapeka West sites (samples 01–12: Pinus; 21–32: Kunzea: 41–52: Nothofagus). b. Amplification products from soil samples from Campbell Island (1–1a: Poa litorosa, Bulbinella and Chionochloa grassland; 3–3a: mixed Chionochloa and Dracophyllum; 6–6a: Dracophyllum shrubland; 8–8a: megaherb field; 9–9a: P. litorosa with a Dracophyllum heath). 1 kb plus ladder is indicated with an ‘M’
Phylogenetic analyses of soil clone MOTUs
An optimal similarity index was determined by plotting the number of sequence clusters obtained at a range of thresholds, from 90 to 99.9 %. The point at which the sequences stop forming close-paired groupings was considered to be the best estimation of the number of total MOTUs. The cluster analysis determined an optimal similarity index of 31 unique MOTUs at 0.975 sequence identity (Fig. 5). Of the total 31 MOTUs, three were shared between all forest types, with 21 MOTUs found from the Kunzea sampling sites, 16 from the Pinus sites and 11 from the Nothofagus sites (Fig. 6a). Four MOTUs were shared between P. radiata and Nothofagus, 12 MOTUs shared between P. radiata and K. ericoides, and four shared between K. ericoides and Nothofagus. The megablast hits for representatives of the MOTUs are all from the genus Cortinarius (Online Resource 3). In both the K. ericoides and P. radiata forest sites, there were a higher number of MOTUs than the number of species that have been recorded for either host throughout New Zealand (Fig. 6b, Online Resource 5).
Venn diagrams illustrating the diversity of Cortinarius. a. Diversity based on numbers of MOTUs from the forest soil sampled in the present study. b. Diversity based on taxonomic descriptions of species recorded in New Zealand (see Online Resource 5 for details) from Pinus radiata, Nothofagus and Kunzea. Kunzea includes species described pre-1989 from Leptospermum, when K. ericoides was L. ericoides, where no newer information is available
Phylogenetic analysis of the representative sequences from each cluster (Online Resource 2) showed that the soil-clones are related to a range of species in the genus Cortinarius with 20 MOTUs related to 11 clades of Northern and Southern Hemisphere species, supported by the Bayesian posterior probabilities >0.97 (Online Resource 4).
Discussion
Through the use of genus-specific primers, we have successfully measured the soil diversity of the ectomycorrhizal fungal genus Cortinarius in three ectomycorrhizal forest types and soil on Campbell Island. The diversity detected in soil is likely to be a superset of diversity on ectomycorrhizal root tips, since we cannot exclude the possibility that some of the MOTUs detected in the present study were from spores in soil, and not from ectomycorrhizas with the host in that soil. This could explain why the molecular diversity of Cortinarius shared between the three forest types was greater than expected. We found unexpectedly high molecular diversity of Cortinarius in forest soil associated with Kunzea and Pinus, including MOTUs restricted to soil of just one host. The molecular diversity of Cortinarius shared between the three forest types was also greater than expected. The range of detection indicates the potential for use of these primers to investigate directly, the molecular ecology of this speciose (~ 2000 described species), but understudied genus. This PCR-based protocol creates the opportunity to focus research on the taxonomic diversity of Cortinarius from different forest communities. The importance of knowing and understanding baseline diversity is crucial where host species are strongly reliant on mycorrhizal associations, such as New Zealand Nothofagus species (Baylis 1967). While the detection of Cortinarius DNA in soil does not necessarily mean that it is forming ectomycorrhizas with that host, it may indicate range of dispersion of these fungi and the availability and diversity of potential inoculum.
This study records the first molecular detection of Cortinarius through amplification of soil DNA in association with ectomycorrhizal host species P. radiata. The cort4/cort5 primer pair successfully amplified Cortinarius in all twenty samples from the two P. radiata sites with 16 unique MOTUs identified within the genus Cortinarius. There are currently three unidentified collections of Cortinarius basidiocarps from P. radiata in New Zealand (from the mid-Canterbury region, voucher: PDD87791, PDD87792 and PDD95864; New Zealand Fungal Herbarium) and no published record of Cortinarius ectomycorrhizas in association with P. radiata in New Zealand (Chu-Chou and Grace 1988, 1990; Walbert et al. 2010a, b). Two of the three unidentified Cortinarius sporocarps have been sequenced (available on GenBank KC153997 and KC153998) and preliminary phylogenetic analyses (not shown) indicated that these new sequences are not closely related to any of our soil clone sequences, nor were they related to collections of named Cortinarius species. We cannot confirm that Cortinarius forms ectomycorrhizas with Pinus radiata, and indeed its absence in previous studies does indicate that it is rare at best in production forests. Nonetheless, it is intriguing that the soil of the Pinus radiata forest harbored unique Cortinarius MOTUs, and when considered with the basidiocarp collections from Pinus radiata forests elsewhere in New Zealand, does indicate a strong possibility that such associations may occur, albeit rarely, and would be worthy of further study.
The similarity of molecular diversity for Cortinarius in the three forest types is in sharp contrast to the diversity based on sporocarp-based descriptions. Nothofagus is known to support a greater ectomycorrhizal fungal diversity than any other New Zealand host (Orlovich and Cairney 2004), with 176 species of Cortinarius described in association with Nothofagus spp. (Online Resource 5), However, the number of MOTUs detected in the present study was relatively even, indicating that the Pinus and Kunzea communities may be taxonomically under-sampled if these MOTUs are indeed ectomycorrhizal with these host trees. While these differences may reflect a taxonomic sampling bias in favor of Nothofagus forests, the high diversity of MOTUs detected in the present study, despite a relatively small study area, indicates that amplification of soil DNA has great potential for the study of the diversity of Cortinarius or other genera.
Although there is poor resolution in the Cortinarius phylogeny a number of the soil-clone MOTUs are related to named species. The ITS region is frequently used for fungal species identification (Bruns et al. 1991; Gardes and Bruns 1993; Peintner et al. 2003; Tedersoo et al. 2003; Schoch et al. 2012). This is because of the high variation found within the internal transcribed spacers, which are subject to faster evolutionary change in comparison to the 18S, 5.8S and 28S genes. The short length of the soil-clone sequences (350–450 bp) may have limited the resolution of the phylogeny. Short variable sequences are sufficient for species identification, DNA barcoding and separation within a phylogeny, but do not necessarily contain enough sequence data or gene coverage to resolve deeper evolutionary relationships. Whilst this is a limiting factor in using the cort4/cort5 primer pair to construct a phylogeny, this is not a limitation for DNA barcoding. Truncating the ITS sequences of the basidiocarps sequenced in this study to the cort4/cort5 region only resulted in one less MOTU cluster, indicating that using a shorter sequence had a minimal effect on the diversity recovered.
The coverage of the ITS region by the cort4/cort5 primer pair has the benefit of including the majority of ITS1, which is often used for sequence identification to species level and is more variable on average in most fungi (Nilsson et al. 2008). Although testing of the primers on ectomycorrhizal basidiocarps revealed some non-specific amplification, no non-Cortinarius sequences were amplified from the soil-DNA. This indicates that while some non-specific amplification of species is possible in single species amplifications, this was not a practical problem in the analysis of the soil samples. While wider sampling of Cortinarius species was beyond the scope of this study, it would be a valuable exercise to further validate the primer pair in regions of different diversity.
The high specificity and sensitivity of the primers to Cortinarius on DNA extracted from soil means that a negative PCR reaction using the PCR-based protocol is indicative of the absence of the target genus. Further in silico and in vitro validation would clarify the boundaries of utility of this primer pair, given the very large phylogenetic diversity of the genus Cortinarius. The absence of Cortinarius in Campbell Island soil is not surprising, since there are no ectomycorrhizal host species present on the island. The cost effectiveness of this PCR-based protocol allows for the assessment of diversity across a wide range of locations or forest types. For example, Peintner et al. (2003) made the first record of Cortinarius from India; the use of specific primers would aid in screening this and other underexplored regions for biodiversity. Microhabitats like canopy soil (Nadkarni 1981; Vance and Nadkarni 1996; Hertel 2010) are known in some cases to support ectomycorrhizal communities (Hertel 2010; Orlovich et al. 2012), but there is little information on the diversity of fungal species in these habitats. The primers developed here could be used to screen soil DNA from such unique soil systems for comparison with terrestrial soils. Surveying Cortinarius in forests of different host trees would be facilitated by using the primers developed here if used on root-tip-extracted DNA, enabling questions of host specificity and local endemism to be addressed. Cortinarius is one of the most commonly occurring and diverse genera on Nothofagus roots in New Zealand (Dickie et al. 2010) but little is known about specificity of Cortinarius to different Nothofagus species, nor to the other two native ectomycorrhizal host genera in New Zealand, Leptospermum and Kunzea. Using genus-specific primers like those described here will permit rapid, cost effective surveys that would inform taxonomic and systematic studies of Cortinarius and other important ectomycorrhizal genera. Our strategy will also be useful in validating next generation sequencing results of ectomycorrhizal soil communities.
References
Anderson IC, Cairney JWG (2004) Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques. Environ Microbiol 6:769–779. doi:10.1111/j.1462-2920.2004.00675.x
Anderson IC, Campbell CD, Prosser JI (2003) Diversity of fungi in organic soils under a moorland—Scots pine (Pinus sylvestris L.) gradient. Environ Microbiol 5:1121–1132. doi:10.1046/j.1462-2920.2003.00522.x
Bastias B, Xu Z, Cairney J (2006) Influence of long-term repeated prescribed burning on mycelial communities of ectomycorrhizal fungi. N Phytol 172:149–158. doi:10.1111/j.1469-8137.2006.01793.x
Baylis GTS (1967) Experiments on the ecological significance of phycomycetous mycorrhizas. N Phytol 66:231–234. doi:10.1111/j.1469-8137.1967.tb06001.x
Baxter JW, Dighton J (2005) Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15:513–523. doi:10.1007/s00572-005-0359-0
Bridge PD, Roberts PJ, Spooner BM, Panchal G (2003) On the unreliability of published DNA sequences. N Phytol 160:43–48. doi:10.1046/j.1469-8137.2003.00861.x
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Pure culture isolation of fungi and production of inoculum. In: Working with mycorrhizas and agriculture. Pirie Printers, Canberra, Australia, pp 233–234
Bruns TD, White TJ, Taylor JW (1991) Fungal molecular systematics. Annu Rev Ecol Syst 22:525–564. doi:10.1146/annurev.es.22.110191.002521
Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, Martin F (2009) 454 pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. N Phytol 184:449–456. doi:10.1111/j.1469-8137.2009.03003.x
Cairney JWG, Chambers SM (1999) Ectomycorrhizal fungi—key genera in profile. Springer, New York
Chu-Chou M, Grace LJ (1988) Mycorrhizal fungi of radiata pine in different forests of the North and South islands in New Zealand. Soil Biol Biochem 20:883–886. doi:10.1016/0038-0717(88)90098-3
Chu-Chou M, Grace LJ (1990) Mycorrhizal fungi of radiata pine seedlings in nurseries and trees in forests. Soil Biol Biochem 22:959–966. doi:10.1016/0038-0717(90)90136-N
Danks M, Lebel T, Vernes K (2010) ‘Cort short on a mountaintop’—Eight new species of sequestrate Cortinarius from sub-alpine Australia and affinities to sections within the genus. Persoonia 24:106–126. doi:10.3767/003158510X512711
Dickie IA, FitzJohn RG (2007) Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza 17:259–270. doi:10.1007/s00572-007-0129-2
Dickie IA, Bolstridge N, Cooper JA, Peltzer DA (2010) Co-invasion by Pinus and its mycorrhizal fungi. N Phytol 187:475–484. doi:10.1111/j.1469-8137.2010.03277.x
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A (2010) Geneious v.5.3. http://www.geneious.com/
Edwards IP, Zak DR (2010) Phylogenetic similarity and structure of Agaricomycotina communities across a forested landscape. Mol Ecol 19:1469–1482. doi:10.1111/j.1365-294X.2010.04566.x
Engels B (2005) Amplify 3 Version 3.14 for MacOS X. http://engels.genetics.wisc.edu/amplify/ University of Wisconsin
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhiza and rusts. Mol Ecol 2:113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
Garnica S, Weiβ M, Oertel B, Oberwinkler F (2005) A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, Agaricales) derived from morphological and molecular data. Can J Bot 83:1457–1477. doi:10.1139/b05-107
Gasparini B, Soop K (2008) Contribution to the knowledge of Cortinarius (Agaricales, Cortinariaceae) of Tasmania (Australia) and New Zealand. Australas Mycol 27:173–203
Hertel D (2010) Tree roots in canopy soil of old European beech trees—An ecological reassessment of a forgotten phenomenon. Pedobiol 54:119–125. doi:10.1016/j.pedobi.2010.11.003
Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F (2007) Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phylogenetics Evol 42:543–555
Høiland K, Holst-Jensen A (2000) Cortinarius phylogeny and possibly taxonomic implications of ITS rDNA sequences. Mycologia 92: 694–710
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10:1855–1871. doi:10.1046/j.0962-1083.2001.01333.x
Jonsson LM, Nilsson M-C, Wardle DA, Zackrisson O (2001) Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93:353–364. doi:10.1034/j.1600-0706.2001.930301.x
Katoh K, Kazuharu M, Kuma K-I, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucl Acids Res 30:3059–3066. doi:10.1093/nar/gkf436
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth & Bisby's Dictionary of the Fungi, 9th Edition. CABI Publishing, UK
Leake JR (2001) Is diversity of ectomycorrhizal fungi important for ecosystem function? N Phytol 152:1–8. doi:10.1046/j.0028-646X.2001.00249.x
Li W, Godzik A (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinforma 22:1658–1659. doi:10.1093/bioinformatics/btl158
McKenzie EHC, Buchanan PK, Johnston PR (2000) Checklist of fungi on Nothofagus species in New Zealand. N Z J Bot 38:635–720. doi:10.1080/0028825X.2000.9512711
McKenzie EHC, Johnston PR, Buchanan PK (2006) Checklist of fungi on teatree (Kunzea and Leptospermum species) in New Zealand. N Z J Bot 44:293–335. doi:10.1080/0028825X.2006.9513025
Nadkarni NM (1981) Canopy roots: convergent evolution in rainforest nutrient cycles. Sci 214:1023–1024. doi:10.1126/science.214.4524.1023
Nilsson RH, Kristiansson E, Ryberg M, Hallenberg N, Larsson K-H (2008) Intraspecific ITS variability in the Kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinforma 4:193–201. doi:10.4137/EBO.S653
O’Brien HE, Parrent JL, Jackson JA, Moncalvo J-M, Vilgalys R (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 71:5544–5550. doi:10.1128/AEM.71.9.5544-5550.2005
Orlovich DA, Cairney JWG (2004) Ectomycorrhizal fungi in New Zealand: current perspectives and future directions. N Z J Bot 42:712–738. doi:10.1080/0028825X.2004.9512926
Orlovich DA, Oliver A-MB (2002) The taxonomic identity of Gymnopilus rubrocastaneus recently described from New Zealand. N Z J Bot 40:481–487. doi:10.1080/0028825X.2002.9512808
Orlovich DA, Draffin SJ, Daly RA, Stephenson SL (2012) Piracy in the high trees: ectomycorrhizal fungi from an aerial “canopy soil” microhabitat. Mycol. doi:10.3852/11-307
Peintner U, Bougher NL, Castellano M, Moncalvo M, Moser M, Trappe J, Vilgalys R (2001) Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). Am J Bot 88:2168–2179
Peintner U, Moser M, Vilgalys R (2002a) Thaxterogaster is a taxonomic synonym of Cortinarius: new names and new combinations. Mycotaxon 81:177–184
Peintner U, Moser M, Vilgalys R (2002b) Rozites, Cuphocybe and Rapacea are taxonomic synonyms of Cortinarius: new names and new combinations. Mycotaxon 83:447–452
Peintner U, Moser MM, Thomas KA, Manimohan P (2003) First records of ectomycorrhizal Cortinarius species (Agaricales, Basidiomycetes) from tropical India and their phylogenetic position based on rDNA ITS sequences. Mycol Res 107:485–494. doi:10.1017/S0953756203007585
Peintner U, Moncalvo J-M, Vilgalys R (2004) Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycol 96:1042–1058
Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2007) Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296:65–75. doi:10.1007/s11104-007-9290-9
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogeny inference under mixed models. Bioinforma 19:1572–1574. doi:10.1093/bioinformatics/btg180
Ryberg M, Nilsson RH, Kristiansson E, Töpel M, Jacobsson S, Larsson E (2008) Mining metadata from unidentified ITS sequences in GenBank: a case study in Inocybe (Basidiomycota). BMC Evol Biol 8:50. doi:10.1186/1471-2148-8-50
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spounge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci USA. doi:10.1073/pnas.1117018109
Segedin BP, Pennycook SR (2001) A nomenclatural checklist of agarics, boletes, and related secotioid and gasteromycetous fungi from New Zealand. N Z J Bot 39:285–348. doi:10.1080/0028825X.2001.9512739
Smit E, Veenman C, Baar J (2003) Molecular analysis of ectomycorrhizal basidiomycete communities in a Pinus sylvestris L. stand reveals long-term increased diversity after removal of litter and humus layers. Microbiol Ecol 45:49–57. doi:10.1016/S0168-6496(03)00109-0
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic, Cambridge
Soop K (2001) Contribution à l’étude de la mycoflore cortinarioïde de Nouvelle-Zélande. Bull Soc Mycol Fr 117:91–132
Soop K (2002) Contribution à l’étude de la mycoflore cortinarioïde de Nouvelle-Zélande. II. Bull Soc Mycol Fr 118:173–194
Soop K (2005) A contribution to the study of the cortinarioid mycoflora of New Zealand, III. N Z J Bot 43:551–562. doi:10.1080/0028825X.2005.9512974
Soop K (2012) Cortinarioid fungi of New Zealand—An Iconography and key, 8th edn. Éditions Scientrix, Mora
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32. doi:10.1006/fgbi.2000.1228
Tedersoo L, Kõljalg U, Hallenberg N, Larsson K-H (2003) Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. N Phytol 159:153–165. doi:10.1046/j.1469-8137.2003.00792.x
Tedersoo L, Hansen K, Perry BA, Kjøller R (2006) Molecular and morphological diversity of pezizalean ectomycorrhiza. N Phytol 170:581–596. doi:10.1111/j.1469-8137.2006.01678.x
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. N Phytol 180:479–490. doi:10.1111/j.1469-8137.2008.02561.x
Vance ED, Nadkarni NM (1996) Microbial biomass and activity in canopy organic matter and the forest floor of a tropical cloud forest. Soil Biol Biochem 22:677–684. doi:10.1016/0038-0717(90)90015-R
Walbert K, Ramsfield TD, Ridgway HJ, Jones EE (2010a) Ectomycorrhizal species associated with Pinus radiata in New Zealand including novel associations determined by molecular analysis. Mycorrhiza 20:209–215. doi:10.1007/s00572-009-0277-7
Walbert K, Ramsfield TD, Ridgway HJ, Jones EE (2010b) Ectomycorrhiza of Pinus radiata (D. Don 1836) in New Zealand—an above- and below- ground assessment. Australas Mycol 29:7–16
Wallander H, Johansson U, Sterkenburg E, Brandström Durling M, Lindahl BD (2010) Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. N Phytol 187:1124–1134. doi:10.1111/j.1469-8137.2010.03324.x
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed) PCR protocols, a guide to methods and applications. Academic Press, San Diego, pp 315–324
Acknowledgments
We thank Shagufta Singh, Yasodha Narayanan, Michael Lucas and Vickey Tomlinson for expert technical assistance. We thank Allison and John Knight for providing access to their property and for logistical support. This research was supported through funding to DAO and PLG from the Shore Fund and Performance-Based Research Fund, University of Otago. SET is supported by a University of Otago Pacific Island Masters Scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 655 kb)
Rights and permissions
About this article
Cite this article
Teasdale, S.E., Beulke, A.K., Guy, P.L. et al. Environmental barcoding of the ectomycorrhizal fungal genus Cortinarius . Fungal Diversity 58, 299–310 (2013). https://doi.org/10.1007/s13225-012-0218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-012-0218-1