Abstract
Climate change affects various facets of life but there is little data on its effects on wild mushroom fruiting. Yunnan Province in China is a rich source of wild mushrooms and has experienced a temperature rise over recent decades. This has resulted in warmer temperatures but the impacts of these changes on mushroom production lack documentation. We collected data on the fruiting of the highly prized matsutake mushroom (Tricholoma matsutake) in West Yunnan, China over an 11 year period from 2000 to 2010. Fruiting phenology and productivity were compared against the driving meteorological variables using Projection to Latent Structure regression. The mushrooms appeared later in the season during the observation period, which is most likely explained by rising temperatures and reduced rain during May and June. High temperature and abundant rain in August resulted in good productivity. The climate response of matsutake production results from a sequence of processes that are possibly linked with regulatory signals and resource availability. To advance the knowledge of this complex system, a holistic research approach integrating biology, ecology, genetics, physiology, and phytochemistry is needed. Our results contribute to a general model of fungal ecology, which can be used to predict the responses of fungi to global climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our planet is undergoing significant climate changes (IPCC 2007) and these changes are having diverse impacts on global biodiversity at various scales (Butchart et al. 2010). Living organisms either develop mechanisms to cope with changing environments (adaptation) or suffer extinction (Thomas et al. 2004; Butchart et al. 2010). Adaptation can take the form of a shift in species’ ranges (Chen et al. 2011; Pearman et al. 2011) or changes in physiological traits, population dynamics, and reproductive behaviour(Hof et al. 2011). Phenology is the science of periodic plant and animal life-cycle events and how these are influenced by seasonal and inter-annual variations in climate. It is one of the primary indicators of the responses of living organisms to climate change (Schwartz et al. 2006; Primack and Miller-Rushing 2011).
Phenological research has traditionally focused mainly on higher plants and animals (Menzel et al. 2006). Due to their elusive traits, long-term monitoring of fungi is generally difficult and expensive and there have been very few studies (Krivtsov et al. 2003). Researchers have only recently begun to analyse mushroom phenology, focusing on fruiting dates and this has been mostly based on collection times of herbarium records. Studies in southern England (Gange et al. 2007) and Norway (Kauserud et al. 2008, 2010) have demonstrated a rapid change of fungal phenology in terms of first fruiting date, last fruiting date, total fruiting duration and mean fruiting date (Kauserud et al. 2008, 2010; Gange et al. 2007). In general, changes are related to temperature and precipitation and are likely to continue to change as the climate changes (Kauserud et al. 2008). Such research has provided useful knowledge, but it often includes inherent errors or biases due to lack of actual observations. The harvesting date, or the time when a specimen is collected, is only a surrogate for the fruiting date. Moreover, the direction and magnitude of change are species and site dependent (Kauserud et al. 2008, 2010). On-site monitoring and species-specific studies are critical for validation, and for filling knowledge gaps (Büntgen et al. 2011).
Yunnan is located in the east of the Himalayan mountain range. It embraces two global biodiversity hot spots, namely the mountains of southwest China and Indo-Burma (Myers et al. 2002). This mountainous region harbours enormous fungal diversity and a host of wild gourmet mushrooms (Yang 2010; Zhang et al. 2010). A market survey revealed 271 marketable wild edible mushrooms in this region (Wang and Liu 2002), amongst which matsutake, morels, truffles, Cordyceps and boletes are the most valuable and traded globally (Sitta and Floriani 2008; Yang et al. 2008; Weckerle et al. 2010; Samils et al. 2008).
Matsutake mushrooms are highly priced, seasonal gourmet items for the Japanese market (Wang et al. 1997). Matsutake is the commercial name for a group of higher fungi belonging to the genus Tricholoma. They are natural mycorrrhizal fungi that have developed symbiotic relationships with certain tree species, mainly pine (Pinus spp.) and oak (Quercus spp.). The principal species is T. matsutake. To date, no artificial cultivation is possible and all mushrooms are extracted from the natural environment. As an item in great demand, the impacts of global change and potential overharvesting are serious resource management concerns (Yang et al. 2008, 2009). In this study we aimed to answer the questions: 1) how have fruiting phenology and productivity of the matsutake mushroom changed in recent years and; 2) does the change relate to changes in climate?
Methods
Study area
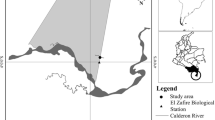
The village of Haitangwa (N 25°16′27.6″, E 99°18′15.0″, Fig. 1) is located in the east of Yunnan Province around 20 km from Baoshan City. Situated along the Gaoligong mountain range, the region has a montane subtropical climate with a clear division between the dry and rainy seasons. Mean monthly temperatures (data from 1971 to 2000) range from 8°C in January to 22°C in June. Total monthly precipitation ranges from 13 mm in December to 173 mm in August. In accordance with global climate warming, temperatures have increased by 0.9°C between 1965 and 2005 (Ma et al. 2009) and showed a slight decrease on annual average temperature in the following years (Fig. 2).
Trend of annual mean temperature at the study site (data source: the China Meteorological Data Sharing Service System, http://cdc.cma.gov.cn/index.jsp)
The forest type producing matsutake is mainly Pinus yunnanensis which grows in mixture with oak trees (Quercus spp.). Canopy cover ranges from 60 to 80%. In order to manage the highly-priced matsutake, the village committee of Haitangwa divided the forest lands among households. Each household is responsible for managing and harvesting mushrooms on the assigned land (Yang et al. 2009). There are 48 patches of forest ranging in size from 0.13 ha to 1.6 ha. The specific forest site under observation encompasses an area of one ha on a south-facing slope with an average inclination of 25°.
Climatic data
Since site-specific weather data is not available at the study location, we used data (1999–2010) from the climate station at Baoshan City, about 20 km away. Data were acquired through the China Meteorological Data Sharing Service System (http://cdc.cma.gov.cn/index.jsp). We tested the correlation of the data collected from the main station in Baoshan with on-site observation from a meteorological station installed at the study site in 2009. The result showed that both temperature (R 2 = 0.92, p < 0.01) and humidity (R 2 = 0.84, p < 0.01) were highly correlated between locations. The temperature in Haitang was (on average) 5°C lower than in Baoshan, however; and relative humidity was 16% higher. The coefficient of determination for precipitation was relatively poor (R 2 = 0.43), even though the correlation was still significant (p < 0.05). On average, monthly precipitation in Baoshan was 30 mm higher than in Haitang. The original dataset included daily temperature (mean, maximum, and minimum), humidity (mean and minimum) and precipitation. Whenever trace quantities of precipitation were reported, we substituted these with 0.01 mm (which is 10% of the minimum recorded precipitation).
Shiro observation
Theoretically, a shiro is a structure of solid, tight aggregate of mycelium and mycorrhiza underground from which fruiting bodies emerge (Peter 2006; Ogawa 1975, 1976, 1977; Yamada et al. 2006). Because of the difficulty of exploring how the mycelium occupies the underground territory, shiro boundaries are typically delineated based on visible fruiting bodies above ground. This was done based on field experience of multiple years. The rule of thumb is spatial distance: the closer the distance (<5 m), the higher the likelihood that a structure belongs to the same shiro (Fig. 3). A total of 56 shiros were observed continuously throughout 2000–2010. Each shiro was coded numerically and classified according to its micro-environment, such as nearby tree and topographic characteristics. Dates of emergence from the soil were noted for each individual fruiting body. The numbers of fruiting bodies were aggregated for each month and year and used as an indicator for productivity. For each shiro, the first fruiting date was registered, when the first matsutake emerged. Likewise, the last fruiting date was when the last matsutake emerged. The fruiting duration was then computed as the difference between last fruiting date and first fruiting date. The mean fruiting date was the average of all observations. In total, the dataset consists of 4578 records of individual fruiting bodies recorded during the observation period.
Statistical analysis
Descriptive statistics and graphs were used to analyse and illustrate the results. The variation in fruiting phenology (mainly referred to as first fruiting date, last fruiting date, fruiting duration, and mean fruiting date) and variation in production were analysed. To explore the relationships between meteorological variables and fruiting behaviour, we used Projection to Latent Structures (PLS; also known as Partial Least Squares) Regression (Wold 1995; Wold et al. 2001) The PLS model coefficients and the variable importance plot (VIP) were used to interpret importance and effects of predictor variables. Predictors with VIP scores above 0.8 were considered important for the PLS model (Wold 1995).
Results
Fruiting phenology and productivity
Matsutake fruiting phenology was highly variable across years, indicating high sensitivity to environmental change (Fig. 4). Generally, fruiting commenced in mid-June and ended in October, with some outlier fruiting bodies emerging up to mid-November. The inter-annual variation of first fruiting date can be greater than one month, with the first fruiting date ranging from 7th June in 2004 to 19th July in 2005. Mean first fruiting date, last fruiting date and mean fruiting date during the observation period occurred on 19th June, 30th October and 22nd August, respectively, resulting in a mean fruiting duration of 133 days.
We observed a pattern of delayed first fruiting date over time (Fig. 4a). The mean fruiting date and last fruiting date fluctuated but did not show a clear trend (Fig. 4a). Interestingly, the period of 2003–2005 presents a wide variation during which fruiting dates were delayed dramatically in 2005 and fruiting duration was extremely long in 2004 (Fig. 4a and b).
Productivity in terms of the number of fruiting bodies produced also demonstrated dynamic variation among study years (2000–2010, Fig. 5a). In the most productive year (2003), a total of 810 fruiting bodies were produced, compared to only 233 in the least productive year (2008). The mean number of observed fruiting bodies over all years was 416 with a standard deviation of 167. Productivity decreased after the exceptionally high production in 2003. In 2009, it recovered slightly. The most productive months were August and September (Fig. 5b). On average, 26, 83, 110, 123, and 56 fruiting bodies emerged from June to October during the period from 2000 to 2010. Productivity levels also differed between shiros, with some consistently producing more fruiting bodies than others (Fig. 5c). For example, shiro 24 produced on average 41 (SD = 17) fruiting bodies per year, whereas shiros 5, 28, 40, 43, and 58 did not fruit every year.
Climate-fruiting relationships
Fruiting characteristics such as first fruiting date and number of fruiting bodies were analysed against temperature, humidity and precipitation. The results (Fig. 6) show that, temperature of 8 months, humidity of 6 months and precipitation of 3 months relates significantly to first fruiting date. In general, high temperature during May-July delayed the first fruiting date, as indicated by positive model coefficients in the Projection to Latent Structures model. Negative model coefficients for temperatures between December and March imply that high temperature during these months advanced first fruiting dates. Likewise, high humidity and precipitation during May-July delays first fruiting. For productivity, temperature of 4 months, humidity of 8 months and precipitation of 3 months are significant responsive variables. High temperature and high precipitation in August were correlated with a high number of fruiting bodies. In contrast, high temperature in June and high humidity from November and December of the preceding year to May were correlated with low numbers of fruiting bodies.
Relationships between first fruiting dates (FFD; a) and the number of fruiting bodies (NFB; b) with temperature (T), humidity (H) and precipitation (P) from November (11–1) of the preceding year to October of the fruiting year, as indicated by PLS regression. The VIP plots indicate the importance of each variable, and model coefficient plots show the direction in which high values for each factor influence the dependent variable
Discussion
We used an 11-year monitoring dataset collected at Haitangwa village in Baoshan Prefecture, Yunnan Province, China. Since 1997, research on promoting matsutake production through forest enclosure has been carried out at this location. This project was conducted by the Baoshan Forestry Bureau and Institute of Tropical Forestry, Chinese Academy of Forestry (Gong et al. 2002). During this period, Pinus yunnanensis forests have been well protected. Harvesting was restricted to methods with low environmental impact. We monitored and recorded the fruiting behaviour of matsutake in the forest between 1999 and 2010. The underlying assumption of the study was that human impact on fruiting is minimised by good management and is not a significant factor impacting variation. We therefore hypothesise that climatic factors drive variation in matsutake fruiting phenology and productivity in the observed forests.
Climate response of matsutake fruiting and underlying mechanisms
Analysis of herbarium data has recently provided new insights into the fruiting phenology of fungi. On-site monitoring has also been intensified, in order to understand the effects of environmental drivers on fungal diversity, fruiting and productivity (Büntgen et al. 2011; Egli 2011; Pinna et al. 2010; Baptista et al. 2010; Newbound et al. 2010; Krebs et al. 2008). Yet studies to date have only presented the pooled result of a collection of fungal species, even though the response of individual species to environmental change may differ. Research focusing on specific species is still limited by a shortage of data from long-term observations. For example, Mihail et al. (2007) present valuable results on spatial and temporal patterns of fruiting, but only for a single species (Morchella esculenta) and for a relatively short period (2001–2005). Our unique dataset on matsutake mushroom allows a species-specific investigation into fruiting phenology and thus contributes to the development and validation of fructification models.
Fruiting results from a complex series of processes, involving stimulation by environmental cues and endogenous regulatory signals (Ugalde 2006; Busch and Braus 2007), which induce expression of genes involved in the fruiting process (Busch and Braus 2007). Furthermore, the fruiting process requires surplus energy and nutrients at the time when fundamental requirements for vegetative growth are met (Busch and Braus 2007). Thus the observed climatic factors affecting fruiting are either directly or indirectly affected by the above-mentioned process. We speculate that the time of fruiting is likely dependent on regulatory signals which might be induced by climate cues; whereas productivity is more related with resource availability.
Similar to patterns found for other fungi, matsutake fruiting is sensitive to changes in environmental conditions. First fruiting date and productivity were sensitive to change, showing active inter-annual and inter-shiro variation. The observed trend towards delayed first fruiting was explained by temperature, humidity and precipitation over several months. To interpret the link between significant variables and first fruiting date, we constrained the efforts to months before and during the early stage of fruiting, as correlations of fruiting with environmental conditions after fruit emergence make no biological or ecological sense. March, May and June appear to be the most important months for determining the onset of matsutake fruiting. The mechanism underlying the response of matsutake fruiting to weather in March is unclear. It may be linked with mycelium development, biomass and nutrient accumulation, which are essential for fruiting. A warm and dry March may favour these processes and induce early fruiting. Prior to fruiting onset, the mycelium aggregates, forms knot structures and then produce primordia. These processes most likely take place in May and June. A further analysis of the climate data revealed a clear temperature increase and precipitation reduction in May (Fig. 7). Moreover, their significant correlation with first fruiting day (Fig. 8) leads to the conclusion that rising temperature and reduced precipitation in May appear to have caused the delay of fruiting onset. Similar patterns were observed for June but with lower significance. The most reasonable biological and ecological explanation for this relationship is a cold shock, as reported by Pinna et al. (2010), or a phenomenon equivalent to the chilling requirement of perennial plants in cold environments (Yu et al. 2010). High temperature may have delayed the fulfilment of chilling requirements for onset of fruiting, while drought enhanced the effect of temperature. The extreme late fruiting of 2005 provided the best evidence, when temperature in May was 20.1°C (0.4°C higher than average) and precipitation was only 5.3 mm (130 mm on average). We speculate that the function of cold shock induces metabolic process and releases chemosensory signals for fruiting.
The positive effect of a warm November and low humidity from November to May on productivity indicates that such conditions favour mycelium development, leading to high productivity. Both temperature and precipitation in August were positively correlated with yield. This is possibly explained by the host-fungi relationship, with high amounts of carbohydrates produced by the host leading to high productivity of the matsutake. It is worth noting that the highest productivity occurring in 2003 was accompanied by the highest temperature in August (22.1°C), and interestingly the lowest precipitation (111.9 mm, compared to a long-term mean of 180 mm from 1951 to 2010) of the same month. This contradictory observation against the above mentioned precipitation effect may reflect the fungi’s response to abnormal climate conditions. As noted in a previous study (Busch and Braus 2007; Ugalde 2006), fungi have two systems for reproduction. In general, growth of vegetative hyphae is sufficient for sustaining the local population. Fructification is not a necessity, but dispersed spores have the function to explore new territories and maintain genetic diversity (Ugalde 2006). In normal years, the number of fruiting bodies depends on resource availability. In an abnormal year, however, fungi may use strategies to lower the risk of extinction. High productivity in a dry August might be one such strategy to cope with an environmental extreme by enhancing the sexual reproduction with the purpose to expand the range and to boost genetic diversity. Similar observations were made in 2010 at Qingcaitang in Chuxiong Prefecture (another research site), where drought conditions in the whole summer postponed fruiting dates but doubled productivity. The tendency to allocate more resources to sexual reproduction system under harsh environmental conditions was well documented in higher plants, e.g. Fan and Yang (2009). Fungi may respond in the same manner. Further research is required on this topic, including during harsher conditions than we observed. While much drier than the annual average, the August of 2003 may still not have been dry enough to trigger survival strategies in matsutake.
We would also like to recommend caution in the use of second hand data, such as herbarium records, for studying fungal phenology. Differences in fruiting dates between shiros can be greater than 2 months even in a small area such as in our study site. This implies that, for species with long fruiting duration, multiple or even full-time field observations rather than one-time or one-spot data collection is required to make an appropriate inference for the dynamics of fungal phenology, which was also suggested by Halme and Kotiaho (2012) from an intensified practical research from Finland.
Challenges for fungal ecology and phenology research
With increasing recognition of the role and function of fungi as important components of ecosystems for element cycling, carbon exchange, economic value and ecosystem equilibrium, an understanding of their biology, ecology, and their responses to climate change is essential. Pickles et al. (2012) summarized the possible responses of ectomycorrhizas under natural and anthropogenic forms of climate change. The publications of Gange et al. (2007) and Kauserud et al. (2008, 2010) have clearly demonstrated the need for research on the response of fungal ecology and phenology in an environment of global change. However, many challenges still exist. Firstly, we currently lack systematic frameworks, methodologies, and techniques to satisfy these needs. There is no standard or well-established method for examining relationships between fungal phenology and climate change. This is due to limited understanding of the fruiting mechanism and its controlling factors, especially in the context of mycorrhizal fungi, few of which are cultivable (Salerni and Perini 2004). Furthermore, the temporary nature of sporocarps and the underground presence of mycelium make research into fungal ecology difficult. Research using model species (categorized by trophic acquisition modes) which can be manipulated experimentally is needed.
Secondly, selection of response variables and corresponding statistical methods is difficult and somewhat arbitrary. The response variables normally used are averages or figures accumulated at certain interval bases. Correlation coefficients and regression are the statistical methods frequently employed. New variables or indices and innovative statistical methods should be developed to understand the covert fungus-environment relationship. Projection to Latent Structures Regression, as employed in the present study, is a step in this direction.
Thirdly, no matter which method and data are used, the most challenging task is interpreting the results in an ecologically sound manner. Do temperature and rainfall affect fruiting behaviour as significantly as the statistics suggest? Have we discovered an actual ecological relationship or is this just a chance finding? If it is an ecological relationship, what mechanism drives it?
Research into fungal ecology and phenology has just begun (Kauserud et al. 2008), and we lack understanding to make confident accurate ecological interpretations. We should, on the one hand, develop generalized models based on site- and taxon-specific cases; and on the other hand incorporate modern technology, such as genetic approaches to test ecological hypotheses. In summary, a holistic approach and research framework integrating biology, ecology, genetics, physiology, and phytochemistry is needed in order to advance this area of research.
References
Baptista P, Martins A, Tavares RM, Lino-Neto T (2010) Diversity and fruiting pattern of macrofungi associated with chestnut (Castanea sativa) in the Tra’s-os-Montes region (Northeast Portugal). Fungal Ecol 3(1):9–19
Büntgen U, Kauserud H, Egli S (2011) Linking climate variability to mushroom productivity and phenology. Front Ecol Environ. doi:10.1890/110064
Busch S, Braus GH (2007) How to build a fungal fruit body: from uniform cells to specialized tissue. Mol Microbiol 64(4):873–876. doi:10.1111/j.1365-2958.2007.05711.x
Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J, Carpenter KE, Carr GM, Chanson J, Chenery AM, Csirke J, Davidson NC, Dentener F, Foster M, Galli A, Galloway JN, Genovesi P, Gregory RD, Hockings M, Kapos V, Lamarque J-F, Leverington F, Loh J, McGeoch MA, McRae L, Minasyan A, Morcillo MH, Oldfield TEE, Pauly D, Quader S, Revenga C, Sauer JR, Skolnik B, Spear D, Stanwell-Smith D, Stuart SN, Symes A, Tierney M, Tyrrell TD, Vié J-C, Watson R (2010) Global biodiversity: indicators of recent declines. Science 328(5982):1164–1168. doi:10.1126/science.1187512
Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333(6045):1024–1026. doi:10.1126/science.1206432
Egli S (2011) Mycorrhizal mushroom diversity and productivity—an indicator of forest health? Ann For Sci 68(1):81–88. doi:10.1007/s13595-010-0009-3
Fan DM, Yang YP (2009) Altitudinal variations in flower and bulbil production of an alpine perennial, Polygonum viviparum L. (Polygonaceae). Plant Biol 11(3):493–497. doi:10.1111/j.1438-8677.2008.00188.x
Gange AC, Gange EG, Sparks TH, Boddy L (2007) Rapid and recent changes in fungal fruiting patterns. Science 316:71
Gong M-q Su, L-j CY, F-z W, J-x C (2002) A study on development of Shiro and productive potentialities of Tricholoma matsutake (in Chinese). For Res 15(4):374–379
Halme P, Kotiaho J (2012) The importance of timing and number of surveys in fungal biodiversity research. Biodivers Conserv 21(1):205–219. doi:10.1007/s10531-011-0176-z
Hof C, Levinsky I, AraÚJo MB, Rahbek C (2011) Rethinking species’ ability to cope with rapid climate change. Global Change Biol 17(9):2987–2990. doi:10.1111/j.1365-2486.2011.02418.x
IPCC (2007) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA
Kauserud H, Stige LC, Vik JO, Økland RH, Høiland K, Stenseth NC (2008) Mushroom fruiting and climate change. Proc Natl Acad Sci 105(10):3811–3814. doi:10.1073/pnas.0709037105
Kauserud H, Heegaard E, Semenov MA, Boddy L, Halvorsen R, Stige LC, Sparks TH, Gange AC, Stenseth NC (2010) Climate change and spring-fruiting fungi. Proceedings of the Royal Society 277:1169–1177. doi:10.1098/rspb.2009.1537
Krebs CJ, Carrier P, Boutin S, Boonstra R, Hofer E (2008) Mushroom crops in relation to weather in the southwestern Yukon. Botany 86(12):1497–1502. doi:10.1139/b08-094
Krivtsov V, Watlingb R, Walkera SJJ, Knottb D, Palfreymana JW, Staines HJ (2003) Analysis of fungal fruiting patterns at the Dawyck Botanic Garden. Ecol Model 170:393–406
Ma X, Xu J, Luo Y, Aggarwal SP, Li J (2009) Response of hydrological processes to land-cover and climate changes in Kejie watershed, south-west China. Hydrological Processes 23:1179–1191
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Og B, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Måge F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišová V, Scheifinger H, Striz M, Susnik A, Vliet AJHV, Wielgolaski F-E, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Global Change Biol 12(10):1969–1976
Mihail JD, Bruhn JN, Bonello P (2007) Spatial and temporal patterns of morel fruiting. Mycol Res 111(3):339–346
Myers N, Mittermeier RA, Mittermeier CG, Faseca GABd, Kent J (2002) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Newbound M, McCarthy M, Lebel T (2010) Phenology of epigeous macrofungi found in red gum woodlands. Fungal Biol 114(2–3):171–178. doi:10.1016/j.funbio.2009.12.001
Ogawa M (1975) Microbial ecology of mycorrhizal fungus, Tricholoma matsutake Ito et Imai (Sing.) in pine forest II, Mycorrhiza formed by Tricholoma matsutake. vol 272. The government forest experiment station, Tokyo, Japan
Ogawa M (1976) Microbial ecology of mycorrhizal fungus-Tricholoma matsutake Ito et Imai (Sing.) in pine forest III, fungal flora in Shiro soil and on the mycorrhiza. The government forest experiment station, Tokyo, Japan
Ogawa M (1977) Microbial ecology of mycorrhizal fungus, Tricholoma matsutake Ito et Imai (Sing.) in pine forest IV, The shiro of Tricholoma matsutake in the fugal community. vol 279. The government forest experiment station, Tokyo, Japan
Pearman PB, Guisan A, Zimmermann NE (2011) Impacts of climate change on Swiss biodiversity: an indicator taxa approach. Biol Conserv 144(2):866–875. doi:10.1016/j.biocon.2010.11.020
Peter M (2006) Ectomycorrhizal fungi – fairy rings and the wood-wide web. New Phytol 171:685–687
Pickles BJ, Egger KN, Massicotte HB, Green DS (2012) Ectomycorrhizas and climate change. Fungal Ecol 5(1):73–84. doi:10.1016/j.funeco.2011.08.009
Pinna S, Gévry MF, Côté M, Sirois L (2010) Factors influencing fructification phenology of edible mushrooms in a boreal mixed forest of Eastern Canada. For Ecol Manage 260(3):294–301. doi:10.1016/j.foreco.2010.04.024
Primack RB, Miller-Rushing AJ (2011) Broadening the study of phenology and climate change. New Phytol 191(2):307–309. doi:10.1111/j.1469-8137.2011.03773.x
Salerni E, Perini C (2004) Experimental study for increasing productivity of Boletus edulis s.l. in Italy. For Ecol Manage 201:161–170
Samils N, Olivera A, Danell E, Alexander S, Fischer C, Colinas C (2008) The socioeconomic impact of truffle cultivation in Rural Spain. Econ Bot 62(3):331–340. doi:10.1007/s12231-008-9030-y
Schwartz MD, Ahas R, Aasa A (2006) Onset of spring starting earlier across the Northern Hemisphere. Global Change Biol 12:343–351
Sitta N, Floriani M (2008) Nationalization and globalization trends in the wild mushroom commerce of Italy with emphasis on Porcini (Boletus edulis and Allied Species). Econ Bot 62(3):307–322. doi:10.1007/s12231-008-9037-4
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Townsend Peterson A, Phillips OL, Williams SE (2004) Extinction risk from climate change. Nature 427 (6970):145–148. doi:http://www.nature.com/nature/journal/v427/n6970/suppinfo/nature02121_S1.html
Ugalde U (2006) Autoregulatory signals in mycelial fungi growth, differentiation and sexuality. In: Kües U, Fischer R (eds) vol 1. The Mycota. Springer, Berlin, pp 203–213. doi:10.1007/3-540-28135-5_11
Wang X-H, Liu P-G (2002) Resources investigation and studies on the wild commercial fungi in Yunnan (in Chinese). Biodivers Sci 10(3):318–325
Wang Y, Hall IR, Evans LA (1997) Ectomycorrhizal fungi with edible fruiting bodies.1. Tricholoma matsutake and related fungi. Econ Bot 51(3):311–327
Weckerle C, Yang Y, Huber F, Li Q (2010) People, money, and protected areas: the collection of the caterpillar mushroom Ophiocordyceps sinensis in the Baima Xueshan Nature Reserve, Southwest China. Biodivers Conserv 19(9):2685–2698. doi:10.1007/s10531-010-9867-0
Wold S (1995) PLS for multivariate linear modeling. In: Waterbeemd VD (ed) Chemometric methods in molecular design: methods and principles in medicinal chemistry, vol 2. Verlag-Chemie, Weinheim, pp 195–218
Wold S, Sjostrom M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometrics Intellig Lab Syst 58:109–130
Yamada A, Maeda K, Kobayashi H, Murata H (2006) Ectomycorrhizal symbiosis in vitro between Tricholoma matsutake and Pinus densiflora seedlings that resembles naturally occurring ‘shiro’. Mycorrhiza 16:111–116. doi:10.1007/s00572-005-0021-x
Yang Z (2010) Inventory of higher fungi in the Hengduan Mountains of southwestern China (in Chinese). Chinese Bulletin of Life Sciences 22(11):1086–1091
Yang X, He J, Li C, Ma J, Yang Y, Xu J (2008) Matsutake trade in Yunnan Province, China: an overview. Econ Bot 62(3):269–277
Yang X, Wilkes A, Yang Y, Xu J, Geslani CS, Yang X, Gao F, Yang J, Robinson B (2009) Common and privatized: conditions for wise management of matsutake mushrooms in Northwest Yunnan province, China. Ecol Soc 14(2):30. doi:http://www.ecologyandsociety.org/vol14/iss2/art30/
Yu H, Luedeling E, Xu J (2010) Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. PNAS 107:22151–22156
Zhang Y, Zhou D, Zhao Q, Zhou T, Hyde K (2010) Diversity and ecological distribution of macrofungi in the Laojun Mountain region, southwestern China. Biodivers Conserv 19(12):3545–3563. doi:10.1007/s10531-010-9915-9
Acknowledgements
This study was sponsored jointly by the National Natural Science Foundation of China (Grant No. 30800158), the 11th Five-Year China Key Science & Technology Project on Silviculture for Carbon Sequestration in Subtropics (Grant No: 2008BAD95B09) and the Ford Foundation (Grant No. 10850639). We are grateful to David Pilz and Edward Grumbine for critical comments and Greta M. Pennington Rana for English editing. We also thank the Global Research Network for Fungal Biology, the National Research Council of Thailand (grant NRCT/55201020007), Mae Fah Luang University (grant MFU/54 1 01 02 00 48) and King Saud University for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Luedeling, E., Chen, G. et al. Climate change effects fruiting of the prize matsutake mushroom in China. Fungal Diversity 56, 189–198 (2012). https://doi.org/10.1007/s13225-012-0163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-012-0163-z