Abstract
The halosphaeriaceous fungi constitute the largest group of marine Ascomycota found predominantly in marine environments, with few transitional species found in freshwater and brackish water habitats. It has been one of the most intensively studied groups of marine fungi, with 126 species in 53 genera. The classification of the halosphaeriaceous fungi is contentious with one school maintaining that they should be regarded as a family in the Microascales, while others continue to retain the order Halosphaeriales. To refine the phylogenetic inter-relationships among the halosphaeriaceous fungi, 36 taxa were sequenced and analysed based on three loci [nuclear small and large subunit (SSU, LSU), the second largest RNA polymerase II subunit (RPB2)]. The halosphaeriaceous fungi constitute a monophyletic group and share a common ancestor with the Microascaceae however, they share few morphological characters. In the Halosphaeriaceae the centrum tissue comprises catenophyses; asci are clavate to fusiform; ascospores are hyaline, unicellular to many septate, usually with appendages, and most are saprobic in aquatic habitats. Whereas, the peridium of the Microascaceae is carbonaceous, frequently bearing hyphal appendages or setae; asci are globose or ovoid; ascospores are reniform, often bear ornamenting ridges or wings. Genera in the Microascaceae are mainly saprobic from soil to rotting vegetation and occasionally found as pathogens, and primarily terrestrial. Based on morphological data the halosphaeriaceous taxa might be considered as a group warranting ordinal status (Halosphaeriales) and this issue is discussed in this study. Sequence data also show clearly that the genera Remispora and Ceriosporopsis are polyphyletic and we propose the erection of three new genera to accommodate C. tubulifera (Toriella), R. crispa (Kochiella) and R. galerita (Tubakiella). Bovicornua intricata is referred to the genus Ceriosporopsis. The molecular data indicate that different phylogenies based on DNA sequences support a hypothesis that ascospore appendage developments e.g. unfurling bipolar appendages, have evolved and been lost several times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Halosphaeriaceae represents the largest and most diverse lineage of marine ascomycetes to date, proposed by Müller and von Arx (1962) and Eriksson (1984) with Halosphaeria as the type genus (Barghoorn and Linder 1944). Members of the Halosphaeriaceae are amongst the most intensively studied ascomycetes at the morphological, ultrastructural and molecular level, with 53 genera (of which 35 are monotypic) and 126 species (Pang 2002; Jones et al. 2009). Most of the taxa referred to the order are marine, with few transitional species found in freshwater and brackish water habitats e.g. Aniptodera, Ascosacculus, Halosarpheia, Lignincola, Luttrellia, Magnisphaera, Nais, Natantispora, Oceanitis and Tirispora (Jones 1995; Pang et al. 2003; Jones et al. 2009).
Morphological characters used to distinguish the genera in the Halosphaeriaceae include: perithecioid ascomata, necks (usually with periphyses), presence of catenophyses that generally deliquesce, unitunicate, thin-walled asci that deliquesce early, asci lacking an apical apparatus and appendages on ascospores (Jones 1995). While studies at the scanning and transmission electron microscope level (SEM, TEM) of ascospore appendage morphology and ontogeny, yielded useful characters for delineation of certain genera in the Halosphaeriaceae (Ceriosporopsis, Corollospora, Marinospora) (Jones et al. 1983b, 1984; Jones 1995), however it fails to distinguish other genera e.g. Halosarpheia, Lulworthia (Campbell et al. 2003, 2005).
DNA sequence analysis to infer phylogenies became common place in fungal systematics, from a single locus to multi loci. Recently, the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) has been accepted in order to recognize fungal species concept based on multiple gene genealogies (Taylor et al. 2000). It provides a powerful tool to investigate fungal evolutionary relationships that cannot be determined using morphological or biological characters alone.
There have been attempts to clarify the phylogeny of the Halosphaeriaceae by examining selected genera and related taxa. For example, Spatafora et al. (1998) proposed a terrestrial origin of this order supported by phylogenetic analysis. Subsequent studies have been undertaken by many authors mainly with a narrower focus on the taxonomic revision of specific genera e.g. Halosarpheia species (Campbell 1999; Kong et al. 2000; Abdel-Wahab et al. 2001b; Anderson et al. 2001; Pang 2001); Halosphaeria (Pang et al. 2004a); Lulworthia and Lindra (Campbell et al. 2003; Pang et al. 2003; Koch et al. 2007); Torpedospora and Swampomyces (Abdel-Wahab et al. 2001a; Sakayaroj 2005; Sakayaroj et al. 2005a); Antennospora (Pang et al. 2008b) and reviewed by Jones et al. (2009). Nevertheless, the higher level classification of the halosphaeriaceous fungi remains contentious with one placing them as a family within the Microascales (Hibbett et al. 2007; Schoch et al. 2007), while others continue to retain the ordinal name Halosphaeriales (Zhang et al. 2006; Tang et al. 2007; Jones et al. 2009).
So far, 97 halosphaeriaceous strains have been sequenced in several studies and their DNA sequences deposited in GenBank, with the following breakdown by gene: 127 LSU, 53 SSU, 7 RPB2, 1 largest subunit of RNA polymerase II (RPB1), 3 elongation factor 1-alpha (EF1-alpha), 1 beta-tubulin and 26 sequences from other genes (data retrieved 4 August 2010). However, resolution of the classification and inter-relationships of many genera within the Halosphaeriaceae, such as Bathyascus, Ceriosporopsis, Lignincola and Remispora remains poor.
The genus Remispora was described by Barghoorn and Linder (1944) (type species: R. maritima) and characterized by mostly hyaline ascomata, 1-septate hyaline ascospores, with pleomorphic polar appendages which arise as fragmentation of an exosporial layer (Johnson et al. 1984). The assignment of this genus has been revised several times based on morphological characteristics with Kohlmeyer (1972) referring it to Halosphaeria (Jones and Moss 1978, 1980; Johnson et al. 1984). Johnson et al. (1984) and Manimohan et al. (1993a, b) demonstrated that the polar appendages of Remispora species were formed by fragmentation of the ascospore exosporium, which consists of two components, a fibrillar material in an amorphous matrix. Currently eight Remispora species are accepted: R. maritima, R. stellata, R. quadri-remis, R. pilleata, R. galerita, R. crispa, R. minuta and R. spitsbergenensis (Jones et al. 2009).
The genus Ceriosporopsis is characterized by light brown to black, subglobose to cylindrical ascomata, clavate, thin-walled asci, ascospores ellipsoidal, 1-septate, hyaline with polar appendages, with the exception of C. tubulifera, which has polar and equatorial appendages. In Ceriosporopsis species, polar appendages uncoil to form long drawn out structures, while C. tubulifera has end chambers or tubes containing mucilage that is released when mounted in water to form long thread-like appendages. There are five Ceriosporopsis species (C. caduca, C. cambrensis, C. capillacea, C. halima (type species), C. tubulifera), but species delineation is unclear due to the variation in the morphology of ascospore appendages (Johnson et al. 1987; Jones et al. 2009). The classification of other genera remains unresolved e.g. Bathyascus, Halosarpheia-like species, Lignincola, as no fresh collections have been available for a molecular study.
The goals of this study were 1) to combine morphology and DNA sequence based phylogenetic analyses of multiple loci (LSU, SSU rDNA and RPB2 sequences) to elucidate the phylogeny and taxonomy of key genera in the Halosphaeriaceae, 2) to consider the evolution of the family and 3) to review its ordinal status based on morphological and biological evidence.
Materials and methods
Collection and growth of fungi
Decaying driftwood was collected from various countries: China, Denmark, Norway, Taiwan, Thailand, USA and Wales (Table 1). Single-spore isolations were made on seawater cornmeal agar (CMA) with added antibiotics following the method of Choi et al. (1999). Axenic cultures and specimens were kept at BIOTEC Culture Collection (BCC, JS) and BIOTEC Bangkok Herbarium (BBH). Additional cultures were obtained from City University of Hong Kong (CY), University of Portsmouth, UK (LP, PP). Fungi were cultured into seawater potato dextrose agar (PDA) before growing in seawater glucose yeast extract peptone (GYP) broth (4 g/l glucose, 4 g/l yeast extract, 2 g/l peptone).
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from both mycelia and ascomata (Pang et al. 2003; Sakayaroj et al. 2005b). Mycelium (~100 mg) was harvested by filtration, washed twice with sterile distilled water, blotted dry by filter paper and immediately frozen in liquid nitrogen. Mycelial pellets were ground into fine powder using a mortar and pestle, and DNA was extracted using a NucleoSpinR Plant DNA extraction kit (Macherey-Nagel). Additional DNA was extracted using 1–2 ascomata following the microwave technique (Pang et al. 2003). Fruiting bodies were picked up with fine forceps or needles, transferred to a tube containing 400 μl lysis buffer (O’Donnell et al. 1997). The tube was sealed with a plastic wrap, and microwaved for 20 s (10 s-5 s-5 s) at maximum power, and then incubated at 70°C for 30 min. The mixture was extracted twice with phenol:chloroform:isoamyl alcohol (25:24:1). The upper liquid phase was precipitated with 7.5 M ammonium acetate and absolute ethanol and kept at 20°C for at least 30 min. Extracted DNA was washed twice with 70% ethanol, air-dried, and the DNA resuspended in 20 μl TE buffer.
Nuclear small and large subunit ribosomal rDNA and RBB2 were amplified using fungal universal primers (White et al. 1990; Bunyard et al. 1994; Landvik 1996; Liu et al. 1999; Lutzoni et al. 2004). PCR reactions were performed in 50 μl using FINNZYMES, DyNAzyme II DNA Polymerase Kit (Macherey-Nagel) in a Perkin Elmer thermal cycler. The PCR products were analyzed by agarose gel electrophoresis and purified using a NucleoSpin Plant DNA Purification Kit (Macherey-Nagel) according to the manufacturer’s instructions. PCR products were sent to Macrogen Inc., Korea, for direct sequencing.
Sequence alignment and phylogenetic analyses
Sequences were defined ambiguity and assembled using BioEdit 7.0.9.0 (Hall 2007). These sequences obtained from GenBank (Table 2) were aligned in Clustal W 1.6 (Thompson et al. 1994), MUSCLE 3.6 (Edgar 2004) and manually adjusted in BioEdit 7.0.9.0. The tree construction procedure was performed in PAUP* 4.0b10 Macintosh and Window versions (Swofford 2002).
The congruence of the three gene regions was tested with the partition homogeneity test (PHT, Farris et al. 1995; Swofford 2002). The LSU dataset was analyzed individually then the combined 3-gene sequences (LSU + RPB2 + SSU) were analyzed using maximum parsimony (MP: heuristic searches with a stepwise starting tree, a random stepwise addition of 100 replicates and TBR branch-swapping algorithm) and neighbor joining (NJ) approaches. All characters were equally weighted. Gaps were treated as missing data. Tree lengths, Consistency Indices (CI), Retention Indices (RI) and Rescaled Consistency Indices (RC) were calculated for each Maximum Parsimonious Tree (MPT) generated. Finally, 1000 replicates of bootstrapping analysis for MP (full heuristic searches, stepwise addition of sequence, 10 replicates of random addition of sequence and TBR branch-swapping algorithm) and NJ analyses were performed (Felsenstein 1985).
MODELTEST v. 2.2 (Nylander 2004) was used to determine the best-evolution for each data set for Bayesian inference (BY). Bayesian inference was calculated with MrBayes v. 3.1.2 with a general time reversible (GTR) model of DNA substitution and a gamma distribution rate variation across sites (Huelsenbeck and Ronquist 2001). Four Markov chains were run for 2 runs from random starting trees for 5 million generations and sampled every 100 generations. The first 5 thousand generations were discarded as burn-in. A majority rule consensus tree of all remaining trees was calculated. The statistical bootstrap support for maximum parsimony (MPBS), neighbor joining (NJBS) and Bayesian posterior probabilities (BYPP) greater or equal than 50% (MPBS, NJBS) and 0.95 (BYPP) are shown above the tree branches, respectively.
Results
DNA sequence alignments and phylogenetic analyses
Forty-one new sequences from 36 taxa were generated in this study (34 LSU, 4 RPB2 and 3 SSU sequences, Table 1). They were analysed along with halosphaeriaceous fungi and other reference sequences from the Hypocreomycetidae (Hypocreales, Coronophorales, Microascales, Melanosporales and TBM lineage, Schoch et al. 2007) sampling from the GenBank (Table 2).
Two introns for LSU sequences, one at position 824 − 1026 and another at 1131 − 1225 for Halosarpheia trullifera (AF396875), H. unicellularis (AF396876), H. fibrosa (U46886), H. marina (AY227125), Panorbis viscosus (AY227133), Remispora quadri-remis (HQ111010), R. stellata (HQ111017), R. crispa (HQ111018, HQ111019, HQ111020), Morakotiella salina (AY864844), Ocostaspora apilongissima (HQ111005, HQ111006, HQ111007), Saagaromyces ratnagiriensis (AF539470), S. abonnis (AF539469), Ascosacculus heteroguttulata (AY227136), Corollospora portsaidica (AB361016), Okeanomyces cullulatus (AY490787) and Etheirophora blepharospora (EF027723). Among the SSU sequences, three introns were observed at position 3193 − 3254, 3322 − 3397 and 4115 − 4525 for Natantispora lotica (AF352080), H. fibrosa (AF352078) and H. marina (AF352082). Inclusion and exclusion of all insertion regions had no effect on the tree topology in all analyses. Therefore, the insertion regions were included in all analyses.
Phylogenetic analyses of two datasets, the individual LSU and the combined 3-gene (LSU + RPB2 + SSU) datasets, were performed. Xylaria hypoxylon and Daldinia concentrica were chosen as the outgroups. The MAXTREES parameter was specified, a MPT limit of 2000 for each replicate was set. The LSU dataset contained 122 taxa of 1691 bases of which 638 were constant (38%) and 524 were parsimony informative (31%). The heuristic searches maximum parsimony analysis gave tree length of 3662 step changes, CI = 0.339, RI = 0.642, RC = 0.218 (tree not shown).
For the 3-gene region, the PHT revealed significant congruence. Based on the PHT, sequence data from the three DNA regions were combined for the phylogenetic analyses. The combined 3-gene dataset included 5095 characters for 122 taxa (1691 bases for LSU, 1136 bases for RPB2 and 2268 bases for SSU). Among these, 2595 were constant (51%) while 1444 were parsimony informative (28%). Parsimony analysis yielded tree length of 8498 step changes, CI = 0.465, RI = 0.575, RC = 0.267 (Fig. 1). All absent genes were coded as missing data: RPB2 and SSU forming 79% and 71% missing data of the total characters, respectively.
One of the most parsimonious tree resulted from maximum parsimony analysis from the combined SSU, LSU rDNA and RPB2 sequences. Bootstrap values greater than 50% from maximum parsimony, neighbor joining and Bayesian Posterior Probabilities are given above the branches. Bar indicates 100 character state changes. Appendage ontogeny:  Fragmentation of exosporium;
Fragmentation of exosporium;  Outgrowth of exosporium;
Outgrowth of exosporium;  Outgrowth and fragmentation;
Outgrowth and fragmentation;  Wall outgrowth and elaboration of exosporium;
Wall outgrowth and elaboration of exosporium;  Direct outgrowth from one or more spore wall layers;
Direct outgrowth from one or more spore wall layers;  Unfurling, exuded through pore;
Unfurling, exuded through pore;  Other type; Catenophyses and periphyses:
Other type; Catenophyses and periphyses:
 Presence of catenophyses;
Presence of catenophyses;  Presence of periphyses; Asci structure:
Presence of periphyses; Asci structure:  Persistent asci;
Persistent asci;  Deliquescing asci;
Deliquescing asci;  Persistent or deliquescing asci;
Persistent or deliquescing asci;  Apical structures; Distribution:
Te = Temperate; Tr = Tropical/Subtropical; STr = Subtropical; W = Widespread; Habitats: Oc = Oceanic; Mg = Mangrove; Ds = Deep sea; Fw = Freshwater; NA = Not applicable
Apical structures; Distribution:
Te = Temperate; Tr = Tropical/Subtropical; STr = Subtropical; W = Widespread; Habitats: Oc = Oceanic; Mg = Mangrove; Ds = Deep sea; Fw = Freshwater; NA = Not applicable
For both datasets, all analyses (MP, NJ and BY) resulted in identical trees, except for the minor difference of the unsupported taxa in the Halosphaeriaceae. These differences do not affect the overall topology of the tree and the conclusions drawn. Therefore, the 3-gene tree is provided in Fig. 1.
Phylogenetic inference from the 3-gene tree
The 3-gene phylogeny provides support for the major ordinal lineages in the Hypocreomycetidae. The Coronophorales is well placed in an early diverging clade with the Melanosporales and the TBM clade. The placement is strongly supported by statistical values for MP and BY (Fig. 1), while the Hypocreales is a sister group to the Microascales. This order split into three groups: the Microascaceae and the Halosphaeriaceae and Ceratocystis species. The Microascaceae comprised key genera such as Petriella, Pithoascus, Pseudallescheria, Scedosporium, Kernia, Scopulariopsis, Enterocarpus and Microascus, while Ceratocystis species always formed a sister group to the Microascaceae and Halosphaeriaceae and well supported by statistical values of 96/75/0.99 for MP, NJ and BY, respectively (Fig. 1).
In every analysis, the phylogeny confirmed congruence of the newly sequenced taxa within the Halosphaeriaceae with other generated trees (Jones et al. 2009). Although major nodes in the Halosphaeriaceae have relatively low support, 10 well resolved subclades can be distinguished (Fig. 1). Our molecular results clearly indicate that the genus Remispora is not monophyletic. The type species, R. maritima, is well placed within a subclade comprising R. pilleata, R. spitsbergenensis, R. stellata and R. quadri-remis. Two isolates of R. maritima are closely related to R. pilleata, while R. spitsbergenensis groups well with R. stellata and R. quadri-remis, respectively. This subclade is strongly supported by the various analyses used and should be referred to as Remispora sensu stricto.
Another two species, R. crispa and R. galerita, are distantly placed in relation to the type species. Three isolates of R. crispa group well within subclade ii comprising Ocostaspora apilongissima and Morakotiella salina, with relatively high support and a short branch length. Remispora galerita strains form a basal subgroup in subclade x within the Halosphaeriaceae, and cluster with the monotypic genera Nautosphaeria and Haligena with high statistical support.
The genus Ceriosporopsis has been shown to be polyphyletic based on our molecular results. Two strains of the type species, C. halima, are well delineated in subclade ix, clustering with the monotypic genus Bovicornua. However, C. tubulifera is distantly placed from C. halima, grouping with the genera Ondiniella and Marinospora in subclade viii. Phylogenetically, M. longissima and M. calyptrata proved to be closely related and monophyletic, forming a sister group to C. tubulifera and Ondiniella torquata. The placement of subclade viii in the Halosphaeriaceae was statistically always well supported.
Our study also showed other stable entities within the Halosphaeriaceae, for example, subclade iii, which comprises the well-supported genera Okeanomyces (and its anamorph Periconia prolifica), Thalespora, Ascosacculus, adjacent to Aniptodera and Nais. Four Oceanitis species form a monophyletic group in subclade iv with the monotypic genus Ophiodeira monosemeia as a sister group. Another stable entity within the family is the placement of Halosarpheia sensu stricto and Lignincola tropica in a well supported subclade v which clustered with a group comprising Humicola siamensis, Pseudolignincola siamensis, Antennospora quadricornuta, Arenariomyces trifurcatus, Haiyanga salina, Cucullosporella mangrovei and a new sequence of Aniptodera longispora. Based on our molecular analyses, A. longispora is distantly placed from the type species A. chesapeakensis. It is highly supported by various statistical values as a monophyletic group with C. mangrovei.
Subclade vi comprises various Corollospora species and their anamorphs: Halosigmoidea parvula, H. marina and Varicosporina ramulosa. The molecular results support their monophyly and placement within the Halosphaeriaceae. Subclade vii shows the placement of the genus Saagaromyces and confirms the monophyly of S. ratnagiriensis, S. glitra and S. abonnis with high statistical values.
The inter-relationship of other halosphaeriaceous taxa was not resolved by this analysis. A newly sequenced fungus Sablecola chinensis was positioned as a separate taxon and a basal branch of subclade i. However, it is not closely related to Remispora sensu stricto, and the data supports the decision to describe it as new genus. Three strains of Naufragella spinibarbata were located basal to subclade ii without any closely related taxa.
Finally, our molecular data show that a monotypic genus Havispora clustered as a sister taxon with Nereiospora comata, N. cristata and Monodictys pelagica. This grouping formed a weak relationship with Magnisphaera species for all analyses.
Discussion
The data presented above, with 41 new sequences, enable a reevaluation of the taxonomy of this group of unitunicate marine fungi at the generic level, as well as consideration of their evolution. We will address the taxonomic changes necessary as a result of this extensive study and review the position of other taxa whose phylogenetic position remains unresolved. Three other areas will be explored: the evolution of marine fungi, their morphological character evolution and the ordinal status of this group of ascomycetes.
Phyletic inference of key genera in the Halosphaeriaceae
Remispora and Ceriosporopsis were inferred to be polyphyletic genera and the following taxonomic changes are proposed to accommodate these displaced species. The taxonomic position of some genera/species with unfurling polar appendages and a number of monotypic genera (Havispora, Ocostaspora, Ondiniella, Nautosphaeria and Sablecola) is reviewed.
Taxonomy
Remispora Linder, Farlowia 1: 409, 1944
R. maritima Linder, Farlowia 1: 410, 1944 (Types species) Fig. 2a, b
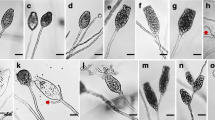
Morphological features of newly sequenced halosphaerialean taxa. a, b Wing-like polar appendages of Remispora maritima; c Superficial black ascomata of R. pilleata on wood; d, e Thick-walled rhomboidal ascospores with subgelatinous appendages of R. pilleata; f: Hyaline-orange ascomata of R. stellata immersed in wood; g Ascospore of R. stellata with bipolar appendages; h Top view of polar radiating appendages of R. stellata; i Ascospore of R. stellata with 5–9 radiating segments; j Hyaline-light brown ascomata of R. quadri-remis; k Ascospore of R. quadri-remis with 4 discrete bipolar appendages; l Radiating segments of appendaged ascospore of R. quadri-remis; m A hyaline ascospore of R. spitsbergenensis with polar appendages; n Superficial hyaline ascomata of Naufragella spinibarbata on wood; o Ascospore of N. spinibarbata with long polar appendages and soft spines; p Ascospore of Havispora longyearbyenensis with bipolar and equatorial appendages; q Ascospore of Sablecola chinensis with with polar and equatorial appendages; r Ascospore of Ocostaspora apilongissima with spoon-shaped polar appendages with equatorial spines; s Ascospore of Nautosphaeria cristaminuta with tufts of polar and equatorial appendages; t Ascospores of Aniptodera longispora. Scale bars: a,b,d,e,g-i,k-m,o-t = 10 μm; c,f = 200 μm; j = 100 μm; n = 300 μm
Synonym: Halosphaeria maritima (Linder) Kohlm., Can J Bot 50: 1956, 1072
Anamorph: Not observed.
Mode of life: saprobic.
Habitat: wood.
Widely distributed in temperate waters.
Accepted species:
R. maritima Linder, Farlowia 1: 410, 1944
R. pilleata Kohlm., Nova Hedw 6: 319, 1963 Fig. 2c–e
R. quadri-remis (Höhnk) Kohlm., Nova Hedw 2: 3332, 1960 Fig. 2j–l
R. spitsbergenensis K.L. Pang & Vrijmoed, Mycologia 101: 531, 2009 Fig. 2m
R. stellata Kohlm., Nova Hedw 2: 334, 1960 Fig. 2f–i
Notes: D.H. Linder (in Barghoorn and Linder 1944) established the genus with R. maritima as the type species. It was characterized by globose, hyaline to brown ascomata, clavate, thin-walled, deliquescing asci, and hyaline, 1-septate ascospores, with wing-like appendages. Kohlmeyer (1972) reduced Remispora to synonomy with Halosphaeria as the ascospores of the “type species of Halosphaeria and Remispora did not differ substantially from each other in morphologic respect”. This was based on the view that the appendages were formed by fragmentation of an epispore. However, SEM and TEM studies supported the acceptance of Remispora as a distinct genus from Halosphaeria (Jones and Moss 1980, 1987; Johnson et al. 1984). Currently eight species have been described with ascospore appendages varying in their morphology, although all appear to be the result of fragmentation of an exosporic sheath (Johnson et al. 1984; Manimohan et al. 1993a, b). Molecular studies indicated that Remispora was polyphyletic (Sakayaroj 2005; Sakayaroj et al. 2004) and this is confirmed by the present study.
Remispora sensu stricto all possess polar appendages that are derived by fragmentation of an exosporial layer of the ascospore cell wall (Johnson et al. 1984). Appendages in R. maritima and R. pilleata are wing-like (Fig. 2a, b, d, e), while those of R. spitsbergenensis, R. quadri-remis, and R. stellata, form a crown of radiating appendages (Fig. 2g, h, i, k, l, m). All these species have appendages that comprise fibrous material in an amorphous matrix (Johnson et al. 1984; Pang et al. 2004b, 2009). Remispora sensu stricto species form a monophyletic group with Sablecola chinensis as a sister taxon with good support. Sablecola chinensis differs from Remispora species in having both polar and equatorial appendages (Pang et al. 2004b). Remispora maritima and R. pilleata formed a well supported group while the three other species formed a sister group. These groupings also reflect the differences observed in ascospore appendage morphology.
Two other Remispora species have been described: R. lobata (Höhnk 1955) and R. spinibarbata (Koch 1989). Meyers (1957) reduced R. lobata to synonymy with R. maritima, yet they differ in ascospore measurements (17.5)21.5 − 27(31.5) × (8)10 − 11.5(12) μm in R. maritima and 16 − 21.6(25.7) μm in R. lobata. They also differ in the morphology of the ascospore appendages. Recent collections made in Denmark, showed that R. maritima and R. lobata are distinguishable and so the taxonomic position of R. lobata should be revisited (J. Koch personal communication). However this observation can not be resolved until collections of R. lobata are sequenced and compared with other Remispora species.
Kohlmeyer and Volkmann-Kohlmeyer (1998) noted that ascospore appendage morphology in R. spinibarbata differed from those of other Remispora species. The former has two types of appendages: gelatinous polar appendages that become strap-like or band-like and up to 90 μm long and subpolar fine bristle-like appendages (Fig. 2o) (Koch 1989), however, Remispora sensu stricto species possess only polar appendages. Subsequently, Kohlmeyer and Volkmann-Kohlmeyer (1998) assigned R. spinibarbata to a new genus Naufragella (type species: N. delmarensis) based on the differences in appendage morphology as described above. Our molecular data confirm that N. spinibarbata does not belong in Remispora sensu stricto with three strains forming a monophyletic group, with Ocostaspora apilongissima and Remispora crispa as a sister group.
Kochiella Sakayaroj, K.L. Pang & E.B.G. Jones gen. nov.
MycoBank MB518769
Etymology: from Koch, in recognition of Dr. Jorgen Koch’s outstanding contribution to Danish marine mycology, and the Latin diminutive suffix—ellus.
Ascocarpis subglobosis, ellispoides, superficialibus vel immersis, ostiolatis, papillatis, coriaceis; asci octosporis, clavatis, leptodermis, deliquescentibus; ascoporis ellispodeis, uniseptatis, hyalines, appendiculatis, pleomorphis.
Typus: Kochiella crispa (Kohlm.) Sakayaroj, K.L. Pang & E.B.G. Jones
Ascomata subglobose to ellipsoidal, superficial to immersed, ostiolate, papillate, periphysate, coriaceous, thin-walled, hyaline to cream coloured; asci ellipsoidal to clavate, thin-walled, deliquescing at maturity; ascospores 1-septate, hyaline, appendages pleomorphic.
Kochiella crispa (Kohlm.) Sakayaroj, K.L. Pang & E.B.G. Jones comb. nov.
MycoBank MB518770
Basionym: Remispora crispa Kohlm., Can J Bot 59: 1317, 1981
Anamorph: Not observed.
Mode of life: saprobic.
Habitat: wood.
Distribution: Denmark, Hawaii, Japan, Liberia, Martinique, Taiwan.
Notes: Kochiella crispa did not group with Remispora species sensu stricto but was placed in clade ii with three monophyletic strains of Ocostaspora apilongissima with high support. However these two fungi are not conspecific and differ markedly in ascospore appendage morphology. Kochiella crispa has polar appendages while O. apilongissima has polar and equatorial appendages. Kochiella differs from Remispora in morphology and substructure of the ascospore appendages. In Remispora species, catenophyses are present, the fibrous component of the appendages is wavy and appendages are wing-like or form a crown of radiating appendages. Conversely, catenophyses are lacking in K. crispa, appendages are pleomorphic and do not completely separate from the ascospore cell wall, while the fibrillar component consists of an inner core continuous with the middle layer of the episporium and the outer sheath of the strand was continuous with the electron-dense layer of the episporium (Manimohan et al. 1993a, b). The species is regarded by Kohlmeyer (1981) as a tropical taxon, however collections have been made in Denmark and Taiwan (this study).
Tubakiella Sakayaroj, K.L. Pang & E.B.G. Jones gen. nov. Fig. 3a–c
Morphological features of new genera and new combination proposed from this study. a The immersed ascomata with hyaline spore mass of Tubakiella galerita oozing on the wood surface; b The ornamented ascospores of Tu. galerita full of oil globules; c Subglobose compact cap-like polar appendages of Tu. galerita; d Superficial black ascomata of Toriella tubulifera; e Ascospores of To. tubulifera with polar end chamber and equatorial appendages; f The immersed orange-creamy ascomata of Ceriosporopsis intricata on wood surface; g Ascospores of C. intricata released from the ascomata when mounted in seawater; h,i Ascospores of C. intricata full of oil globules with curved polar appendages. Scale bars: a,d,f = 200 μm; g = 50 μm; b,c,e,h,i = 10 μm
MycoBank MB518772
Etymology: from Tubaki, in recognition of Professor Keiush Tubaki, distinguished mycologist and an early contributer to marine mycology in Japan, and the Latin diminutive suffix—ellus.
Ascocarpis globosis vel subglobosis, superficialibus vel immerses, membranaceis, hylainis, flavidis vel pallide fuscis, rotrie elongate cylindricus, centricis, ostiolatis, leptodermis; ascis clavatis, leptodermis, octosporis, mox deliquescentibus; ascoporis ellipsoides, tegimentis mucosis tectis, uniseptatis, hyalines; appendiculis gelatinosis, plerumques ger germinatis galeriformibusque, deniques striates.
Typus: Tubakiella galerita (Tubaki) Sakayaroj, K.L. Pang & E.B.G. Jones
Ascomata globose, subglobose to pyriform, superficial to immersed, ostiolate, papillate, coriaceous, thin-walled, hyaline to yellowish or light brown; asci ellipsoidal or ovoid, thin-walled, deliquescing at maturity; ascospores ellipsoidal, 1-septate, hyaline, thick-walled at the apices; ascospore appendages at first a gelatinous sheath covers ascospores completely, later only a subglobose subgelatinous appendages.
Tubakiella galerita (Tubaki) Sakayaroj, K.L. Pang & E.B.G. Jones, comb. nov.
MycoBank MB518773
Basionym: Remispora galerita Tubaki, Publ Seto Mar Bio Lab 15: 362, 1967.
Synonym: Halosphaeria galerita (Tubaki) I. Schmidt in Kohlm. & E. Kohlm., In: Marine Mycology, The Higher fungi 291, 1979.
Anamorph: Not observed.
Mode of life: saprobic
Habitat: wood
Distribution: France, Denmark, Germany, Japan, Taiwan, USA, Yugoslavia
Notes: Tubakiella was distantly placed from Remispora sensu stricto with the two sequenced strains forming a monophyletic group with Nautosphaeria cristaminuta and Haligena elaterophora as a sister group with weak support. Tubakiella has appendages formed by fragmentation of an exosporic layer and morphologically has little in common with either of the associated species. Haligena elaterophora has strap-like polar appendages that initially are wrapped around the ascospore, while Nautosphaeria has both polar and equatioral tufts of hair-like appendages (Jones 1964; Farrant and Jones 1986, Fig. 2s).
Ceriosporopsis Linder, Farlowia 1: 408, 1944
C. halima Linder, Farlowia 1: 408, 1944 (Type species).
Anamorph: Not observed
Mode of life: saprobic
Habitat: wood
Distribution: widely distributed in temperate waters
Accepted species:
C. caduca E.B.G. Jones & Zainal, Mycotaxon 32: 238, 1988
C. capillacea Kohlm., Can J Bot 59:1314, 1981
C. intricata (Jorg. Koch & E.B.G. Jones) Sakayaroj, K.L. Pang & E.B.G. Jones, Fungal Divers. this volume
Doubtful species:
C. cambrensis I.M. Wilson, Trans Br Mycol Soc 37:276, 1954
Rejected species: C. tubulifera (Kohlm.), P.W. Kirk ex Kohlm., Can J Bot 50: 1953, 1972
Notes: The genus Ceriosporopsis is characterized by subglobose to cylindrical ascomata, immersed, ostiolate, papillate, coriaceous or subcarbonaceous, light brown to black, catenophyses, deliquescing, asci clavate, pedunculate, unitunicate, thin-walled, deliquescing early, ascospores ellipsoidal, 1-septate, hyaline, with appendages. Appendage morphology variable depending on the species (Johnson et al. 1984, 1987; Jones et al. 1986; Yusoff et al. 1993).
Ceriosporopsis intricata (Jorg. Koch & E.B.G. Jones) Sakayaroj, K.L. Pang & E.B.G. Jones comb. nov. Fig. 3g–i
MycoBank MB518771
Anamorph: Not observed.
Mode of life: saprobic
Habitat: wood
Distribution: Denmark, Wales
Notes: Bovicornua intricata is characterised by globose to subglobose ascomata, ostiolate with short conical necks, grayish white, erumpent, membranous, gregarious, centrum pseudoparenchyma breaks down, no catenophyses, asci broadly clavate, pedunculate, unitunicate, thin-walled, early deliquescing, no apical pore, ascospores unequally 1-septate, slightly curved, constricted at the septum, hyaline and appendaged. At each pole there is a single appendage enclosed within an outer sheath which swells when mounted in seawater.
At the light microscope level, the ascospores of Bovicornua intricata with their polar appendages and enveloping exosporic sheath resemble those of Ceriosporopsis species, but differed in the complexity of the exosporium. This was considered sufficient evidence for the erection of a new genus Bovicornua (Yusoff et al. 1993). Ascospore walls of both genera consist of a mesosporium, episporium and exosporium, the polar appendage arising as an outgrowth of the spore wall. The exosporium also surrounds the polar appendage but in C. halima it ruptures and forms a cupule-like structure at the base of the polar appendage. However the structure of the ascospore wall and appendage in B. intricata is much more elaborate than that observed for any Ceriosporopsis species (Yusoff et al. 1993, 1994). Molecular data place B. intricata in the Ceriosporopsis clade ix with strong support, and so the species is transferred to Ceriosporopsis.
Toriella Sakayaroj, K.L. Pang & E.B.G. Jones gen. nov. Fig. 3d–f.
MycoBank MB518774
Etymology: Tor from the Thai for tube, in reference to the tube-like end chamber and the Latin diminutive suffix—ellus.
Ascocarpis globosis ad cylindricis vel elongatis, immersis, ostiolatis, papillatis, coriaceis, pallide brunea vel nigris, solitaris or gregarious, catenophyses absens; asci octosporis, clavatis, pedicellati, sine pori apicalibus, leptodermis, mox deliquescentibus; ascoporis ellispodeis, uniseptatis, hyalines, appendiculis, cum appendices ad polum et aequatorem quatuor adepisporium.
Typus: Toriella tubulifera (Kohlm.) Sakayaroj, K.L. Pang & E.B.G. Jones
Ascomata globose, cylindrical or elongate, immersed, ostiolate, papillate, coriaceous, brown to black, solitary or gregarious, no catenophyses; asci thin-walled, unitunicate, clavate, pedunculate, no apical pore, deliquescing early; ascospores hyaline, ellipsoidal, 1-septate, with equatorial and polar appendages. Ascospore appendage ontogeny: exosporium folds to form an annulus-like equatorial appendage while the polar appendages are formed inside and end chamber that consist of two electron-dense layers.
Toriella tubulifera (Kohlm.) Sakayaroj, K.L. Pang & E.B.G. Jones comb. nov.
MycoBank MB518775
Basionym: Halosphaeria tubulifera Kohlm., Nova Hedw 2: 312, 1960.
Synonym: Ceriosporopsis tubulifera (Kohlm.) P.W. Kirk ex Kohlm., Can J Bot 50: 1953, 1972
Anamorph: Not observed.
Mode of life: saprobic.
Habitat: wood
Distribution: Canada, Denmark, England, Germany, Iceland, Norway, Sweden, USA, Wales.
Notes:Ceriosporopsis tubulifera was initially described as a Halosphaeria species (Kohlmeyer 1960), but transferred to Ceriosporopsis based on the development of the appendages: “an exosporic sheath is pierced apically (or also laterally) by the outgrowth of mucilaginous appendages” (Kohlmeyer 1972). However, in C. tubulifera the exosporium folds to form an annulus-like equatorial appendage and the polar appendage is formed inside an end chamber that consists of two electron-dense layers (Johnson et al. 1987). The polar appendage is not an outgrowth of the spore wall and the end chamber is persistent (Johnson et al. 1987; Jones 1995). Johnson et al. (1987) pointed out that a new genus might be required to accommodate C. tubulifera and this is supported by the molecular data which placed it distantly from the type species C. halima (Fig. 1, clade viii).
Toriella formed a sister group to Ondiniella with strong bootstrap support, a genus characterized by elongate-cylindrical or subglobose ascomata, immersed or superficial, ostiolate, papillate, membranous, ranges from hyaline to pale brown, necks short and cylindrical, no periphyses, catenophyses deliquescing, asci clavate to subfusiform, apiculate, pedunculate, unitunicate, thin-walled, no apical apparatus, deliquescing, ascospores broadly ellipsoidal, 1-septate, slightly constricted at the septum, hyaline with appendages. Two types of appendages: spine-like polar appendages and an annulus-like equatorial appendage, arise as outgrowths of the episporium, with amorphous material within the appendages and lacking an exosporium (Jones et al. 1984). Although O. torquata groups consistently with C. tubulifera, they are not congeneric.
Marinospora calyptrata was initially described as a Ceriosporopsis species (Kohlmeyer 1960), transferred to Ceriosporella (generic name occupied) (Cavaliere and Johnson 1966), to Marinospora (Cavaliere and Johnson 1966) and referred back to Ceriosporopsis by Kohlmeyer (1972) based on the structure of the appendages. Johnson et al. (1984) re-established the genus based on the ontogeny of ascospore appendage ontogeny. Appendages arise as outgrowths of the spore wall, the exosporium fragments leaving remnants as cup-like structures at their tips (Johnson et al. 1984; Jones 1995). Ceriosporopsis species (C. halima, C. capillacea C. caduca) also have appendages that arise as outgrowths of the spore wall, but the exosporial sheath persists around the spore body and forms a collar at the base of the polar appendages (Johnson et al. 1987; Yusoff et al. 1994). No cup-like exosporial remnants are found on the primary appendages in Ceriosporopsis. Two Marinospora species have been described, M. calyptrata (type species) and M. longissima (Kohlmeyer 1962). The two species can be distinguished by ascospore size and the length of the primary polar appendages. Marinospora longissima ascospores are always enveloped by a layer of mucilage and has polar appendages which are longer than the equatorial ones. In M. calyptrata, the length of primary and secondary appendages are equal (Johnson et al. 1984).
Ascomycetes with unfurling polar appendages
A number of marine and freshwater ascomycetes have ascospores with initially hamate appendages that are closely adpressed to the spore cell wall, later uncoiling in water to form long bipolar thread-like sticky appendages (Kohlmeyer and Kohlmeyer 1977). Kong et al. (2000) indicated that the genus Halosarpheia was polyphyletic, an observation confirmed by Anderson et al. (2001). Subsequently a number of species were transferred to new genera based on sequence data (Abdel-Wahab et al. 2001b; Campbell et al. 2003; Pang et al. 2003; Pang and Jones 2004). However, the taxonomic position of a number of Halosarpheia species remained unresolved: H. bentotensis, H. culmiperda, H. kandeliae, H. marina, H. minuta and H. phragmiticola, primarily because fresh material was not available for a molecular study (Jones et al. 2009). That this group is polyphyletic is well demonstrated by their placement in different positions in the Halosphaeriaceae (Fig. 1). Some formed well supported clades e.g. clade iv—Oceanitis species; clade v—Halosarpheia sensu stricto; clade vii—Saagaromyces species. However, the position of other taxa is not resolved e.g. Aniptodera lignatilis, A. chesapeakensis and Ascosacculus species in clade iii, although the clade has high support.
Another group that is unresolved includes the genera/species Halosarpheia marina, Panorbis viscosus and Phaeonectriella species. Halosarpheia marina and H. kandeliae are two species that clearly do not belong in Halosarpheia sensu stricto. Halosarpheia marina forms a sister group to P. viscosus with weak support, while H. kandeliae forms a sister group to a clade comprising Saagaromyces species and Halosphaeriopsis mediosetigera with low support. However further taxon sampling is required before they can be assigned to new genera.
Halosarpheia kandeliae Abdel-Wahab & E.B.G. Jones, Mycologia 103:1500, 1999
This species is characterized by ellipsoidal, membranous, ostiolate, papillate ascomata, lateral necks, periphysate, catenophyses present, asci 8-spored, thin-walled, unitunicate, with narrow long stalks, persistent, lacking an apical apparatus, ascospores ellipsoidal, 1-septate, not constricted at the septum, hyaline, thin-walled, with bipolar apical appendages (Abdel-Wahab et al. 1999). It differs from many Halosarpheia species in having asci with long drawn out stalks (tail-like), and polar appendages that initially appear amorphous and only later form the characteristic thread-like bipolar appendages (Abdel-Wahab et al. 1999).
Genera with bipolar unfurling appendages have evolved a number of times within the family and are found in both freshwater and marine habitats. Although morphologically similar they differ radically at the ultrastructural and molecular level. Marine taxa are distributed within six of the ten clades in our analysis of three genes. Species may possess one (e.g. Tirispora unicaudata, Oceanitis scuticella, O. unicaudata, Nohea umiumi) or two polar appendages (e.g. Cucullosporella mangrovei, Panorbis viscosus). Most have 1-septate ascospores, while others are multiseptate (e.g. Oceanitis cincinnatula, Magnisphaera spartinae), the majority are hyaline although Phaeonectriella species have brown spores. Ultrastructurally ascospore appendages are produced through a single apical pore (Magnisphaera spartinae, Jones 1962), a hood-like structure (Cucullosporella mangrovei, Alias et al. 2001) or through a series of discontinuities in the episporium layer (McKeown et al. 1996; Baker et al. 2001). Three basic ontogenetic processes have been reported for ascospores with uncoiling appendages: in Trichomaris invadens they arise from the episporium (Porter 1982); in Tunicatispora australiensis from the mesosporium (McKeown et al. 1996) or their origin is not resolved, but released through discontinuities in the episporium and probably derived from the mesosporium (Saagaromyces (as Halosarpheia) ratnagiriensis, Aniptodera (as Halosarpheia aquadulcis) (Hsieh et al. 1995; Baker et al. 2001). The appendage may initially be amorphous in appearance and later differentiate; or comprise a single tightly coiled thread or composed of two distinct components (Jones 2006). Some are a single stout thread while others comprise a series of threads.
Some eleven marine genera have ascospores with uncoiling polar appendages (Jones et al. 2009) while 9 genera (e.g. Ascosacculus) with similar spores are known from freshwater habitats (Cai et al. 2006). However, these are not confined to the Halosphaeriaceae, as the Annulatascaceae and Lasiosphaeriaceae have similar appendages: Annulatascus, Cataractispora, Diluviocola, Proboscispora (Wong et al. 1998a, b). Cataractispora bipolaris has a polar pad-like appendage that eventually forms long, drawn out strands, and Diluviocola capensis has a cone-shaped hood that separates to form narrow threads (Hyde et al. 1998; Hyde and Goh 2003).
Clearly taxa with ascospores with unfurling bipolar appendages are not monophyletic and have occurred many times during the evolution of the ascomycetes (Campbell et al. 2003; Pang and Jones 2004; Pang et al. 2003) and similar appendages also occur in the Trichomycetes (Moss 1979; Jones 1994). The possession of such appendages must confer an environmental advantage and the result of convergent evolution (Jones 1995, 2006). The long thread-like appendages are sticky and wrapped around debris/substrata or adhere the spore to surfaces (Jones 1994, 2006).
Monotypic and unresolved genera: Havispora longyearbyenensis, Ocostaspora apilongissima, Ondiniella torquata, Nautosphaeria cristaminuta, Sablecola chinensis and Aniptodera longispora.
Havispora longyearbyenensis K.L. Pang & Vrijmoed, Mycologia 100: 293, 2008 Fig. 2p
This genus was described from Longyearbyen, Norway with subglobose to ellipsoidal ascomata, immersed, coriaceous, solitary or gregarious, lacking periphyses, catenophyses present deliquescing, long perithecial necks, asci clavate, pedunculate, unitunicate, thin-walled, persistent, ascospores ellipsoidal, hyaline, thin-walled, 3-septate, constricted at the septa, with tufts of appendages (Pang et al. 2008a). Ascospores have a polar appendage and four at the central septum that are string-like and composed of intertwining strands (Pang et al. 2008a). Morphologically this genus has similar tufts of polar and equatorial appendages as Nautosphaeria and Nereiospora species (Jones 1964; Jones et al. 1983b). However the ascospores of N. cristaminuta are unicellular while Nereiospora species have ascospore with brown central cells and hyaline end cells. The phylogenetic position of Havispora longyearbyenensis is distantly placed from Nautosphaeria, but has affinities with the Nereiospora/Monodictys grouping.
Ocostaspora apilongissima E.B.G. Jones, R.G. Johnson & S.T. Moss, Bot Mar 26: 353, 1983 Fig. 2r
This genus was described and placed in the Halosphaeriales by Jones et al. (1983a). This fungus possesses unique ascospore appendage morphology with long irregular amorphous polar appendage, with many equatorial appendages, spoon-shaped, with a fibrillar component. The appendage ontogeny has been referred to as type III: appendages formed by fragmentation of the outer exosporial layer of the spore wall (Jones 1995). Morphological data supports that O. apilongissima is not congeneric with Kochiella crispa, although they share a common ancestor in clade ii (Fig. 1).
Nautosphaeria cristaminuta E.B.G. Jones, Trans Br Mycol Soc, 47: 97, 1964. Fig. 2s
This species was described from material collected on wood in Wales and referred to the Halosphaeriales (Jones 1964). This fungus has spherical ascocarps, hyaline to cream-colored, and anchored with brownish hyphae to the substratum. The asci are broadly clavate or ellipsoidal, pedunculate and without an apical apparatus. Ascospores are one-celled, ellipsoidal, hyaline, possess a tuft of bristle-like appendages at each end and four tufts around the equator. Ascospore appendage ontogeny falls into type VII: formed as direct outgrowths of the spore wall, however, it has not been studied at the TEM level (G.C. Hughes personal communication; Jones 1995). Ascospore appendages resemble those of Nereiospora, with the tufts of polar and equatorial appendages. However, these two genera appear to be different at the SEM level (Jones and Moss 1987; Hyde and Jones 1989). Moreover, the molecular result confirms that N. cristaminuta and Nereiospora species are not related. Additionally N. cristaminuta has little in common with the sister taxa Haligena elaterophora and Tubakiella galerita.
Sablecola chinensis E.B.G. Jones, K.L. Pang & Vrijmoed, Can J Bot 82: 486, 2004. Fig. 2q
Pang et al. (2004b) described this genus from southern China growing on wood with ellipsoidal to subglobose ascomata, immersed to partly immersed, yellow to brown, ostiolate, papillate, necks short and periphysate, lacking catenophyses, asci clavate, pedunculate, unitunicate, thin-walled, no apical apparatus, persistent to deliquescing. This genus is characterized by its unique bipolar and quadri-equatorial appendages, which disintegrate when mounted in seawater. The appendages are obclavate, tapered to a fine point, appear striated when released from the ascus but later become fibrillar (Pang et al. 2004b). This species groups in clade i with various Remispora species with weak support and its phylogenetic position remains unresolved.
Aniptodera longispora K.D. Hyde, Bot Mar 33: 335, 1990 Fig. 2t
This species is characterized by pyriform to subglobose ascomata, ostiolate, papillate, hyaline to light-brown, catenophyses present, asci clavate to cylindrical, pedunculate, unitunicate, thin-walled, with a refractive pore or plug-like structure at the apex, with a retracting plasmalemma subapically, persistent asci, ascospores ellipsoidal, 1-septate, hyaline, thin-walled, longer than 35 μm (Fig. 2t). Ascospores are released from the asci through a fissure made at the refractive pore or plug-like structure (Hyde 1990). In our analysis, Aniptodera longispora was distantly placed from A. chesapeakensis (the type species) but formed a robust clade with Cucullosporella mangrovei with high support. These two genera share morphological similarities with clavate, pedunculate, unitunicate, thin-walled asci, thickened apically with a lens-shaped refractive region. They differ in that C. mangrovei has well developed bipolar appendages composed of two components, a fibrillar material in an amorphous matrix. Molecular data suggests A. longispora should be assigned to Cucullosporella, but this is not formally proposed here until a more detailed study of marine and freshwater Aniptodera species is undertaken.
The evolution of marine fungi
In considering the evolution and transition of fungi from land to the marine environment, a number of observations can be made based on their morphological adaptation and phylogenetic data. It is multifaceted, with many unique lineages now identified, ranging from the Basidiomycota (e.g. Halocyphina villosa, Haloaleurodiscus mangrovei, Mycoaureola dilsea, Binder et al. 2006); dothideomycetous Ascomycota (e.g. Aigialus, Biatriospora, Halotthia, Verruculina, Suetrong et al. 2009b); and sordariomycetous Ascomycota (most of the genera in the Halosphaeriaceae, Groenhiella, Swampomyces, Torpedospora). This transition from land to an aquatic milieu was accompanied by varying morphological changes, reduction in the size of the basidiome (H. villosa, Calathella mangrovei, Binder et al. 2006), and production of appendaged basidiospores (Nia vibrissa, Digitatispora species, Jones and Choeyklin 2008) in the basidiomycetes; loss of active spore dispersal in the ascomycetes (particularly in the unitunicate ascomycetes: Halonectria milfordensis, Halosphaeriopsis mediosetigera, Torpedospora radiata, Moss and Jones 1977), development of appendaged ascospores (Eiona tunicata, Halosarpheia spp., Groenhiella bivestita, Jones 1995), or multibranched or appendaged conidia for anamorphic fungi (Orbimyces spectabilis, Varicosporina anglusa, Abdel-Wahab et al. 2009; Jones et al. 2009).
Terrestrial ascomycetes were initially thought to have evolved from a marine ancestor as outlined in the Floridean hypothesis (Sachs 1874; de Bary 1887) and with the discovery of marine ascomycetes this theory gained nominal support for at least some taxa (Demoulin 1974, 1985; Kohlmeyer 1975; Kohlmeyer and Kohlmeyer 1979; Kohlmeyer 1986). However, molecular phylogenetic data clearly show that the Halosphaeriaceae evolved independently from a terrestrial origin (Spatafora et al. 1998). A plausible explanation of the origin of marine ascomycetes could be migration from terrestrial habitats to freshwater and brackish water, then to marine environments (Shearer 1993; Jones 1995). This gradual transition may have brought about morphological diversity and changes in response to environmental conditions. For example, Savoryella species are found in freshwater and marine habitats, and preliminary data indicates that phylogenetically the two groups separate into distinct subclades (Boonyuen et al. in press). Many morphological adaptations to aquatic habitats are pertinent and include: deliquescing asci, lack of an apical ascal structure, passive release of ascospores, and modification of pseudoparenchymatous tissue into catenophyses and presence of appendaged ascospores (Shearer 1993; Jones 1995; Pang 2002). The possession of appendaged ascospores may confer and enable ascospores to stick onto substrata, and remain attached often under turbulent water movement and also as an aide for flotation and dispersal of spores (Fazzani and Jones 1977; Hyde et al. 1986; Hyde and Jones 1989; Hyde et al. 1993; Jones 1994).
It is however apparent that marine ascomycetes have made the transition into the sea many times and bitunicate and unitunicate taxa may have followed different evolutionary pathways. The majority of unitunicate ascomycetes is found in temperate oceanic and coastal waters, and possesses deliquescing asci, passive spore release and often with elaborate appendaged ascospores. Many lineages can be identified: e.g. Halosphaeriaceae, Xylariales, Hypocreales, Lulworthiales and taxa that currently do not fall into any family or order e.g. TBM clade (Schoch et al. 2007; Jones et al. 2009). The TBM clade comprises the genera Etheirophora, Juncigena, Swampomyces and Torpedospora, all marine taxa, that constitute a clade that forms a sister group to the Hypocreales in the Hypocreomycetidae (Schoch et al. 2007). On the other hand bitunicate ascomycetes are predominantly tropical, in coastal waters, with active spore discharge and ascospores lacking appendages or with a mucilaginous sheath that swells in water (Suetrong et al. 2009a). Tropical species occur on mangrove wood and herbaceous plants (Alias and Jones 2009), while temperate taxa are found on maritime and monocotyledonous plants, e.g. Atriplex, Halimione, Juncus, and Spartina (Kohlmeyer and Kohlmeyer 1979; Kohlmeyer and Volkmann-Kohlmeyer 2002).
Morphological character evolution in the Halosphaeriaceae
Ascomatal and hamathecium structure
The morphological variables in the family are the possession of periphyses and catenophyses. Periphyses have been observed and well defined for a wide range of genera, in particular in clades ii, iii, iv, v, vii (Fig. 1). However, the possession of periphyses varies between some genera in these clades and even within a genus (e.g. Remispora sensu stricto). Taxa in clade vi, which are mostly associated with sand grains (Corollospora, Nereiospora, Arenariomyces), lack periphyses or these are degenerate at maturity.
Catenophyses are persistent chains of utricular, thin-walled cells formed by the vertical separation of the pseudoparenchyma in the centrum (Kirk et al. 2008). Presence of catenophyses has been observed in a wide range of genera e.g. Aniptodera, Marinospora, Haligena, Halosarpheia, Remispora, Lignincola, Nais, Phaeonectriella, Tirispora, Naufragella and Morakotiella (Fig. 1). This character can be used to distinguish between certain genera, such as, Remispora from Halosphaeria, Marinospora from Ceriosporopsis, but may not be used to characterize such genera as Halosarpheia from Saagaromyces and Ascosacculus. Catenophyses have not been reported in the arenicolous genera Corollospora, Nereiospora, and Arenariomyces. Therefore, presence or absence of periphyses and catenophyses, whilst informative, may not be indicators of phylogenetic relationships of taxa within the Halosphaeriaceae. Gain or loss of these morphological features may be the result of adaptation to different habitats or substrata, but observation of these features depends upon the age of specimens.
Ascus structure
The ancestral ascus may have been persistent, with or without an apical structure, and this variation is found in a number of genera in the Halosphaeriaceae: Aniptodera, Halosarpheia (selected species only), Natantispora, Neptunella, Panorbis, Tirispora, Phaeonectriella, Saagaromyces and Halosarpheia kandeliae (Pang 2002). In most of these genera the persistent ascus has retraction of the plasmalemma around the apical pore (e.g. Aniptodera chesapeakensis, Saagaromyces abonnis) (Baker et al. 2001; Pang et al. 2003). These appear in a number of different subclades within the family so is this a retained or reacquired character? while some may be more common on intertidal and freshwater substrata (e.g. Aniptodera chesapeakensis, Natantispora retorquens). Development of asci lacking an apical pore and active spore release may have followed but still with a persistent ascus (e.g. Lignincola laevis). Evanescent asci with early deliquescence of the centrum and the passive release of the ascopores are the predominant feature of the family. Genera found to be primarily submerged generally have deliquescent asci and passive release of the ascospores e.g. Bovicornua (as Ceriosporopsis), Ceriosporopsis, Corollospora, Marinospora and most are in early diverging lineages in the tree (Fig. 1).
Spatafora et al. (1998) drew attention to terrestrial ascomycetes with evanescent asci, e.g. Ceratocystis, Microascus and Petriella species; however, in the Microascaceae ascospores are insect dispersed. This is not the only group with deliquescing asci, as this feature has evolved several times in the ascomycetes. Cain (1972) opined that “the plasticity of the ascus morphology and dehiscence is consistent with a character that is strongly affected by selection pressures”. This may well account for the great variation observed in the occurrence of this character within the family.
Presence of apical structures (apical pore, apical plate, apical thickening and retraction of the plasmalemma) evolved independently from different common ancestors (Fig. 1), and is a difference between the Ceratocystis spp. and the Halosphaeriaceae. Genera possessing these structures include Phaeonectriella, Tirispora, Neptunella, Halosarpheia marina, Aniptodera, Cucullosporella and Saagaromyces, but these characters do not give any clue of their phylogenetic relationships (Fig. 1). However many of these taxa are common in intertidal habitats, e.g. Cucullosporella mangrovei, Tirispora unicaudata, Saagaromyces glitra, suggesting the retention of a primary character. Ascal apical structures are thought to be characteristic of terrestrial ascomycetes that serve as an aide for active discharge of ascospores (Ingold 1975). Within terrestrial ascomycetes they can be quite distinctive at the ordinal/family level, e.g. J+ and well developed rings in the Xylariales and J- with huge rings in the Annulatascaceae (Wong et al. 1998a).
Although the ascus shape in the Halosphaeriaceae is commonly clavate, it can vary from fusiform, ellipsoidal, saccate, clavate-broadly fusoid, elongate clavate, clavate to ellipsoidal and cylindrical. Pang (2002) suggested that the length to width ratio of the asci should be closely considered in the delineation of genera. Ascus stalk length should also be examined for a relationship between different taxa e.g. long stalks are present in Halosarpheia kandeliae, Saagaromyces abonnis and S. ratnagiriensis, while short stalks are a feature in Halosarpheia sensu stricto.
Ascospore appendages
Jones et al. (1986) and Jones (1995) suggested that ascospore shape and ascospore appendage ontogeny are of primary importance as taxonomic characters in the delineation of genera in the Halosphaeriaceae. Halosphaeriaceous ascospores are usually hyaline (with the exception of Phaeonectriella, Carbosphaerella and Nereiospora), with ascospore morphology varying from ellipsoidal (Morakotiella, Remispora, Neptunella), cylindrical to ellipsoidal (Haligena), or long cylindrical or fusiform (Ascosalsum, Magnisphaera). Ascospore septation is also variable: unicellular (Nautosphaeria), 1-septate (Morakotiella, Remispora, Neptunella), or multi-septate (Haligena, Ascosalsum, Magnisphaera). Therefore, there is no clear pattern for ascospore morphology within the Halosphaeriaceae.
Although it has been shown that marine ascomycetes evolved from a terrestrial ancestor (Spatafora et al. 1998), no hypothesis has been proposed for the evolution of appendaged ascospores within the Halosphaeriaceae. The basic ascospore wall in marine fungi has three layers: epi- (outermost), meso- (central) and endo- (inner) sporium (Kirk 1976, 1986; Johnson et al. 1984). Spore appendages are in turn derived from these three wall layers by different processes: fragmentation of one or more layers (e.g. Corollospora species, Halosphaeriopsis mediosetigera, Remispora species); outgrowths of one or more layers and elaboration of the exosporium (e.g. Ceriosporopsis, Halosphaeria) and wall outgrowth (e.g. Ondiniella torquata).
Speculation as to which of these appendage types are primitive or derived characters have been voiced (Kirk 1986; Jones 2006). It is assumed that the ancestral ascospore lacked appendages or any spore wall ornamentation (Jones 2006). Kirk (1986) proposed a scheme representing genera that might share a close relationship, based on ascospore appendage morphology and histochemistry of the cell wall layers. He suggested they evolved along two separate lines: 1. a group where the exosporium is absent (e.g. Ondiniella torquata) and 2. those with an exosporium (e.g. Remispora spp.). However, he cautioned that “because selection is most rigorous where a prototype crosses an adaptive threshold, a few intermediate forms remain and close homologies are difficult to find”.
Our DNA sequence analyses of various taxa suggest that appendages have evolved and then been lost several times during evolutionary times, or has a primitive character been retained in some genera? (Fig. 1). Examples of non-appendaged fungi include: Aniptodera sensu stricto species, Aniptodera longispora, Lignincola laevis, L. tropica, Pseudolignincola siamensis, Neptunella longirostris, Okeanomyces cucullatus, Nais inornata and Saagaromyces glitra. Of the 77 species sequenced here (Fig. 1), only 8 lack appendages, and some also of these have persistent asci. Aniptodera chesapeakensis has been reported with and without polar appendages and molecular data is required to determine if this constitutes one taxon (Shearer 1989). Saagaromyces glitra and Lignincola tropica are the only species in a clade-genus lacking appendages, and may suggest the retention of a primitive character or the loss of a character. Ascospores of Thalespora appendiculata on release passively from the ascus lack appendages but when mounted in seawater produce apical appendages (Jones et al. 2006), but not in freshwater. A similar condition has been noted for the aquatic species Savoryella appendiculata and Halosarpheia aquatica (Jones and Hyde 1992; Hyde 1992).
Appendages that arise as an outgrowth of the exosporium (Haligena, Morakotiella); direct outgrowth from one or more spore wall layers (Nereiospora, Arenariomyces, Nautosphaeria); or exuded through pores (Halosarpheia sensu stricto, Ascosacculus, Ophiodeira, Magnisphaera) are present in number of genera throughout the family. The appendage type that seems to be stable for certain genera, and include appendages that arise as an outgrowth and fragmentation of the spore wall (Corollospora), or as an outgrowth of the spore wall and with the elaboration of an exosporium (Marinospora, Ceriosporopsis).
Marine fungi were thought to be unusual in that they possessed ascospores with elaborate appendages, however with better microscopes and mounting material in water, other groups have been shown to be equally endowed with appendaged spores (Jones 2006). Hyde et al. (2000) documented 100 palm fungal genera, the majority of which (67) have some kind of appendages or sheaths. Ascospores with mucilaginous sheaths predominated (52), 20 genera had appendages and 10 genera produced polar mucilaguinous pads or a drop of extruded mucilage from the spore wall. Also coelomycetes have conidia that show infinite variation in the type of appendages produced and have been well documented by Nag Raj (1993). Of some 7000 coelomycetes described, 420 have appendaged conidia and were characterised into 11 developmental patterns by Nag Raj (1993). Appendaged spores are produced by a wide range of taxa, freshwater and terrestrial ascomycetes, hyphomycetes, coprophilous fungi and Trichomycetes (Jones 2006). So development of appendages or sheaths is not confined to fungi in aquatic habitats.
Review of the ordinal status of the Halosphaeriaceae
The monophyly of the Halosphaeriaceae has been confirmed and our results are concordant with other studies (Spatafora et al. 1998; Chen et al. 1999; Kohlmeyer et al. 2000; Kong et al. 2000), with the family closely related to the terrestrial Microascales. Members of the Halosphaeriaceae and Microascales share certain features in common e.g. perithecial ascomata, ascomal position, absence of paraphyses, evanescent asci and passively discharged ascospores (Table 3). Unlike the Halosphaeriaceae, however, the Microascaceae is mainly saprobic in terrestrial habitats (e.g. soil, plant debris, rotting vegetation, dung) while a few are plant (e.g. Ceratocystis fimbriata) or animal (Pseudallescheria) pathogens. There are few reports of the Microascales sensu lato from marine habitats (Jin and Jones in press). The halosphaerialean fungi are saprobic on decaying woody and herbaceous substrata in aquatic environments. The Microascales sensu lato is also associated with insects, whereas the Halosphaeriaceae may have lost their arthropod association ascospore dispersal on migration to the sea (Spatafora et al. 1998). Moreover, the ascomal type in the Halosphaeriaceae is typically perithecioid, while many genera in the Microascales sensu lato possess cleistothecia, sometimes with well developed setae. Ascus apical structures are present in many genera in the Halosphaeriaceae, while there is none in the Microascales sensu lato. Moreover, the asci and ascospore morphology are different: in the Microascales sensu lato asci are globose or ovoid, often produced in chains; with reniform ascospores, which often bear ornamenting ridges or wings. Halosphaeriaceous genera possess clavate to fusiform asci; hyaline ascospores, unicellular to many septate, usually with appendages (Table 3). Another feature that separates them is the paucity of anamorphs in the Halosphaeriaceae (Cirrenalia macrocephala, Clavatospora bulbosa, Varicosporina anglusa, Trichocladium achrasporum, Monodictys pelagica, Humicola siamensis) all possessing dematiaceous conidia (Shearer and Crane 1977; Mouzouras and Jones 1985). In the Microascales sensu lato anamorphs include Scopulariopsis, Graphium and Scedosporium, with hyaline annellidic or aleuro-conidia (Abbott et al. 1998).
Sequence data support three entities in the order Microascales: the Halosphaeriaceae, Microascaceae and a group of Ceratocystis species, all in a well supported clade (Fig. 1). However, the morphological differences noted above between the Halosphaeriaceae and Microascaceae raise the issue should they be referred to the same order?
In conclusion, this study of all the major genera in the Halosphaeriaceae has shown that while most are well resolved (e.g. Corollospora, Oceanitis, Halosarpheia sensu stricto, Saagaromyces), others require further investigation (e.g. Aniptodera, Nereiospora, Alisea). Taxa with unfurling polar appendages have evolved many times within the family and may indicate a special adaptation to an aquatic exsistence. While appendages have evolved in response to environmental conditions, they may also have been lost (e.g. Saagaromyces glitra). Sequence data showed that the genera Ceriosporopsis, Remispora were polyphyletic and the genera Kochiella, Torriella and Tubakiella are introduced to accommodate the displaced taxa.
References
Abbott SP, Singler L, Currah RS (1998) Microascus brevicaulis sp. nov., the teleomorph of Scopulariopsis brevicaulis, supports placement of Scopulariopsis with the Microascaceae. Mycologia 90:297–302
Abdel-Wahab MA, Jones EBG, Vrijmoed LLP (1999) Halosarpheia kandeliae sp. nov. on intertidal bark of the mangrove tree Kandelia candel in Hong Kong. Mycol Res 103:1500–1504
Abdel-Wahab MA, El-Sharouney HM, Jones EBG (2001a) Two new intertidal lignicolous Swampomyces species from Red Sea Mangroves in Egypt. Fungal Divers 8:35–40
Abdel-Wahab MA, Pang KL, El-Sharouney HM, Jones EBG (2001b) Halosarpheia unicellularis sp. nov. (Halosphaeriales, Ascomycota) based on morphological and molecular evidence. Mycoscience 42:255–260
Abdel-Wahab MA, Nagahama T, Abdel-Aziz FA (2009) Two new Corollospora species and one new anamorph based on morphological and molecular data. Mycoscience 50:147–155
Alias SA, Jones EBG (2009) Marine fungi from mangroves of Malaysia. Monograph No. 8, Institute of Ocean and Earth Sciences, Malaysia
Alias SA, Moss ST, Jones EBG (2001) Cucullosporella mangrovei, ultrastructure of ascospores and their appendages. Mycoscience 42:405–411
Anderson JL, Chen W, Shearer CA (2001) Phylogeny of Halosarpheia based on 18S rDNA. Mycologia 93:897–906
Baker TA, Jones EBG, Moss ST (2001) Ultrastructure of ascus and ascospore appendages of the mangrove fungus Halosarpheia ratnagiriensis (Halosphaeriales, Ascomycota). Can J Bot 79:1–11
Barghoorn ES, Linder DH (1944) Marine fungi: their taxonomy and biology. Farlowia 1:395–467
Barr ME (2001) Ascomycota. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The mycota VII part a systematics and evolution. Springer-Verlag, Berlin Heidelberg, pp 161–177
Binder M, Hibbett DS, Wang Z, Farnham WF (2006) Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycetes pathogen of a subtidal Rhodophyte. Am J Bot 93:547–556
Bunyard BA, Nicholson MS, Royse DJ (1994) A systematic assessment of Morchella using RFLP analysis of the 28S ribosomal RNA gene. Mycologia 86:762–772
Cai L, Ji KF, Hyde KD (2006) Variation between freshwater and terrestrial fungal communities on decaying bamboo culms. Anton Leeuw Int J G 89:293–301
Cain RF (1972) Evolution of the fungi. Mycologia 64:1–14
Campbell J (1999) Molecular phylogeny of the Halosphaeriaceae, Ascomycota. University of Portsmouth, UK, Ph.D. Dissertation
Campbell J, Anderson JL, Shearer CA (2003) Systematics of Halosarpheia based on morphological and molecular data. Mycologia 95:530–552
Campbell J, Volkmann-Kohlmeyer B, Gräfenhan T, Spatafora JW, Kohlmeyer J (2005) A reevaluation of Lulworthiales: relationships based on 18S and 28S rDNA. Mycol Res 109:556–568
Cavaliere AR, Johnson TW (1966) Marine ascomycetes: ascocarp morphology and its application to taxonomy and evaluation. Nova Hedw 10:453–461
Chen W, Shearer CA, Crane JL (1999) Phylogeny of Ophioceras spp. based on morphological and molecular data. Mycologia 91:84–94
Choi YW, Hyde KD, Ho WH (1999) Single spore isolation of fungi. Fungal Divers 3:29–38
de Bary A (1887) Comparative morphology and biology of the fungi, mycetozoa and bacteria. Clarendon, Oxford, pp 1–525
Demoulin V (1974) The origin of ascomycetes and basidiomycetes. The case for a red algal ancestry. Bot Rev 40:315–345
Demoulin V (1985) The red algal-higher fungi phylogenetic link: the last ten years. BioSystems 18:347–356
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Eriksson OE (1984) Outline of the ascomycetes. Systema Ascomycetum 3:1–72
Farris JS, Källersjö M, Kluge AG, Bult C (1995) Testing significance of incongruence. Cladistics 10:315–319
Farrant CA, Jones EBG (1986) Haligena salina: a new marine pyrenomycete. Bot J Linn Soc 93:405–411
Fazzani K, Jones EBG (1977) Spore release and dispersal in marine and brackish water fungi. Material Org 12:235–248
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Hall T (2007) BioEdit 7.0.9.0. Department of Microbiology, North Carolina State University. (http://www.mbio.ncsu.edu/BioEdit/bioedit.html)
Hibbett DS, Binder M, Bischoff JF et al (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Höhnk W (1955) Studien zur Brack-und Seewassermykologie. V. Höhere Pilze des sub-mersen Holzes. Veroeff Inst Meeresforsch Bremerhaven 3:199–227
Hsieh S-Y, Chang HS, Jones EBG, Read SJ, Moss ST (1995) Halosarpheia aquadulcis sp. nov., a new lignicolous, freshwater ascomycete from Taiwan. Mycol Res 99:49–53
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755 (http://morphbank.ebc.uu.se/mrbayes/download.php)
Hyde KD (1990) A new marine ascomycete from Brunei Aniptodera longispora sp. nov. from intertidal mangrove wood. Bot Mar 33:335–338
Hyde KD (1992) Tropical Australian freshwater fungi IV. Halosarpheia aquatica sp. nov., Garethjonesia lacunosispora gen. et sp. nov. and Ophioceras dolichostomum (Ascomycetes). Aust Syst Bot 5:407–414
Hyde KD, Jones EBG (1989) Observations on ascospore morphology in marine fungi and their attachment to surface. Bot Mar 32:205–218
Hyde KD, Goh TK (2003) Adaptations for dispersal in filamentous freshwater fungi. Fungal Divers Res Ser 10:231–258
Hyde KD, Jones EBG, Moss ST (1986) How do fungal spores attach to surfaces? In: Barry S, Houghton DR, Llewellyn GC, O’Rear CE (eds) Biodeterioration 6. CAB International Mycological Institute and the Biodeterioration Society, London, pp 584–589
Hyde KD, Greenwood R, Jones EBG (1993) Spore attachment in marine fungi. Mycol Res 97:7–14
Hyde KD, Wong SW, Jones EBG (1998) Diluviocola capensis gen. and sp. nov., a freshwater ascomycete with unique polar caps on the ascospores. Fungal Divers 1:133–146
Hyde KD, Fröhlich J, Taylor JE (2000) Genera of ascomycetes from palms. Fungal Divers Res Ser 2:1–247
Ingold CT (1975) An illustrated guide to aquatic and water-borne Hyphomycetes (Fungi Imperfecti) with notes on their biology. Scientific, England
Johnson RG, Jones EBG, Moss ST (1984) Taxonomic studies of the Halosphaeriaceae: Remispora Linder, Marinospora Cavaliere and Carbosphaerella Schmidt. Bot Mar 27:557–566
Johnson RG, Jones EBG, Moss ST (1987) Taxonomic studies of the Halosphaeriaceae: Ceriosporopsis, Haligena and Appendichordella gen. nov. Can J Bot 65:931–942
Jones EBG (1962) Haligena spartinae sp. nov. A pyrenomycete on Spartina townsendii. Trans Br Mycol Soc 45:246–248
Jones EBG (1964) Nautosphaeria cristaminuta gen. et sp. nov., a marine pyrenomycete on submerged wood. Trans Br Mycol Soc 47:97–101
Jones EBG (1994) Fungal adhesion. Mycol Res 98:961–981
Jones EBG (1995) Ultrastructure and taxonomy of the aquatic ascomycetous order Halosphaeriales. Can J Bot 73(Suppl 1):S790–S801
Jones EBG (2006) Form and function of fungal spore appendages. Mycoscience 47:167–183
Jones EBG, Choeyklin R (2008) Ecology of marine and freshwater basidiomycetes. In: Boddy L, Frankland JC, van West P (eds) Ecology of Saprotrophic Basidiomycetes, British Mycological Society Symposia Series. Elsevier, London, pp 301–324
Jones EBG, Hyde KD (1992) Taxonomic studies on Savoryella Jones et Eaton (Ascomycotina). Bot Mar 35:83–91
Jones EBG, Moss ST (1978) Ascospore appendages of marine ascomycetes: an evaluation of appendages as taxonomic criteria. Mar Biol 49:11–26
Jones EBG, Moss ST (1980) Further observations on the taxonomy of Halosphaeriaceae. Bot Mar 23:483–500
Jones EBG, Moss ST (1987) Key and notes on genera of the Halosphaeriaceae examined at the ultrastructural level. Systema Ascomycetum 6:179–200
Jones EBG, Johnson RG, Moss ST (1983a) Ocostaspora apilongissima gen. et sp. nov. A new marine pyrenomycete from wood. Bot Mar 26:353–360
Jones EBG, Johnson RG, Moss ST (1983b) Taxonomic studies of the Halosphaeriaceae: Corollospora Werdermann. Bot J Linn Soc 87:193–212
Jones EBG, Johnson RG, Moss ST (1984) Taxonomic studies of the Halosphaeriaceae: Halosphaeria Linder. Bot Mar 27:129–143
Jones EBG, Johnson RG, Moss ST (1986) Taxonomic studies of the Halosphaeriaceae philosophy and rationale for the selection of characters in the delineation of genera. In: Moss ST (ed) The biology of marine fungi. Cambridge University Press, UK, pp 211–230
Jones EBG, Chatmala I, Pang KL (2006) Two new genera isolated from marine habitats in Thailand: Pseudolignincola and Thalespora (Halosphaeriales, Ascomycota). Nova Hedw 83:219–232
Jones EBG, Sakayaroj J, Suetrong S, Somrithipol S, Pang KL (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–203
Kirk PW (1976) Cytochemistry of marine fungal spores. In: Jones EBG (ed) Recent advances in aquatic mycology. Elek Science, London, pp 177–193
Kirk PW (1986) Evolutionary trends within the Halosphaeriaceae. In: Moss ST (ed) The biology of marine fungi. Cambridge University Press, UK, pp 263–274
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth and Bisby’s dictionary of the fungi, 9th edn. CABI, London
Koch J (1989) Remispora spinibarbata sp. nov., a marine lignicolous ascomycete from Denmark. Nord J Bot 8:517–520
Koch J, Pang KL, Jones EBG (2007) Rostrupiella danica gen. et sp. nov., a Lulworthia-like marine lignicolous species from Denmark and the USA. Bot Mar 50:1–8
Kohlmeyer J (1960) Wood-inhabiting marine fungi from the Pacific Northwest and California. Nova Hedw 2:293–343
Kohlmeyer J (1962) Halophile Pilze von den Ufern Frankreichs. Nova Hedw 4:389–420
Kohlmeyer J (1972) A revision of Halosphaeriaceae. Can J Bot 50:1951–1963
Kohlmeyer J (1975) New clues to the possible origin of Ascomycetes. BioScience 25:86–93
Kohlmeyer J (1981) Marine fungi from Martinique. Can J Bot 59:1314–1321
Kohlmeyer J (1986) Taxonomic studies of the marine Ascomycotina. In: Moss ST (ed) The biology of marine fungi. Cambridge University Press, UK, pp 199–210
Kohlmeyer J, Kohlmeyer E (1977) Bermuda marine fungi. Trans Br Mycol Soc 68:207–219
Kohlmeyer J, Kohlmeyer E (1979) Marine mycology. The higher fungi. Academic, New York
Kohlmeyer J, Volkmann-Kohlmeyer B (1998) Naufragella a new genus in the Halosphaeriaceae. Systema Ascomycetum 16:9–16
Kohlmeyer J, Volkmann-Kohlmeyer B (2002) Fungi on Juncus and Spartina: New marine species of Anthostomella, with a list of marine fungi known from Spartina. Mycol Res 106:365–374
Kohlmeyer J, Spatafora J, Volkmann-Kohlmeyer B (2000) Lulworthiales, a new order of marine Ascomycota. Mycologia 92:453–458
Kong RYC, Chan JYC, Mitchell JI, Vrijmoed LLP, Jones EBG (2000) Relationships of Halosarpheia, Lignincola and Nais inferred from partial 18S rDNA. Mycol Res 104:336–343
Landvik S (1996) Neolecta, a fruit-body-producing genus of the basal ascomycetes, as shown by SSU and LSU rDNA sequences. Mycol Res 100:199–202
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among Ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Lutzoni F, Kauff F, Cox CJ et al (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Manimohan P, Jones EBG, Moss ST (1993a) Ultrastructural studies of ascospores of some Remispora species. Can J Bot 71:385–392
Manimohan P, Moss ST, Jones EBG (1993b) Ultrastructure of the ascospore wall and appendages of Remispora galerita. Mycol Res 97:1190–1192
McKeown TA, Moss ST, Jones EBG (1996) Ultrastructure of ascospores of Tunicatispora australiensis. Mycol Res 100:1247–1255
Meyers SP (1957) Taxonomy of marine pyrenomycetes. Mycologia 49:475–528
Moss ST (1979) Commensalism in the Trichomycetes. In: Batra LP (ed) Insect-fungus symbiosis. Allanheld, Osmum & Co. Montclair, New Jersey, pp 175–222
Moss ST, Jones EBG (1977) Ascospore appendages of marine Ascomycetes: Halosphaeria mediosetigera. Trans Br Mycol Soc 69:313–315
Mouzouras R, Jones EBG (1985) Monodictys pelagica, the anamorph of Nereiospora cristata (Halosphaeriaceae). Can J Bot 63:2444–2447
Müller E, von Arx JA (1962) Die Gattungen der didymosporen Pyrenomyceten. Beiträge. Kryptogamenflora Schweiz 11:1–922
Nag Raj TR (1993) Coelomycetous anamorphs with appendages-bearing conidia. Mycologue, Waterloo
Nylander JAA (2004) MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University
O’Donnell K, Cigelnik E, Weber NS, Trappe JM (1997) Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89:48–65
Pang KL (2001) Molecular systematics and phylogenetic biogeography of the Halosphaeriales (Ascomycota), City University of Hong Kong, Hong Kong SAR, Ph.D. Dissertation
Pang KL (2002) Systematics of the Halosphaeriales: which morphological characters are important? In: Hyde KD (ed) Fungi in marine environments. Fungal Diversity Press, Hong Kong, pp 35–57
Pang KL, Jones EBG (2004) Reclassifications in Halosarpheia and related genera with unfurling ascospore appendages. Nova Hedw 78:269–271
Pang KL, Vrijmoed LLP, Kong RYC, Jones EBG (2003) Lignincola and Nais, polyphyletic genera of the Halosphaeriales (Ascomycota). Mycol Prog 2:29–39
Pang KL, Jones EBG, Vrijmoed LLP, Vikineswary S (2004a) Okeanomyces, a new genus to accommodate Halosphaeria cucullata (Halosphaeriales, Ascomycota). Bot J Linn Soc 146:223–229
Pang KL, Jones EBG, Vrijmoed LLP (2004b) Two new marine fungi from China and Singapore, with the description of a new genus, Sablecola. Can J Bot 82:485–490
Pang KL, Chiang MWL, Vrijmoed LLP (2008a) Havispora longyearbyenensis gen. et sp. nov., an arctic marine fungus from Svalbard, Norway. Mycologia 100:291–295
Pang KL, Jones EBG, Vrijmoed LLP (2008b) Autecology of Antennospora (Ascomycota, Fungi) and its phylogeny. Raffles Bull Zool 19:1–10
Pang KL, Chiang MWL, Vrijmoed LLP (2009) Remispora spitsbergenensis sp. nov., a marine lignicolous ascomycete from Svalbard, Norway. Mycologia 101:531–534
Porter D (1982) The appendaged ascospores of Trichomaris invadens (Halosphaeriaceae), a marine ascomycetous parasite of the tanner crab, Chinocetes bairdi. Mycologia 74:363–375
Sachs J (1874) Lehrbuch der Botanik, 4th edn. Engelmann, Leipzig
Sakayaroj J (2005) Phylogenetic relationships of marine Ascomycota. Prince of Songkla University, Thailand, Ph.D. Dissertation
Sakayaroj J, Jones EBG, Chatmala I, Phongpaichit S (2004) Thai marine fungi. In: Jones EBG, Tantichareon M, Hyde KD (eds) Thai fungal diversity. BIOTEC, Thailand, pp 107–117
Sakayaroj J, Pang KL, Phongpaichit S, Jones EBG (2005a) A phylogenetic study of the genus Haligena (Halosphaeriales, Ascomycota). Mycologia 97:804–811
Sakayaroj J, Pang KL, Jones EBG, Vrijmoed LLP, Abdel-Wahab MA (2005b) A systematic reassessment of marine ascomycetes Swampomyces and Torpedospora. Bot Mar 48:395–406
Samuels GL, Blackwell M (2001) Pyrenomycetes-fungi with perithecia. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The mycota VII part a systematics and evolution. Springer-Verlag, Berlin, pp 221–255
Schoch CL, Sung G-H, Volkmann-Kohlmeyer B, Kohlmeyer J, Spatafora JW (2007) Marine fungal lineages in the Hypocreomycetidae. Mycol Res 110:257–263
Shearer CA (1989) Aniptodera (Halosphaeriaceae) from wood in freshwater habitats. Mycologia 81:139–146
Shearer CA (1993) The freshwater Ascomycetes. Nova Hedw 56:1–33
Shearer CA, Crane JL (1977) Fungi of the Chesapeake Bay and its tributaries. VI. Trichocladium achrasporum, the imperfect state of Halosphaeriopsis mediosetigera. Mycologia 69:1218–1223
Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J (1998) Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). Am J Bot 85:1569–1580
Suetrong S, Sakayaroj J, Phongpaichit S, Jones EBG (2009a) Morphological and molecular characteristics of a poorly known marine ascomycete Manglicola guatemalensis (Jahnulales, Pezizomycetideae, Dothideomycetes Incertae sedis) A new lineage of marine ascomycetes. Mycologia 102:83–92
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009b) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Swofford DL (2002) PAUP: Phylogenetic Analysis Using Parsimony, version 4.0b10. Sinauer Associates, Sunderland, Massachusetts
Tang AMC, Jeewon R, Hyde KD (2007) Phylogenetic utility of protein (RPB2, β-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi). Anton Leeuw Int J G 91:327–349
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JS, White TJ (eds) PCR Protocol: A guide to methods and applications. Academic, San Diego, pp 315–322
Wong SW, Hyde KD, Jones EBG (1998a) Annulatascaceae, a new ascomycete family from the tropics. Systema Ascomycetum 16:17–25
Wong SW, Hyde KD, Jones EBG (1998b) Ascospore ultrastructure of Halosarpheia heteroguttulata sp. nov. from tropical streams. Can J Bot 76:1857–1862
Yusoff M, Koch J, Jones EBG, Moss ST (1993) Ultrastructure observations in a marine lignicolous ascomycete Bovicornua intricata gen. et sp. nov. Can J Bot 71:346–352
Yusoff M, Jones EBG, Moss ST (1994) A taxonomic reappraisal of the genus Ceriosporopsis based on ultrastructure. Can J Bot 72:1550–1559
Zhang N, Castlebury LA, Miller AN, Huhndorf S, Schoch CL, Seifert K, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung S-H (2006) Sordariomycetes systematics: an overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98:1076–1087
Acknowledgments
This work was supported by the TRF/BIOTEC Special Program for Biodiversity Research and Training Grants BRT R_245002, R_251006 and R_351004. JS would like to thank Graduate School-Prince of Songkla University, BIOTEC LGS scholarship for partial financial support. We acknowledge Dr. Kanyawim Kirtikara, Dr. Lily Eurwilaichitr and Dr. Souwalak Phongpaichit for continued support. JS thanks Prof. Lilian L.P. Vrijmoed for providing cultures and laboratory facilites; Ms. Satinee Suetrong, Mr. Anupong Klaysuban and Ms. Sita Preedanon for field and the molecular work assistance. We are grateful to Dr. Jorgen Koch for his contribution in supplying the cultures for this study. KLP would like to thank National Science Council of Taiwan (Project No. NSC 98-2621-B-019-002-MY3) and Center of Excellence for Marine Bioenvironment and Biotechnology (CMBB), National Taiwan Ocean University. We thank an anonymous reviewer for his valued comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakayaroj, J., Pang, KL. & Jones, E.B.G. Multi-gene phylogeny of the Halosphaeriaceae: its ordinal status, relationships between genera and morphological character evolution. Fungal Diversity 46, 87–109 (2011). https://doi.org/10.1007/s13225-010-0072-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-010-0072-y