Abstract
Biosurfactant-producing bacteria were isolated from mangrove sediment samples collected in the southern part of Thailand by an enrichment-culture technique in which lubricating oil was the sole carbon source. A total of 1,600 colonies were obtained, which were screened for biosurfactant production using the qualitative drop-collapsing test in a mineral salts medium containing 1% of different carbon sources (commercial sugar, glucose, molasses, and used lubricating oil). Ninety-five isolates were positive for biosurfactant production based on the results of this test, among which 20 could reduce the surface tension of the 48-h culture supernatant. The phylogenetic position of these 20 isolates was evaluated by 16S rRNA gene sequence analysis. The production of biosurfactants was determined for strains representative of eight different bacterial genera. Leucobacter komagatae 183, one of the newly isolated strains showing biosurfactant production, produced extracellular biosurfactants which reduced the surface tension of the culture supernatant from 72.0 to 32.0 m/Nm. Eighteen strains released extracellular emulsifiers able to stabilize the emulsion formed. Among these, the strains L. komagatae 183 and Ochrobactrum anthropi 11/6 exhibited emulsification activities comparable to those of synthetic surfactants. Overall, the new biosurfactant-producing strains isolated in this study display promising features for the future development and use in economically efficient industrial-scale biotechnological processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Mangroves, dominant inter-tidal wetlands found along coastlines of tropical and subtropical regions, are considered to be significant sinks for pollution from freshwater discharges as well as from contaminated tidal water (Bernard et al. 1996). The wetlands are also particularly susceptible to oil pollution since they are usually situated in regions active in oil production, transportation, and other anthropogenic activities generating spilled and stranded oil (Burns et al. 1993). When oil spreads in an environment, low-molecular-weight hydrocarbons are volatilized while polar components are dissolved in water. However, most of the oil hydrocarbons remain on the water surface or adhere to soil particles due to their low solubility (Karanth et al. 1999). Evaporation and photo-oxidation play an important role in oil detoxification, with ultimate and complete degradation being accomplished mainly by microbial activity (Batista et al. 2006).
Diverse groups of indigenous microorganisms capable of utilizing and degrading contaminants such as hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) might be present in contaminated sediment (Ke et al. 2003). Hydrocarbon-degrading bacteria release biosurfactants that facilitate the assimilation of these insoluble substrates (Bicca et al. 1999). However, intrinsic biodegradation often takes a long time to complete because of the low water solubility of hydrocarbon (Snape et al. 2006). The biodegradation process is maximized when the water-insoluble substrate is dissolved or emulsified, which are the mechanisms by which biosurfactants are capable of increasing the bioavailability of hydrocarbons that dissolve poorly.
Biosurfactants are either extracellular compounds or localized on the cell surface (Chayabutra et al. 2001). In the latter case, the microbial cell itself is a biosurfactant and adheres to hydrocarbon (Maneerat 2005). There are many areas of application where biosurfactants can be used, such as in industries and environmental restoration, including the removal of heavy metal and organic compounds from soil and water. Biosurfactants also increase both the water-insoluble uptake of microorganisms and the efficiency of bioremediation. Whereas many studies on biosurfactants have been performed in the last few decades (Maneerat et al. 2006; Rodrigues et al. 2006; Maneerat and Phetrong 2007; Kebbouche-Gana et al. 2009; Anandaraj and Thivakaran 2010; Gudina et al. 2010; Burgos-Diaz et al. 2011; Darvishi et al. 2011), relatively fewer reports have been published on biosurfactants produced by mangrove sediment microorganisms. The objectives of this study were to isolate and characterize new biosurfactant-producing bacteria from mangrove sediments. The utilization of renewable and cheap carbon sources for biosurfactant production by isolated strains was also studied.
Materials and methods
Microorganism isolation
Samples (100 g) of mangrove sediment (depth: 0–5 cm) were collected from five different sites (10 samples/site) along the east and west coast of southern Thailand: (1) Palain District, Trang Province; (2) Sikao District, Trang Province; (3) Thungwa District, Satun Province; (4) Ranot District, Songkhla Province; (5) Huasai District, Nakhonsrithammarat Province. The enrichment and isolation of the biosurfactant-producing bacterial consortium was performed by using used lubricating oil (ULO) as the sole carbon and energy source. Initially, the bacterial consortium was enriched by adding 1 g of soil sample to 50 ml of minimal salt medium [MSM (g/l): K2HPO4, 0.8; KH2PO4, 0.2; CaCl2, 0.05; MgCl2, 0.5; FeCl2, 0.01; (NH4)2SO4, 1.0; NaCl, 5.0; ULO, 10; Yin et al. 2005] in a 250-ml Erlenmeyer flask. This mixture was shaken (150 rpm) at 30°C for 5 days or until an oil emulsion was observed. A 1-ml aliquot of the culture broth was then transferred to 50 ml of fresh MSM in a 250-ml flask and incubated under the same conditions as described above. This procedure was repeated five times.
Sediment-enriched cultures were diluted in a sterile 0.85% saline solution and plated on MSM agar using glucose (1%, w/v) or ULO (1%, w/v) as the carbon source for the isolation of microorganisms. Morphologically distinct colonies were re-isolated by transfer onto fresh glucose- or ULO-containing agar plates at least three times to obtain pure cultures and subsequently Gram-stained. Pure cultures were stored at −20°C in MSM mixed with sterile glycerol at a final concentration of 30%.
Screening of potential biosurfactant-producing strains
In total, 1,600 isolates were streaked on MSM agar containing 1% (w/v) of ULO or glucose for 48 h at 30°C. One loop of each isolate was then transferred to test tubes containing 5 ml of nutrient broth (NB) and shaken (150 rpm) at 30°C for 24 h. A 100-μl sample of each cell culture was transferred to 5 ml of MSM medium supplemented with 1% (w/v) of different carbon sources [commercial sugar (CS, sucrose), glucose, molasses, and ULO) in a rotary shaker (Vision Scientific, Daejon, Korea) at 30°C and 150 rpm for 24 h. Screening for biosurfactant-producing isolates was performed by using the qualitative drop-collapsing test and by testing for the emulsification activity (E24) of the culture supernatant after centrifugation at 8,500 rpm and 4°C for 10 min.
Evaluation of biosurfactant production
Twenty bacterial isolates were evaluated for biosurfactant production in 250-ml Erlenmeyer flasks containing 50 ml of MSM supplemented with 1% (w/v) of the chosen carbon source. The carbon sources used when testing for biosurfactant production were CS, glucose, glycerol, molasses, n-hexadecane, and ULO. The isolates were activated by growing them on MSM agar containing 1% (w/v) of ULO or glucose for 48 h at 30°C. One loop of each isolate was then transferred to test tubes containing 5 ml of NB and shaken (150 rpm) at 30°C for 24 h. Cell suspensions were adjusted to an optical density (OD) at 600 nm of 0.10 ± 0.05 (103 CFU/ml), and 1 ml of each suspension was used as the starter. The flasks were incubated at 30°C, and growth was monitored by reading the OD600 on a Libra S22 spectrophotometer (Biochrom, Cambridge, UK). Biosurfactant activities were measured by using the qualitative drop-collapsing test and by testing for the E24 and surface tension by the duNouy method using a ring tensiometer (OS; Torsion Balance, Warwickshire, UK). The activity of the synthetic surfactants sodium dodecyl sulfate (SDS; Sigma Chemicals, St. Louis, MO) and Tween 80 (Sigma Chemicals) (10 g/l) was tested at concentrations higher than their critical micelle concentrations (2.0 and 0.16 g/l, respectively). MSM medium supplemented with the different carbon sources without inocula was used as a negative control.
16S rRNA gene sequence analysis
Selected isolates were incubated for 48 h at 30°C on MSM agar supplemented with 1% (w/v) of ULO or glucose and subsequently Gram-stained. For 16S rRNA gene amplification, selected bacterial isolate chromosomal DNA was isolated using a Roche kit (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions. The 16S rRNA gene was amplified using a PCR method with 1 U of Taq DNA polymerase (Bio-Lab, Auckland, New Zealand) and universal bacterial primers UFUL (GCCTAACACATGCAAGTCGA) and URUL (CGTATTACCGCGGC TGCTGG) (Nilsson and Strom 2002). These two primers target two highly conserved regions of the prokaryotic 16S rRNA gene (Phalakornkule and Tanasupawat 2006) and produced a PCR product of about 450–500 bp. The 16S rRNA gene was sequenced by using the ABI Prism BigDye terminator kit (Perkin-Elmer Applied Biosystems, Waltham, MA), according to the manufacturer’s protocol, with UFUL as primer. The 500-bp 16S rRNA gene sequences obtained were aligned along with the sequences of type strains obtained from the GenBank by using the program ClustalW (Thompson et al. 1997). Sequence homologies were examined using BLAST ver. 2.2.12 of the National Center for Biotechnology Information (NCBI), and a consensus neighbor-joining tree was constructed using Molecular Evolutionary Genetics Analysis (MEGA) software ver. 4.0 (Tamura et al. 2007). The 16S rRNA gene sequence was submitted to GenBank with an accession number.
Analytical methods
Growth
Growth was monitored by measuring the OD of the culture broth at 600 nm.
Drop-collapsing test
The drop collapse test was performed as described by Youssef et al. (2004).
Emulsification activity (E24) assay
The E24 assay was performed as described by Plaza et al. (2006).
Surface tension measurement
Assessment of surface tension was performed as described by Jachimska et al. (1995).
Statistical analysis
All experiments were carried out at least in triplicate. Statistical analysis was performed using Statistical Package for Social Science ver. 10.0 for Windows (SPSS, Chicago, IL).
Results
Microorganism isolation
The 89 sediment samples screened for biosurfactant producers were collected from the east and west coasts of southern Thailand by an enrichment-culture technique using ULO as a sole carbon source. The enrichment-culture technique was repeated five times, resulting in the isolation of 1,600 colonies by spreading on MSM supplemented with 1% of glucose or ULO as the carbon source. These 1,600 isolates were screened for biosurfactant production in MSM containing 1% of the different carbon sources (CS, glucose, molasses, or ULO). Ninety-five isolates tested positive for biosurfactant production according to the qualitative drop-collapsing test. These 95 isolates also showed promising biosurfactant activity by exhibiting a surface tension reduction of more than 10 mN/m. Accordingly, 20 isolates were selected for further testing: 19 of these grew on MSM containing glucose or molasses as the sole carbon source, 18 grew on MSM supplemented with CS or ULO as the sole carbon source, and only three grew on MSM containing n-hexadecane (Table 1).
Of these 20 bacterial isolates, 16 (80%) were Gram-negative bacteria. This result is in accordance with previous reports that most bacteria isolated from sites with a history of contamination by oil or its byproducts are Gram-negative bacteria due to the presence of outer membranes which act as biosurfactants (Bicca et al. 1999; Bodour et al. 2003; Batista et al. 2006). However, we found that selected isolates produced extracellular biosurfactants since culture supernatants exhibited biosurfactant activity based on the results of the drop-collapsing and E24 tests (Table 1).
16S rRNA gene sequence analysis
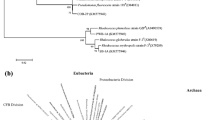
The 20 strains selected for further testing were genetically characterized as belonging to eight different genera (Table 2). Their sequences were deposited in DDBJ/EMBL/GenBank (accession numbers are given in parenthesis in Fig. 1) and compared to those of the biosurfactant-producing strains described in the literature. A phylogenetic tree was reconstructed (Fig. 1). Among the analyzed strains, 19 isolates (2/3, 7, 9/4, 11, 11/6, 33, 54, 57 79, 213, 318, 319, 418, 1106, 1033, 1291, 1297, 1310, and 1457) belonged to genera that have been previously been reported and characterized for the production of biosurfactants or bioemulsifiers (Kebbouche-Gana et al. 2009; Anandaraj and Thivakaran 2010; Gudina et al. 2010; Burgos-Diaz et al. 2011; Darvishi et al. 2011). One isolate (183) belonged to the genus Leucobacter. To the best of our knowledge, this is the first report on the capacity of the genus Leucobacter to produce a biosurfactant.
Unrooted phylogenetic tree based on 16S rRNA gene comparison of the bacterial strains featured in this study (bold) and microorganisms previously described in literature for biosurfactant production. Bootstrap probability values of <50% were omitted from the figure. Scale bar indicates substitutions per nucleotide position. GenBank accession numbers are given in parenthesis
Evaluation of biosurfactant production
The 20 strains testing positive for biosurfactant production were further examined for their capability to grow and produce biosurfactants in shake flasks using the same carbon sources used for the small-scale assay. The same results were obtained under the two cultural conditions, thus demonstrating the reliability of the small-scale growth test to screen for substrates supporting the growth of the isolates (Table 3). Among the five tested carbon sources, n-hexadecane supported the growth of a small number of strains after 48 h of incubation. It was noted that only Acinetobacter sp. 33, Enterobacter sp. 1033, and Pseudomonas putida 1106 grew on n-hexadecane (Table 1). In order to widen the spectrum of substrates, glycerol, a waste from biodiesel production, was also tested in a flask culture as a carbon source for biosurfactant production.
Table 3 shows the emulsification activity and the surface tension of the culture supernatants of the 20 strains testing positive for biosurfactant production in the preliminary screening. These were grown on CS, glucose, glycerol, molasses, or ULO. Emulsification activities significantly higher than those of the culture medium supplemented with each carbon sources were found in all strains (Table 3). Among the 20 selected isolates, the emulsification activity evaluated by the E24 ranged from 5.5 to 69.8%. Most of isolates demonstrated 20–50% of emulsification. The highest emulsification activity was observed in Ochrobactrum anthropi 11/6. In our study, the specificity of emulsion formation was highly variable, depending on the carbon source used in the growth medium of the culture.
Biosurfactants produced by the selected 20 bacterial isolates were affected by type of carbon source. When the medium contained a water-insoluble substrate (ULO), 18 isolates produced bioemusifiers which were capable of stabilizing emulsions toward xylene. Ochrobactrum anthropi 2/3, Acinetobacter sp. 33, Bacillus cereus 54, Acinetobacter sp. 57, and Acinetobacter sp. 79 exhibited either surface tension reduction or E24. The highest surface tension reduction was obtained from Acinetobacter sp. 79 (25.8 mN/m) when ULO was used as a carbon source. However, some isolates (isolate 7, 9/4, 11, 11/6, 33, 54, 57, 213, 318, 319, 418, 1033, 1106, 1291, 1297, 1310, and 1457) showed only E24, and the highest E24 was obtained from A. calcoaceticus 418 (64.3%).
When the medium was a single phase one (CS, glucose, glycerol, or molasses was used as the carbon source), with no requirement for an emulsifier to make an insoluble substrate more accessible, few isolates produced a potent extracellular bioemulsifier. Among the latter strains, O. anthropi 11/6, B. cereus 54, L. komagatae 183, and P. putida 1106 produced stable xylene-supernatant emulsions showing an E24 comparable to those of the synthetic surfactants SDS (63%) and Tween 80 (61%) when CS was used as the carbon source. The highest E24 was obtained from O. anthropi 11/6 (69.8%) when CS was used as carbon source. The surface tension of the culture supernatants of four (isolate 183, 318, 1291, and 1297), 13 (isolate 9/4, 11, 11/6, 183, 213, 319, 418, 1033, 1106, 1291, 1297, 1310, and 1457), two (isolate 183 and 318), and three isolates (isolate 7, 79, and 318) were reduced when CS, glucose, glycerol, and molasses were used as the carbon source, respectively. Strains which had a high surface tension reduction ability (strains: Leucobacter komagatae 183) were not generally able to form the emulsions with xylene when glucose was used as the sole carbon source (Table 3). According to Cooper (1986), a microorganism is considered to be a promising biosurfactant producer if it is able to reduce the surface tension to values of <40 mN/m. A decrease in surface tension below this threshold was found in some of the culture supernatants, namely, those of A. calcoaceticus 7, Acinetobacter sp. 79, L. komagatae 183, Bacillus subtilis 318, and P. putida 1106 when molasses, ULO, CS and glucose, molasses, and glucose were used as the sole carbon source, respectively.
Discussion
Microbial molecules which exhibit a high surface and emulsifying activity are classified as biosurfactants/bioemulsifiers. These molecules reduce the surface and interfacial tensions in both aqueous solutions and hydrocarbon mixtures making them potential agents for bioremediation (Banat et al. 2000). With the advantage of environmental compatibility, the demand for biosurfactants has been steadily increasing and may eventually replace their chemically synthesized counterparts. Several recent studies have reported the screening of new biosurfactant-producing isolates from terrestrial and marine environments (Das et al. 2009; Gandhimathi et al. 2009; Das et al. 2010). However, few studies have addressed the diversity of biosurfactant-producing bacteria (Ruggeri et al. 2009), particularly those isolated from mangrove sediment. In this work, we used an experimental approach which reduced the time and costs for screening new biosurfactant-producing bacteria. Rational choices were made for the different samples and enrichment procedures that were carried out in order to broaden the spectrum of the isolates. After isolation, strains were phylogenetically characterized and their capability to produce molecules with surface and emulsifying activity were analyzed. The biosurfactant production by strains belonging to well-characterized genera gave results comparable to those previously reported in the literature (Gudina et al. 2010; Burgos-Diaz et al. 2011; Darvishi et al. 2011).
An estimate of the frequency of biosurfactant-producing strains within a microbial population cannot be easily determined as it depends on the experimental procedures used. It has been reported that 2–3% of screened populations in uncontaminated soils are biosurfactant-producing microorganisms and that this increases to 25% in polluted soils (Bodour et al. 2003). However, enrichment culture techniques specific for hydrocarbon-degrading bacteria may lead to a much higher detection of biosurfactant producers, with estimates of up to 80% (Rahman et al. 2002). The principle of enrichment culture is to provide growth conditions that are very favorable for the organisms of interest and as unfavorable as possible for competing organisms. Thus, the microbes of interest are selected and enriched. In our study, we obtained isolates showing a large reduction in surface tension and emulsification activity by an enrichment culture technique.
Biosurfactant activity can be measured by changes in surface and interfacial tensions and emulsification/emulsion stabilization. Microbial candidates for biosurfactant production are expected to reduce surface tension to around 40 mN/m or lower (Cooper 1986; Olivera et al. 2003). In our work, we achieved a reduction in surface tension that was lower than that threshold with A. calcoaceticus 7, Acinetobacter sp. 79, L. komagatae 183, B. subtilis 318, and P. putida 1106. Another approach for screening potential biosurfactant-producing microorganisms is to estimate the emulsification activity (E24). All isolates could form an emulsion with xylene, but this depended on the carbon source. Some strains reduced the surface tension to <40 mN/m but could not emulsify xylene. Our results show that the reduction of surface tension and emulsion formation were not correlated. Among the strains tested, three released emulsifiers, O. anthropi 11/6, L. komatagae 183, and P. putida 1106 These strains efficiently stabilized emulsion forms even if they did not reduce the surface tension of the medium when CS was used as the carbon source (Table 2). These results are similar to those reported by Willumsen and Karlson (1997) and Plaza et al. (2006). Polymeric biosurfactants with emulsification abilities are produced by a number of bacteria, Archaea, and yeast (Bodour and Maier 2002). In general, polymeric biosurfactants do not significantly lower the surface tension. The polymeric biosurfactant with the best characteristics is the complex acylated polysaccharide emulsan, which is produced by Acinetobacter calcoaceticus RAG I. Rosenberg et al. (1979) identified a protein associated with the polymers that is required for emulsification activity. The production of extracellular polymers has been extensively demonstrated in rhizobia, even though the surface properties and applicability of these compounds have not yet been investigated (Skorupska et al. 2006).
According to Willumsen and Karlson (1997), a good bioemulsifier had an E24 of >50%. In our study, we obtained an E24 of >50% with O. anthropi 11/6, B. cereus 54, Acinetobacter sp. 57, L. komagatae 183, Acinetobacter sp. 213, B. subtilis 318, A. calcoaceticus 418, Enterobacter sp. 1033, and P. putida 1106. The stability of the emulsions has been reported to be important for both the performance and the effectiveness of the emulsifier (Willumsen and Karlson 1997). In this study, stable and compact emulsions of xylene-supernatant were observed after 1 hour and they were found to be stable for up to 48 h (O. anthropi 11/6, B. cereus 54, L. komagatae 183, A. calcoaceticus 418, and P. putida 1106) (data not shown). Based on these results, it is possible to suggest that the bioemulsifier from this study would be useful in applications designed for the biodegradation of hydrocarbons or other water-immiscible substrates and for the enhancement of oil recovery. These properties are important in order to be able to reduce the capillary forces that are entrapping oil within the pores of rocks. They can also be considered for use as a mobility control agent to improve the sweep efficiency of a water flood in the petroleum industry (De Acevedo and McInerney 1996). Consequently, we suggest that we have isolated another promising microbial candidate for use in biosurfactant/bioemulsifier production.
Bacillus subtilis 318 and Pseudomonas putida 1106, both isolated in this study, displayed a substantial capacity to decrease surface tension and increase emulsification activity, respectively. Members of Bacillus species are some of the most studied industrial microorganisms. Saimmai et al. (2011) reported that a Bacillus spp. isolated from mangrove sediment by using only molasses as a whole medium lowered the water surface tension to 28.5 mN/m. This Bacillus isolate produced two surface active agents, namely, a polymer containing D-glucosamine, which stabilized thick oil-in-water emulsions, and a mixture of saturated monoglycerides, which lowered the surface tension of water (Cooper et al. 1979). Among the major types of biosurfactants produced by microorganisms, surfactin is one of the best known products with a commercial application. Bacillus amyloliquefaciens (Singh et al. 2011), B. licheniformis (Rivardo et al. 2009), B. mojavensis (From et al. 2007), B. pumilus, and B. subtilis (Banat et al. 2000) have been reported as surfactin producers. The majority of hydrocarbon-degrading bacteria reported in the literature belong to the genus P. (Widada et al. 2002). In our study, isolate 1106 was similar to P. putida (Table 2), and isolate 57 was similar to Acinetobacter sp.; members of both of these genera have been reported to produce surface-active polymers (Rosenberg and Ron 1998) and surface active agents (Huy et al. 1999).
Among the bacteria tested, L. komagatae 183 produced extracellular biosurfactants which reduced the surface tension of culture supernatant from 72.0 to 32.0 m/Nm. The strain also produced extracellular emulsifiers able to stabilize xylene-supernatant emulsions. To the best our knowledge, our work provides the first description of a biosurfactant-producing strain belonging to the genus Leucobacter. Interestingly, terrestrial subsurface environments have been reported as a source of new microorganisms even if they have not been previously investigated for biosurfactant production (Blume et al. 2002). The majority of the biosurfactant-producing strains identified in this work were assigned to the alpha subdivision of Proteobacteria, a division which includes Gram-negative bacteria. In fact, it is well documented that the majority of bacteria isolated from contaminated environments are Gram-negative bacteria (Batista et al. 2006; Ruggeri et al. 2009). Biosurfactants exhibit properties as emulsifying or dispersing agents, favoring the release of hydrophobic contaminants absorbed in organic matter or increasing the surface area of the contaminant available as the substrate (Mercade et al. 1996). These may be a characteristics that contribute to the survival of Gram-negative bacteria in harsh environments (Batista et al. 2006).
The carbon source generally used in biosurfactant production can be divided into two categories, namely, water-insoluble and water-soluble carbon sources (Desai and Banat 1997). Water-insoluble carbon sources, such as oil or hydrocarbon compounds, are widely used for biosurfactant production. Abouseoud et al. (2008) reported the production of biosurfactant by Pseudomonas fluorescens only in the presence of water-insoluble carbon, such as hexadecane and olive oil. This strain was able to utilize glucose as a substrate but without biosurfactant synthesis. Darvishi et al. (2011) also found that the presence of olive oil supports the biosurfactant production from Enterobacter cloacae and Pseudomonas sp. In our study, we found that some strains (O. anthropi 2/3 and Acinetobacter sp. 79) preferred ULO as the carbon source to produce biosurfactant/bioemulsifier over a water-soluble carbon source (CS, glucose, glycerol, or molasses). Alternatively, many studies have shown that a water-soluble substrate is suitable for biosurfactant production by P. aeruginosa SP4 (Pansiripat et al. 2010) and Pseudozyma hubeiensis SY62 (Konishi et al. 2011). We found that 13 isolates were able to reduce the surface tension of the culture supernatant when glucose was used as the carbon source. In addition, the highest surface tension reduction (41.8 mN/m from L. komagatae 183) and E24 (69.8% from O. anthropi 11/6) were obtained when CS was used as the carbon source.
Overall, the new biosurfactant-producing strains characterized in our study display important characteristics which make them potential candidates for use in the development of economically efficient industrial-scale biotechnological processes. These strains were able to produce and release extracellular biosurfactant into the culture medium, which should simplify recovery procedures. In addition, bacterial growth and biosurfactant production were supported by low-cost renewable substrates, such as molasses and glycerol, both of which are wastes from biodiesel production. The use of cheap raw materials and wastes will contribute to the reduction of processing costs. Finally, our results should stimulate further evaluation of potential applications of biosurfactants and bioemulsifiers synthesized by the new strains.
References
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A (2008) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Anandaraj B, Thivakaran P (2010) Isolation and production of biosurfactant producing organism from oil spilled soil. J Biosci Tech 1:120–126
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Batista SB, Mounteer AH, Amorim FR, Totola MR (2006) Isolation and characterization of biosurfactant/bioemulsifier-producing bacteria from petroleum contaminated sites. Bioresource Technol 97:868–875
Bernard D, Pascaline H, Jeremie JJ (1996) Distribution and origin of hydrocarbons in sediments from lagoons with fringing mangrove communities. Mar Pollut Bull 32:734–739
Bicca FC, Fleck LC, Ayub MAZ (1999) Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Rev Microbiol 30:231–236
Blume E, Bischoff M, Reichert JM, Moorman T, Konopka A, Turco RF (2002) Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl Soil Ecol 20:171–181
Bodour AA, Maier RM (2002) Biosurfactants: types, screening methods and application. In: Bitton G (ed) Encyclopedia of environmental microbiology. Wiley, New York, pp 750–769
Bodour AA, Drees KP, Raina MM (2003) Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid Southwestern soils. Appl Environ Microbiol 69:3280–3287
Burgos-Diaz C, Pons R, Espuny MJ, Aranda FJ, Teruel JA, Manresa A, Ortiz A, Marques AM (2011) Isolation and partial characterization of a biosurfactant mixture produced by Sphingobacterium sp. isolated from soil. J Colloid Interf Sci 361:195–204
Burns KA, Garrity SD, Levings SC (1993) How many years until mangrove ecosystems recover from catastrophic oil-spills. Mar Pollut Bull 26:239–248
Chayabutra C, Wu J, Ju LK (2001) Rhamnolipid production by Pseudomonas aeruginosa under denitrification: effects of limiting nutrients and carbon substrates. Biotechnol Bioeng 72:25–33
Cooper DG (1986) Biosurfactants. Microbiol Sci 3:145–149
Cooper DG, Zajic JE, Gerson DF (1979) Production of surface active lipids by Corynebacterium lepus. Appl Environ Microbiol 37:4–10
Darvishi P, Ayatollahi S, Mowla D, Niazi A (2011) Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloid Surface B 84:292–300
Das P, Mukherjee S, Sen R (2009) Substrate dependent production of extracelullar biosurfactant by a marine bacterium. Bioresouce Technol 100:1015–1019
Das P, Mukherjee S, Sivapathasekaran S, Sen R (2010) Microbial surfactants of marine origin: potentials and prospects. Adv Exp Med Biol 672:88–101
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
De Acevedo GT, McInerney MJ (1996) Emulsifying activity in thermophilic and extremely thermophilic microorganisms. J Ind Microbiol 16:1–7
From C, Hormazabal V, Hardy SP, Granum PE (2007) Cytotoxicity in Bacillus mojavensis is abolished following loss of surfactin synthesis: Implications for assessment of toxicity and food poisoning potential. Int J Food Microbiol 117:43–49
Gandhimathi R, Kiran GS, Hema TA, Selvin J, Raviji TR, Shanmughapriya S (2009) Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess Biosyst Eng 32:825–835
Gudina EJ, Teixeira JA, Rodrigues LR (2010) Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid Surface B 76:298–304
Huy NQ, Jin S, Amada K, Haruki M, Huu NB, Hang DT, Ha DT, Imanaka T, Morikawa M, Kanaya S (1999) Characterization of petroleum degrading bacteria from oil-contaminated sites in Vietnam. J Biosci Bioeng 88:100–102
Jachimska B, Lunkenheimer K, Malysa K (1995) Effect of position of the functional group on the equilibrium and surface properties of butyl alcohols. J Colloid Interf Sci 176:31–38
Karanth NGK, Deo PG, Veenanadig NK (1999) Microbial production of biosurfactant and their importance. Curr Sci 77:126–166
Ke L, Wang WQ, Wong TW, Wong YS, Tam NF (2003) Removal of pyrene from contaminated sediments by mangrove microcosms. Chemosphere 52:1581–1591
Kebbouche-Gana K, Gana ML, Khemili S, Naimi FF, Bouanane NA, Penninckx M, Hacene H (2009) Isolation and characterization of halophilic archaea able to produce biosurfactants. J Ind Microbiol Biotechnol 36:727–738
Konishi M, Nagahama T, Fukuoka T, Morita T, Imura T, Kitamoto D, Hatada Y (2011) Yeast extract stimulates production of glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma hubeiensis SY62. J Biosci Bioeng 111:702–705
Maneerat S (2005) Biosurfactants from marine microorganisms. Songklanakarin J Sci Technol 27:1263–1272
Maneerat S, Phetrong K (2007) Isolation of biosurfactant-producing marine bacteria and characteristics of selected biosurfactant. Songklanakarin J Sci Technol 29:781–791
Maneerat S, Bamba T, Harada K, Kobayashi A, Yamada H, Kawai K (2006) A novel crude oil emulsifier extracted in the culture supernatant of a marine bacterium, Myroides sp. SM7. Appl Microbiol Biotechnol 70:254–259
Mercade ME, Monleon L, de Andres C, Rodon I, Martinez E, Espuny MJ, Manresa A (1996) Screening and selection of surfactant-producing bacteria from waste lubricating oil. J Appl Bacteriol 81:161–166
Nilsson WB, Strom MS (2002) Detection and identification of bacterial pathogens of fish in kidney tissue using terminal restriction length polymorphism (T-RFLP) analysis of 16S rRNA genes. Dis Aquat Org 48:175–185
Olivera NL, Commendatore MG, Delgado O, Esteves JL (2003) Microbial characterization and hydrocarbon biodegradation potential of natural bilge waste microflora. J Ind Microbiol Biotechnol 30:542–548
Pansiripat S, Pornsunthorntaweea O, Rujiravanit R, Kitiyanana B, Somboonthanate P, Chavadej S (2010) Biosurfactant production by Pseudomonas aeruginosa SP4 using sequencing batch reactors: effect of oil-to-glucose ratio. Biochem Eng J 49:185–191
Phalakornkule C, Tanasupawat S (2006) Characterization of lactic acid bacteria from traditional Thai sausages. J Cult Collect 5:46–57
Plaza GA, Zjawiony I, Banat IM (2006) Use of different methods for detection of thermophilic biosurfactant-producing bacteria from hydrocarbon contaminated and bioremediated soils. J Pet Sci Eng 50:71–77
Rahman KSM, Banat IM, Thahira J, Thayumanvan T, Akshmanaperumalsamy P (2002) Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Bioresource Technol 81:25–32
Rivardo F, Turner RJ, Allegrone G, Ceri H, Martinotti MG (2009) Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl Microbiol Biotechnol 83:541–553
Rodrigues LR, Teixeira JA, van der Meib HC, Oliveira R (2006) Isolation and partial characterization of a biosurfactant produced by Streptococcus thermophilus A. Colloid Surface B 53:105–112
Rosenberg E, Ron EZ (1998) Surface active polymers from the genus Acinetobacter. In: Kaplan DL (ed) Biopolymers from renewable resources. Springer, Berlin, pp 281–291
Rosenberg E, Ziclerberg A, Rubinowitz C, Gutnick DL (1979) Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl Environ Microbiol 37:402–408
Ruggeri C, Franzetti A, Bestetti G, Caredda P, La Colla P, Pintus M, Sergi S, Tamburini E (2009) Isolation and characterization of surface active compound-producing bacteria from hydrocarbon-contaminated environments. Int Biodeter Biodegr 63:936–942
Saimmai A, Sobhon V, Maneerat S (2011) Molasses a whole medium for bosurfactants production by Bacillus strains and their application. Appl Biochem Biotech 165:315–335
Singh BR, Dwivedi S, Al-Khedhairy AA, Musarrat J (2011) Synthesis of stable cadmium sulfide nanoparticles using surfactin produced by Bacillus amyloliquifaciens strain KSU-109. Colloid Surface B 85:207–213
Skorupska A, Janczarek M, Marczak M, Mazur A, Krol J (2006) Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb Cell Fact 16:5–7
Snape I, Ferguson SH, Harvey PM, Riddle MJ (2006) Investigation of evaporation and biodegradation of fuel spills in Antarctica: II Extent of natural attenuation at Casey Station. Chemosphere 63:89–98
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibbons TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTALX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Widada J, Nojiri H, Kasuga K, Yoshida T, Habe H, Omori T (2002) Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl Microbiol Biotechnol 58:202–209
Willumsen PA, Karlson U (1997) Screening of bacteria isolated from PAH-contaminated soils for production of biosurfactants and bioemulsifiers. Biodegradation 7:415–423
Yin B, Gua JD, Wana N (2005) Degradation of indole by enrichment culture and Pseudomonas aeruginosa Gs isolated from mangrove sediment. Int Biodeter Biodegr 56:243–248
Youssef NH, Dunacn KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganism. J Microbiol Meth 56:339–347
Acknowledgments
The last author would like to thank the Office of the Higher Education Commission, Thailand for financial support for this work through a grant funded under the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree. This work was also funded by the Faculty of Agro-Industry and Graduate School, Prince of Songkla University, and further supported by the TRF/BIOTEC Special Program for Biodiversity Research and Training grant BRT R651178.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saimmai, A., Tani, A., Sobhon, V. et al. Mangrove sediment, a new source of potential biosurfactant-producing bacteria. Ann Microbiol 62, 1669–1679 (2012). https://doi.org/10.1007/s13213-012-0424-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0424-9