Abstract
Ethanol was added to submerged cultures of Phanerochaete chrysosporium to enhance the production of laccase and improve the decolorization of triarylmethane dye. The addition of 10 g/l ethanol resulted in a 4.7-fold increase in laccase production, while higher ethanol concentrations caused a sharp decrease in activity. The protein content increased with the increase in ethanol concentrations; on the other hand, pellet diameter and fungal biomass wet weight decreased. The addition of ethanol to the cultivation media affected both the mycelial morphology and fungal wall permeability. Although catalase assay indicated a stress response behavior, the lipid peroxidation decreased, suggesting the presence of a protective protein compound which appears in the presence of ethanol. The partial purification of the extracellular fluid (ECF) of ethanol-amended cultures resulted in a low molecular weight fraction (<5 kDa), UV–visible spectrum for this fraction showed a single sharp peak at 356 nm under ethanol stress; this peak represents glutathione, a reductive peptide which increased 2-fold after the fungus was exposed to ethanol stress. The glutathione assay, reductive activity, and the in vitro decolorization confirm that glutathione is responsible for dye reduction (65.1%) as compared to decolorization in in vivo conditions (23.8%). The use of ethanol could improve the performance of Phanerochaete chrysosporium through laccase induction for oxidation or reductive activity depending on the concentration and mode of addition of ethanol.
Similar content being viewed by others
Introduction
Although fungi are known for their capability of degrading a wide variety of dyes, some dye classes are considered as inhibitors of the fungal enzymatic system, limiting their degrading abilities. The bioremediation processes could be improved by increasing microbial enzymatic-related degradation (Papagianni et al. 2001) by adding certain inducers which result in an up-regulation process for these degrading enzymes. Ethanol, an aliphatic alcohol and a low cost agro-industrial product, is known to induce laccase in some fungal strains (Lomascolo et al. 2003; Alves et al. 2004). This enzyme is a widely used ligninolytic enzyme, commonly produced by white rot fungi; it catalyses the oxidation of phenolic and non-phenolic compounds in the presence of the appropriate redox mediator (Alves et al. 2004). There are different hypotheses to the role of ethanol in enzyme production which are summarized as follows: (1) increase in membrane permeability and promotion of protein secretion, (2) inhibition of melanin formation and consequent increase in phenolic monomers, and (3) activation of an oxidative stress which could be directly responsible for laccase production. Ethanol is also said to be involved in growth and laccase gene expression, and as protease inhibitor (Meza et al. 2007).
The presence of combined stresses such as nutrient starvation and xenobiotics has been proven to help in xenobiotic degradation (Mykytczuk et al. 2007). Stress response results in the production of catalase and laccase which are stress enzymes correlated with the decolorization process in Phormidium valderianum (Palanisami and Lakshmanan 2010). These stress enzymes help the microbial cells in the degradation of recalcitrant compounds. In a previous study, the lignin-degrading laccase enzyme produced from Phanerochaete chrysosporium did not show high decolorization results for Victoria blue, a triaryl methane dye, one of the most recalcitrant dye classes (Gomaa et al. 2008). This dye class requires reduction besides oxidation for a complete degradation process to be accomplished (Tacal and Ozer 2007). A number of small, low molecular weight molecules have a reductive activity; glutathione is one of the known reducing molecules which could be used in vitro (Tacal and Ozer 2007), or as a molecule produced in in vivo cultures of Phanerochaete chrysosporium under stress (Hu et al. 2009). Therefore, the aim of the present article is to study the role of ethanol as a stress-inducing factor in Phanerochaete chrysosporium cultures in terms of laccase production, oxidative response, reductive activity, and Victoria blue decolorization
Materials and methods
Microorganism and cultivation conditions
The fungal strain Phanerochaete chrysosporium ATCC 34541 was maintained through periodic (monthly) transfer on potato dextrose agar, Oxoid (PDA). The strain was stored at 4°C.
The cultivation was performed in Malt extract medium which was used to inhibit production of manganese peroxidase (MnP) and lignin peroxidase (LiP) activities. The growth medium consisted of the following constituents in g/l: malt extract, 20; glucose, 20; peptone 1. The pH was adjusted to 7 before autoclaving. Once inoculated, flasks were incubated on an orbital shaker at 150 rpm and 30°C for 48 h. Ethanol was added to separate cultures under aseptic conditions using Millipore sterilization filters (0.45 μm) in the following concentrations: 10, 20, 30 and 40 g/l. The cultures were incubated for a further 2 days, after which they were used for the following assays.
Fungal growth and pellet diameter
After the cultures were incubated for a total of 4 days with different ethanol concentrations, the fungal mycelia for all cultures were filtered; excess water was removed by blotting with Whatmann no.1, and cultures were weighed to monitor the effect of ethanol addition on fungal growth. Pellet diameter was measured; the data recorded were the mean value of 20 pellets.
Protein determination
Protein concentrations were determined by the method of Lowry et al. (1957) using bovine serum albumin as a standard. The data recorded are the mean values for triplicate separate experiments.
Scanning Electron Microscopy (SEM)
Scanning electron micrographs of the nature of the pellets was carried out using a JOEL JMS 5600 scanning electron microscope; the pellets of both control cultures and ethanol-amended cultures (40 g/l) were collected and dried in an oven at 50°C. The pellets were then glued separately on to brass stubs using a double-sided adhesive tape and were coated with a thin layer of gold under reduced pressure. The images were captured at magnifications of ×1,500 using an electron beam high voltage of 30 kV.
Fungal wall permeability using dielectric measurements
The fungal wall permeability (measured as conductance) of the fungal pellets grown in an ethanol-free and an ethanol-amended medium (40 g/l) was performed using the dielectric measurements. The procedure was carried out using LCR meter type HIOKI 3531, manufactured in Japan. The measuring cell is a parallel plate conductivity cell with platinum electrodes, coated with a platinum black layer to reduce electrode polarization (Iwamoto and Kumagai 1998); it covers an area of 4 cm2 and a separating distance of 2 cm. The conductance (measured in Siemens) was detected at different frequencies over a range from 100 to 100,000 Hz.
Biochemical assays
Laccase activity
Laccase was determined for all ethanol-amended cultures spectrophotometrically using ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] as the substrate (Srinivasan et al. 1995). One unit of activity is defined as the amount of enzyme which leads to the transformation of 1 mM of ABTS per minute.
Catalase assay
Catalase was measured according to the method of Beers and Sizer (1952) in which the disappearance of peroxide is followed spectrophotometrically at 240 nm. One unit decomposes one micromole of H2O2 per minute at 25°C (pH 7.0) under the specified conditions. The reaction mixture reaction contained 0.05 M potassium phosphate buffer pH 7 containing 0.059 M hydrogen peroxide.
Lipid peroxidation
Lipid peroxidation was calculated as the concentration of malondialehyde (MDA) (the end product of lipid peroxidation) in the cell wall of fungal mycelium of ethanol-free and ethanol-amended cultures (40 g/l) was determined as thiobarbituric acid reactive substance (TBARs) according to Yoshika et al. (1979).
Glutathione (GSH)
Glutathione was measured in the extracellular fraction for ethanol-free and ethanol-amended cultures, using 5,5′dithio-bis-(2-nitrobenzoic acid) (DNTB) as the substrate, and read at wave length 412 nm according to the method of Prins and Loose (1969).
The reductive activity
The reductive activity for both ethanol-free and ethanol-amended cultures (40 g/l) was measured using a modified method of Hu et al. (2009). The assay mixture contained citrate buffer (pH 5) 50 mM; 2 deoxy D-ribose 4 mM; ferric chloride 0.1 mM; hydrogen peroxide 10 mM and 200 μl of the extracellular filtrate. The reaction was incubated at 37°C for 1 h, 0.25% thiobarbituric acid (TBA) and 0.7% trichloroacetic acid (TCA) were added and heated at 100°C for 15 min. The absorbance was recorded at 532 nm and the reductive capability was indicated as the decrease in absorbance as compared to the control. The obtained reading was calculated in percentages.
Partial purification of extracellular fluid (ECF)
The low molecular weight fraction was obtained from the crude ECF of a 4-day culture filtrate of control, ethanol, and ethanol- and mannitol-amended medium by ultrafiltration using Amicon MWCO >5 kDa (Millipore, MA, USA). Mannitol (0.5 M) was added to the culture media prior to sterilization. The resulting 200-μl filtered components were screened by UV–vis spectrophotometer (Schimadzu UV 2100 spectrophotometer) in the range of 200–500 nm.
The removal of Vicotria blue (Vb)
Victoria blue (C.I name Basic blue 26) was used as a model triaryl methane dye, its peak was monitored at the maximum absorbance which was 618 nm. The dye was added to the medium before cultivation for in vivo experiments, and added to the ECF of a 4-day ethanol-amended culture for in vitro experiments. The residual dye was measured after removing pellets from the medium for in vivo experiments and direct measurements were performed using the ECF for in vitro measurements. The spectra were monitored using UV–vis spectrophotometer (Schimadzu UV 2100 spectrophotometer). The decolorization of VB was calculated from the following equation:
where A o is the initial dye absorption on the day of inoculation; A is the final dye absorption after incubation (Jadhav et al 2010).
Statistical treatment of data
The analysis of the variance of the data (ANOVA) has been performed using MS Excel statistiXL v.1.8.
Results and discussion
Ethanol effect on laccase production and protein content
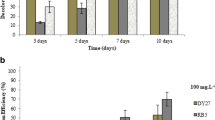
The addition of ethanol to the medium in different concentrations affected the whole culture in different ways. Laccase activity of the medium upon the addition of different ethanol concentrations illustrates an increase of laccase activity from 19.4 in ethanol free cultures to 92.1 U/mg protein when 10 g/l ethanol was added to the cultivation medium; this was followed by a decrease to 14.4 U/mg protein at 40 g/l ethanol as represented in Fig. 1. Different studies showed that the addition of some chemical compounds, such as aliphatic alcohols, to fungal cultures during cultivation could cause a myriad of reactions on several cellular and sub-cellular levels. The use of ethanol as an inducer for laccase production in white rot fungi was reported earlier in some studies (Lomascolo et al. 2003; Alves et al. 2004; Meza et al. 2007) using ethanol as an inducer in Pycnoporus cinnabarinus and causing a 9-fold increase in laccase productivity. In the present study, ethanol was used with the aim of increasing laccase production; one of the first observations noticed in this work is that the timing of ethanol addition affected the fungal growth. The addition of ethanol on the day of inoculation caused an inhibition of fungal growth. A similar study reported some morphological and growth differences between Trischosporon cutaneum grown in solvents as compared to those grown in glucose medium (Tileva et al. 2007). In general, laccase enzyme production is highly controlled by media constituents (Kapdan et al. 2000). Therefore, ethanol was added to the cultures after 48 h of cultivation and incubated for another 48 h, to ensure that fungal pellets are formed and that the changes exerted on the fungal pellets are a true reflection of ethanol addition to the cultivation medium and laccase induction. The pattern of laccase release in the medium after the addition of ethanol is governed by the initial fluidity effect which is followed by adaptive rigidification (Mykytczuk et al. 2007). Although laccase decreased with the increase of ethanol, the protein content of the medium showed an increase; this increase shows that forms of protein are secreted in the medium in response to ethanol addition.
Ethanol effect on mycelial morphology and permeability
Ethanol, as a stressor, could affect the uniformity of fungal pellets and/or the mycelial cell wall permeability, and, therefore, both morphological and permeability assessments were conducted.
The pellet diameter decreased from 3 to 0.5 mm, while the wet weight showed only a slight decrease under the same conditions (Fig. 2). Scanning electron micrographs demonstrate the difference in the intact fungal pellets for control cultures (Fig. 3a) as compared to the loosely bound fungal pellets in ethanol grown cultures (Fig. 3b). SEM photos show that fungal pellets were morphologically altered, but not inhibited. The growth of fungal pellets in ethanol-amended media was governed by the time of ethanol addition, adding ethanol on the day of inoculation would have inhibited the growth, while its addition after 48 h growth caused loose pellets due to the alteration caused to the fungal cell wall. There is a difference between adding glucose or ethanol in the cultivation medium. Glucose enters the metabolic network through the glycolitic pathway, while ethanol enters through the gluconeogenic pathway (Mogensen et al. 2006). The gluconeogenic genes require the function of some protein kinases (Randez-Gil et al. 1997) which are thought to govern the morphology of filamentous fungi. Cell wall changes could be studied in terms of measuring the dielectric properties of the cells. Cell wall permeability is a parameter which proves to be important in investigating any occurring modifications in the overall biological cell wall structure in cases of disease or due to external agents. Figure 3c represent the changes occurring on the cell wall from a biophysical point. The results show a 27% decrease in cell wall permeability upon the addition of ethanol. The use of dielectric measurements, represented in this study by the conductance, has been found to describe the influence of solvents on the microbial cell wall (Markx and Davey 1999; Tileva et al. 2007). Some microbial cells undergo fluidity adjustments under ethanol stress conditions (Da Silveira et al. 2003), which explains the decrease in the cell wall permeability, with ethanol attacking the cell wall and causing solubilization of the cell wall at the point of contact which was quickly followed by rigidification.
Ethanol oxidative response
Some stress response parameters were evaluated in the presence and absence of ethanol. Catalase activity increased from 7 to 28.6 nKat/mg protein. Catalase is one of the stress response enzymes; its marked increase confirms that ethanol caused stress to Phanerochaete chrysosporium pellets while lipid peroxidation decreased from 93.71 to 53.55 nmole/g fungi. Although it was expected that the addition of ethanol would trigger the formation of hydroxyl radicals which would attack the lipid fraction in the cell wall resulting in increased lipid peroxidation (Comporti et al. 2010), the results obtained contradict this theory. In contrast, the lipid peroxidation decreased, this could probably be due to the presence of a molecule which acted as a protectant against oxidative stress. There was no detection of the protease activity in both ethanol-free and ethanol-amended cultures. Glutathione, a low molecular tripeptide molecule, is considered a protectant molecule; the 2-fold increase in glutathione assay from 2.055 to 4.11 mg/100 ml seems to be the reason for the decrease in lipid peroxidation under ethanol stress. Low molecular weight compounds were reported to act as reductive molecules (Hu et al. 2009). The results show that the reductive activity increased to reach 54% in ethanol-amended cultures as compared to 2% in control ethanol-free cultures (Table 1).
Reductive activity and its role in decolorization
The reductive fraction present in the ECF was partially purified for control, ethanol-amended and ethanol- and mannitol-amended culture medium using ultrafiltration, and the resultant fraction (<5 kDa) was used for UV–visible analysis. The results shown in Fig. 4 demonstrate the differences in the spectra for the three cultures; a sharp peak at 356 nm was evident when ethanol was added to the culture medium. Anderson (1989) described this peak as glutathione, a low molecular weight tri-peptide molecule which is known for its induction when hydroxyl radicals are present and which is also known for its reductive activity. There was about 50% reduction in the peak in the presence of mannitol. Mannitol is known for its potent hydroxyl radical scavenging effect (Forney et al. 1982). The peak was absent in control cultures which confirms its appearance only as the result of adding a stress-inducing compound.
The role of ethanol in the release of a low molecular weight compound and the decolorization of dyes was studied in both in vivo and in vitro cultures. The in vitro addition of ethanol culture showed a decolorization of 65.1% for VB compared to both control cultures and in vivo addition of ethanol. On the other hand, in vivo decolorization of Phanerochaete chrysosporium was 23.8% under the abovementioned cultivation conditions (Fig. 5). The increase in decolorization confirms that the low molecular weight compound which has the reductive activity could reduce the dye in vitro. TAM dyes are widely used in industry and as antimicrobial and antiparasitic agents as well as in some medicines. It is known that there is a need for reduction of TAM to ensure dye clearance (Tacal and Ozer 2007). Obviously, the addition of ethanol to VB dye differs in vivo and in vitro, and this difference could be attributed to the action of ethanol directly on the fungal cell wall triggering the formation of a low molecular weight compound, and not as a reducing molecule itself, which is contradictory to the addition of ethanol as an electron donor for the reduction of radionuclide-contaminated sediments (Akob et al. 2008). The addition of aliphatic alcohol to the cultivation media increases the transcription of genes involved in the oxidative stress response (Hogan 2006). Stress response represented by increase in the synthesis of oxidative stress enzymes such as catalase and laccase were directly linked to decolorization (Palmanisami and Lakshmanan 2010).
At this point, the interpretation of the above data lies in the following context: adding ethanol to the medium affects both the culture morphology and the stress response of fungal cells. It induced laccase production at low concentrations and generated hydroxyl radical formation (oxidative stress) at high concentrations, this in turn triggered the formation of a low molecular weight protein molecule which could be directly related to decolorization via reduction. The primary relationship of ethanol to Phanerochaete chrysosporium cultivated under the previously mentioned conditions and the decolorization of VB dye lies in the changes induced by ethanol on the lipid content of the fungal mycelia, thus releasing glutathione to protect itself, and at the same time acting as a reducing agent which increases decolorization.
Conclusion
The use of ethanol in fungal cultures could induce different stress responses which may act as a simple but effective tool in bioremediation; the addition of ethanol to the medium inoculated with Phanerochaete chrysosporium acted as a signal to regulate culture growth and morphology. It induced the production of laccase at low concentrations and a low molecular weight molecule with reductive activity at high concentrations. The induction of a low molecular weight compound could be used in degradation of recalcitrant compounds which require reduction as well as oxidation to obtain an enhanced bioremediation process.
References
Akob DM, Mills HJ, Gihring TM, Kerkhof M, Stucki JW, Anastacio AS, Chin K-J, Kusel K, Palumbo AV, Watson DB, Kostka JE (2008) Functional diversity and electron donor dependence of microbial populations capable of U(VI) reduction in radionuclide-contaminated subsurface sediments. App Environ Microbiol 74:3159–3170
Alves AMCR, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JGH, Wosten HAB (2004) Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol 70:6379–6384
Anderson ME (1989) Enzymatic and chemical methods for the determination of glutathione. In: Dolphin D, Poulson R, Avramovic O (eds) Glutathione: chemical, biochemical and medical aspects, Vol A. Wiley, New York, pp 339–365
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:130–140
Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B (2010) Ethanol-induced oxidative stress: basic knowledge. Genes Nutr 2:101–109
Da Silveira M, Golovina EA, Hoeskstra FA, Rombouts FM, Abee T (2003) Membrane fluidity adjustments in ethanol-stresses Oenococcus oeni cells. Appl Environ Microbiol 69:5826–5823
Forney LJ, Reddy CA, Tien M, Aust SD (1982) The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem 257:11455–11462
Gomaa O, Linz J, Reddy CA (2008) Decolorization of Victoria blue by Phanerochaete chrysosporium. World J Microbiol Biotechnol 24:2349–2356
Hogan DA (2006) Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell 5:613–619
Hu M, Weican Z, Wu Y, Gao P, Lu X (2009) Characteristics and function of a low-molecular-weight compound with reductive activity from Phanerochaete chrysosporium in lignin biodegradation. Bioresour Technol 100:2077–2081
Iwamoto S, Kumagai H (1998) Analysis of the dielectric relaxation of a gelatin solution. Biosci Biotechnol Biochem 62:1381–1387
Jadhav JP, Kalyani DC, TelkeAA PSS, Govindwar SP (2010) Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour Technol 101:165–173
Kapdan IK, Kargi F, McMullan G, Marchant R (2000) Effect of environmental conditions on biological decolorization of textile dyestuff by C. versicolor. Enzyme Microb Technol 36:381–387
Lomascolo A, Record E, Herpoel-Gimbert I, Delattre M, Robert JL, Georis J, Dauvrin T, Sigoillot JC, Asther M (2003) Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J Appl Microbiol 94:618–624
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1957) Protein measurement with phenol-folin reagent. J Biol Chem 193:265–275
Markx GH, Davey CL (1999) The dielectric properties of biological cells at radiofrequencies: application in biotechnology. Enzyme Microb Technol 25:161–171
Meza JC, Auria R, Lomascolo A, Sigoillot JC, Casalot L (2007) Role of ethanol on growth, laccase production and protease activity in Pycnoporus cinnabarinus ss3. Enzyme Microb Technol 41:162–168
Mogensen J, Nielsen HB, Hofmann G, Nielsen J (2006) Transcription analysis using high-density micro-arrays of Aspergillus nidulans wild-type and CreA mutant during growth on glucose or ethanol. Fungal Genet Biol 43:593–603
Mykytczuk NCS, Trevors JT, Leduce LG, Ferroni GD (2007) Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog Biophys Mol Biol 95:60–82
Palanisami S, Lakshmanan U (2010) Role of copper in poly R-478 decolorization by the marine cyanobacterium Phormidium valderianum BDU140441. World J Microbiol Biotechnol 27:669–677
Papagianni M, Nokes SE, Filers K (2001) Submerged and solid-state phytase fermentation by Aspergillus niger: effect of agitation and medium viscosity on phytase production, fungal morphology and inoculum performance. Food Technol Biotechnol 39:319–326
Prins HK, Loose JA (1969) Glutathione, Chapter 4. In: Yunis JJ (ed) Biochemical methods in red cell genetics. Academic, London, pp 126–129
Randez-Gil R, Bojunga N, Proft M, Entian K (1997) Glucose derepression of gluconeogenic enzymes in Saccharomyces cerevisiae correlates with phosphorylation of the gene activator Cat8p. Mol Cell Biol 17:2502–2510
Srinivasan C, D’souza T, Boominathan K, Reddy CA (1995) Demonstration of laccase in the white rot basidiomycete Phanerochaete chrysosporium BKM-F-1767. Appl Environ Microbiol 61:4274–4277
Tileva FP, Yotiva LK, Markx GH (2007) Dielectric measurement of the resistance of Trichosporon cutaneum against toxic chemicals. Afr J Microbiol Res 1:117–120
Tacal O, Ozer I (2007) An assessment of the role of intracellular reductive capacity in the biological clearance of triarylmethane dyes. J Haz Mat 149:518–522
Yoshika T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 135:372–376
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomaa, O.M. Ethanol induced response in Phanerochaete chrysosporium and its role in the decolorization of triarylmethane dye. Ann Microbiol 62, 1403–1409 (2012). https://doi.org/10.1007/s13213-011-0390-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0390-7