Abstract
When the variables (inoculum size, methanol and yeast extract) identified to affect phytase production by Bacillus subtilis US417 using Plackett-Burman design were optimized by RSM, a high enzyme production of 112 U/g of wheat bran was attained. Overall, a 5-fold improvement in phytase production was achieved. In SSF, on the other hand, a 4-fold enhancement in enzyme titer was attained (85 U/g of wheat bran). Based on these findings, phytase productivity was higher in SF [2.3 U/(g × h)] than in SSF [1.2 U/(g × h)].
Similar content being viewed by others
Introduction
Phytases are widely distributed in nature and have been identified in plants, microorganisms and certain animal tissues. They catalyze the release of phosphate from phytic acid (myo-inositol-1,2,3,4,5,6-hexakisphosphate) and the salts of phytic acid (phytates) to generate inorganic phosphate and myo-inositol phosphate derivatives. According to the carbon in the myo-inositol ring of phytate at which dephosphorylation is initiated, phytases could be grouped into 3-phytases (EC 3.1.3.8) and 6-phytases (EC 3.1.3.26) (Sung et al. 2005).
Phytate represents the primary storage form of organic phosphorus in plants and accounts for 88% of the total phosphorus in seeds and cereals (Haefner et al. 2005). Monogastric animals, such as poultry and pigs, consume diets that contain high levels of phytate-phosphorus, which is poorly digested, due to negligible phytase activity in their digestive tract (Greiner and Egli 2003). For this reason, monogastric diets have often been supplemented with large quantities of inorganic phosphorus to meet the phosphorus needs of the animal. Excess phosphorus is excreted in manure and poses serious environmental problems (Xiong et al. 2005a, b). Furthermore, phytates can chelate several divalent cations of major nutritional importance, such as calcium, iron, zinc, magnesium, and manganese, thus making them unavailable (Xiong et al. 2005a, b). Several studies have demonstrated that the dephosphorylation of phytates reduces the antinutritive effects of these salts (Greiner and Egli 2003). For this reason, the supplementation of monogastric diets with exogenous phytases can improve the availability of phytate phosphorus, limit the instances of mineral deficiency and, hence, increase the feed nutritional value. It can also decrease the excretion of phosphorus and, therefore, reduce environmental problems. Phytases have also been advocated for several applications and used as bicatalysts in the production of special isomers of different lower phosphate esters of myo-inositol (Xiong et al. 2005a, b). In fact, current estimates indicate that the annual global sales of enzymes described as phytases or phytate-degrading enzymes have reached about €150 million (Greiner and Konietzny 2006).
Due to their potential for industrial applications, phytases have triggered great interest in the last few decades. Several bacteria, fungi and yeasts have been reported to produce phytases. In fact, the commercial production of phytases for the animal feed industry has so far focused primarily on aspergilli and yeasts (Haefner et al. 2005). Recently, phytases from Bacillus species have been reported to have the advantage of being naturally thermostable and being strictly specific for phytates (Kim et al. 1998; Choi et al. 1999; Farhat et al. 2008). Nevertheless, and partly due to inefficient enzyme production methods, Bacillus phytases have not been applied on a large scale (Kerovuo and Tynkkynen 2000).
Two major processes have been utilized for the production of microbial phytases (Vats and Banerjee 2004), namely solid state fermentation (SSF) (Ebune et al. 1995; Krishna and Nokes 2001; Roopesh et al. 2006) and submerged fermentation (SF) (Nampoothiri et al. 2004). SSF systems have gained a lot of interest in the last few years, mainly because they involve less energy, lower wastewater output, simpler fermentation media, easier aeration, and reduced bacterial contamination (Vats and Banerjee 2004). The culture conditions, type of strain, nature of substrate and availability of nutrients should be taken into account for the selection of a particular production technique since they are the most critical factors affecting the production yield. SF, on the other hand, continues to be studied at the laboratory scale using new strains (Ebune et al. 1995) and substrates. The most economical substrates that have so far been used are wheat bran (Nampoothiri et al. 2004), full-fat soybean flour (Krishna and Nokes 2001), canola meal (Ebune et al. 1995), cane molasses (Singh and Satyanarayana 2008) and oil cakes (Roopesh et al. 2006).
In view of the ever increasing demand for Bacillus phytases, it is necessary to select an appropriate fermentation technology and to optimize the parameters affecting phytase production. Statistically based experimental designs are useful tools in this optimization (Singh and Satyanarayana 2008). Several studies have applied them for the optimization, modeling and control of phytase production (Singh and Satyanarayana 2008).
Accordingly, the present study combined experimental design methodologies with statistical optimization tools to optimize the production of extra-cellular phytase (PHY US417) by a recently isolated and characterized strain of Bacillus subtilis US417 (Farhat et al. 2008) in submerged and solid state fermentation. PHY US417 was chosen because it is optimally active at neutral pH and shows high thermal stability and specificity for phytic acids, features which make this enzyme a strong candidate for application as a feed additive (Farhat et al. 2008). Various operational parameters were evaluated in terms of their individual and synergistic effects on phytase production in SF using a Plackett–Burman design and the most significant factors were further investigated and optimized using a Box-Behnken design. In the case of phytase production in SSF, a central composite design was used to optimize the moisture content (a specific factor of SSF) and the crucial variables from the investigation. SF and SSF processes have been compared in terms of their suitability for Bacillus subtilis US417 phytase production.
Materials and methods
Micro-organism and culture conditions
Bacillus subtilis US417, which was previously described as a potent producer of phytase (Farhat et al. 2008), was routinely grown at 30°C on Luria-Bertani (LB) agar, and was stored at 4°C and −20°C in glycerol.
Substrates and chemicals
Wheat bran (wb), gruel (a by-product of wheat) and glucose syrup DE 45 (DE: dextrose equivalent in percentage of reducing sugars) were obtained from local suppliers. Phytic acid sodium salt hydrate from rice (P0109) was purchased from Sigma-Aldrich (France). All other chemicals used in this study were of analytic grade and applied without further purification.
Submerged fermentation
Prior to optimization, the basal medium used for phytase production by Bacillus subtilis US417 in SF contained (% w/v): 2 wb, 0.04 (NH4)2SO4, 0.02 MgSO4·7H2O and 0.22 CaCl2 at pH 6. Cultures of 100 ml, carried out in 250-ml erlenmeyer flasks, were inoculated at 0.1 OD600 with a 16-h-old culture grown on LB and incubated under shaking conditions (210 rpm) at 30°C for 48 h. Experiments relative to the screening and optimization of the crucial variables in SF were performed using wheat bran at 5% as a substrate added to the components of the medium.
Solid state fermentation
The experiments were performed in 250-ml erlenmeyer flasks with 20 g of wheat bran. The type and concentration of the nutritional factor in the medium were defined according to the experimental design. Distilled water was added to attain the required moisture level. The substrate was autoclaved for 20 min at 121°C. The flasks were carried out in a static horizontal position at 30°C for 72 h with an initial moisture content varying from 65 to 75% (wet basis) and an inoculum size ranging from 107 to 108 CFU/ml, previously grown for 15 h in LB broth. All the experiments were performed in triplicate and average values are reported.
Enzyme extraction
In the case of SSF, the crude enzyme was extracted by mixing the Erlenmeyer content with a quantity of distilled water (containing 0.1% Tween-80) equal to three times the weight of the fermented matter. The mixture was homogenized on a rotary shaker (210 rpm) for 1 h at 30°C. Then, 1 ml of the suspension was centrifuged for 10 min at 7,000 g at 4°C and the supernatant was kept for further use in subsequent enzymatic assays.
Phytase assay
Phytase activity assays were performed as described by Engelen et al. (1994). One phytase unit (U) was defined as the amount of enzyme capable of releasing 1 μmol of inorganic phosphate from phytic acid per minute under the reaction conditions cited by Farhat et al. (2008). Phytase production was expressed in U/ml or U/g of wheat bran.
Identification of critical culture variables in SF using Plackett–Burman design
A Plackett–Burman design was used to determine the significant culture variables affecting phytase production by Bacillus subtilis US417. A total of 15 variables (n) were studied at two levels, high and low, denoted by (+) and (−), respectively (Table 1). These variables were screened in 16 trials (n + 1). The effect of each variable represented the difference between the measurement averages of the high and low levels of each factor, which was determined using the following equation:
where E(X i ) is the concentration effect of the tested variable. Y i+ and Y i- are the phytase activities from the trials where the measured variable (X i ) was present at the high and the low concentration, respectively, and N is the number of trials. Standard error (SE) of the concentration effect was the square root of the variance of an effect and the significant level (p-value) of the effect of each concentration was determined using Student’s t test as given by the following equation:
where E (Xi) is the effect of variable X i and t(X i ) is the variance of the estimator.
Optimization of screened variables in SF using RSM
The Box-Behnken design under a Response Surface Methodology (RSM) was applied to find the optimum levels of the significant variables identified by the Plackett–Burman design as affecting phytase production in SF [i.e. inoculum size (I), yeast extract (YE) and methanol (M)]. A total of 13 experiments were conducted (Kammoun et al. 2009). Upon completion of the experiments, phytase production was taken as the response (Y). The data were subjected to multiple regression analysis and the following empirical second order polynomial equation was used to relate the measured response to the independent variables:
where Y is the predicted response, β 0 the intercept, β 1 , β 2 , and β 3 the linear coefficients, β 11 , β 22 , and β 33 the squared coefficients, β 12 , β 23 , and β 13 the interaction coefficients and x i and x j the independent variables.
Optimization of phytase production in SSF using RSM
An RSM methodology based on central composite design was then applied to further enhance phytase production in SSF. It aimed to optimize four variables: the three critical variables so far identified in the investigation of SF (I, YE and M) and a fourth factor specific to SSF, namely moisture content (H). The minimum and maximum values of the variables investigated are listed in Table 4 and a total of 28 trials was carried out. Phytase production at the end of the fermentation was taken as the response (Y). The data were then subjected to a multiple regression analysis to obtain an empirical model that related the measured response to the independent variables. A second order polynomial equation, similar to that generated for the SF, was obtained for a four-factor system.
Validation of the experimental model
For SF, the statistical model was validated with respect to phytase production under the optimized conditions predicted by the model in 0.25- to 0.5-l shake-flasks. The fermentation was operated at 30°C and 210 rpm, with pH = 6. The culture supernatants were used for the determination of phytase activity.
Software tools
The Statistical Package for Social Sciences (SPSS, v.11.0.1 2001; LEAD Technologies, USA) was used in order to analyze the experimental data and EXCEL (v.2003; Microsoft, USA) was employed to generate the response surface curves that determine the optimum values of the variables selected and support maximum enzyme titres.
Results
Selection of basal substrate for phytase production prior to statistical optimization
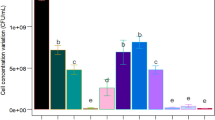
A Bacillus subtilis US417 strain has been reported to produce an extracellular phytase activity (PHY US417) at only 0.64 U/ml in SF when grown for 20 h with wheat bran at 2% as a substrate (Farhat et al. 2008). In order to become competitive with currently commercialized ones, enzyme production levels must be maximized and production costs minimized. With this concern in mind, the present study involved a series of preliminary assays that aimed to identify the best type and concentration of basal substrate prior to the statistical optimization of Bacillus subtilis US417 phytase production. Three substrates, namely wheat bran, gruel and glucose syrup, were assayed at 2%. The maximum titre of phytase production (0.78 U/ml) was achieved with wheat bran after 24 h fermentation (data not shown). Further assays were performed with the aim of optimizing the wheat bran content of the culture and the highest phytase production yield of 1.10 U/ml (equivalent to 22.0 U/g of wheat bran) was achieved after 48 h fermentation when wheat bran was used at 5% (data not shown). This level of phytase production was, therefore, retained as an analytical value of reference against which all subsequent experimental data were compared.
Screening of critical culture variables in SF
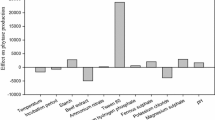
A set of 15 variables, namely temperature, initial pH, inoculum size, agitation speed and a total of 11 culture components (Table 1), were evaluated in terms of their effects on PHY US417 production in SF using the Plackett–Burman design as described by Kammoun et al. (2009). A total of 16 trials were conducted. The results showed a wide variation in the levels of phytase production, ranging from 0.001 to 3.454 U/ml (equivalent to 0.02–69 U/g of wheat bran). The values of the contrast coefficients were calculated based on the enzyme content in terms of all the variables tested (Table 1). The results from statistical analysis showed that phytase production was higher in cultures having large inoculum sizes (I: log10 CFU/ml) and yeast extract levels (YE: g/l) and, to a lesser extent, high methanol levels (M: ml/l). Accordingly, these factors were further optimized by RSM using Box-Behnken design. Further investigations were performed wherein the temperature (30°C), initial pH (6.5) and agitation (210 rpm) parameters were maintained constant.
Optimization of the selected variables for maximum phytase production in SF
Following screening, RSM was applied using the Box-Behnken design described by Kammoun et al. (2009) to find the optimal levels of the most significant variables affecting SF PHY US417 production (I, YE and M). The mean values predicted by the model and observed experimental responses are presented in Table 2. The phytase production level varied from 48.1 to 110.3 U/g of wheat bran. The regression model and significance of each term were determined (data not shown). The data were then subjected to analysis of variance (ANOVA) and the following regression equation was obtained to describe the level of phytase Y (U/g of wheat bran ) produced as a function of inoculum density and yeast extract and methanol contents:
The coefficient of determination R 2 was 0.916 for phytase production. The coefficient of determination (Adjusted R 2), the proportion of variation in the independent variable that can be explained by the predictors in the regression model, was 0.874 for phytase production. The statistical testing of the model was performed using Fisher’s test for the analysis of variance (ANOVA) (Table 3). The F value corresponding to phytase production was 21.7, which was greater than the tabulated F value at the 0.0002 level of significance, and the null hypothesis (i.e. that the factors are not affecting enzyme production) was, therefore, rejected. This indicated that the probability to accept the null hypothesis was of 0.0002.
A Student’s t test was performed to determine the significance of the coefficients (Heck et al. 2006). The coefficients of I and I2 were highly significant (p < 0.038 and p < 0.034, respectively). Using the submerged process, an increase in inoculum concentration, at a level of 1.6 ⋅107 CFU/ml, had a positive effect on enzyme production. The model revealed significant interactions between I and M (p < 0.003) and between M and YE (p < 0.0002).
Phytase production was linked to inoculum size and methanol level interaction especially at an inoculation density level ranging from 1 × 107 to 3.16 × 107 CFU/ml. Fig. 1 shows that the yield of phytase increased as the YE content increased from 0.25 to 0.75%. At high levels of YE, phytase secretion became more abundant with the increase of the M content from 0.25 to 0.75%. Similarly, the contour plot of the calculated response surface, for the interaction between inoculum size (I) and methanol (M) while keeping the yeast extract (YE) variable at its ‘0’ level, showed that, at each level of inoculum size, a moderate linear increase in phytase titer with increase in methanol concentration of up to the ‘+1’ level (0.75%) (RSM not presented). In addition, the contours were slightly inclined to the horizontal, indicating a limited interaction between the two variables.
Optimization of the PHY US417 production in SSF
RSM was used to optimize phytase production in SSF. Three of the independent variables were those that were identified in the SF investigation (namely, I, YE and M) and the fourth one was the moisture content (H: ml/l), a specific factor for SSF. This phase of the study involved the application of a 24 factorial design with eight star points and four replicates at the central points. The values of the independent variables are given in Table 4. The production yield varied from 14.4 to 78.11 U/g of wheat bran, depending on the culture conditions. The regression equation obtained after the analysis of variance (ANOVA) provided the levels of phytase produced as a function of the initial values of H, I, M and YE.
The coefficient of determination R 2 was calculated as 0.93 for phytase production indicating that the statistical model can explain 93% of the variability in the response. This is also supported by data generated from the analysis of variance (ANOVA) (data not shown). The computed model F value of 46.38 was higher than the tabulated value of F 0.05(6,21) = 2.57, demonstrating that the validity of the model was significant at a confidence level of 5%.
The significance levels of the coefficients are determined by P values and the Student’s t test (data not shown). Phytase production was significantly (p < 0.05) affected by I, I2, YE, M and the interaction between M and YE. The quadratic term of moisture content (H2) had a lesser effect. Within the range of the values tested, moisture content also had a limited effect on phytase production. When the moisture content was maintained at a low level, phytase production endured a decrease of about 15%.
Similar to Fig. 1., response surface curves plotted to evaluate the interaction between the variables and to determine the optimum level required for each variable shows that the contours were diagonal to both axes, suggesting a significant interaction between the two parameters (response curve not included). As in the case of SF, phytase production was maximized in the presence of high levels of methanol. This behavior was similar to that observed in SF (Fig. 1). However, unlike SF, at a low level of YE, phytase production increased by increasing the M content from 0.25 to 0.75%.
SF and SSF comparison
The RSM optimization of phytase production was achieved by using the SF and SSF production processes. Our results showed that both were function of I (I, I2) and the interaction between M and YE. Also, yeast extract and particularly methanol play a crucial role in phytase production. Besides, SSF was governed by YE, M and to a less extent by the quadratic term of moisture content (H2). On the other hand, SF was slightly controlled by the interaction between I and M. It should be noticed that the phytase productivity was higher in SF [112 U/g of wb; 2.33 U/(g × h)] than in SSF [85 U/g of wb; 1.18 U/(g × h)]. According to our results, SF seems more convenient for abundant enzyme production.
Validation of the optimum conditions
To validate the results predicted by the experimental methodology, fermentation experiments were conducted using the optimized culture conditions. For SF, the optimum combination was found to be: I of 1.6 × 107 CFU/ml, M and YE at 0.75 g/g of wheat bran. For SSF, on the other hand, it was: H of 75%, I of 1 × 108 CFU/ml, M of 0.75 g/g of wheat bran and YE 0.25 g/g of wheat bran. Table 5 shows the comparison between the yield of phytase from original and optimized media under the experimental conditions. The yields obtained, of 112 and 85 U/g, were very close to the predicted ones of 115 and 92 U/g, respectively. These results indicated a close agreement between the predicted and the experimental responses.
Discussion
In the present study, statistically based experimental designs were employed to optimize the culture medium and to assess the effectiveness of SF and SSF processes for phytase production. This approach allowed us to attain a relatively high production yield (112.0 U/g) when wheat bran was used, a low-cost phytate source that has been frequently reported in the literature as a basal medium component for the production of phytase (Ebune et al. 1995; Nampoothiri et al. 2004). The activity produced, which was equivalent to 5.60 U/ml, was actually greater than phytase titers achieved by Bacillus laevolacticus (2.96 U/ml) (Gulati et al. 2007) and Escherichia coli expressing the phytase gene from Bacillus sp. (4.29 U/ml) (Rao et al. 2008) under optimized conditions. It can also be considered relatively high compared to other yields previously attained using other by-products. For instance, phytase titers of 44.5 and 64 U/g substrate were obtained by Mucor racemosus NRRL 1994 and Rhizopus oryzae NRRL 1891 using a mixture of wheat bran/sesame oil cake (Roopesh et al. 2006) and coconut /sesame oil cake (Ramachandran et al. 2005), respectively.
A wide array of ingredients has previously been reported in literature to exert significant effects on phytase production, including carbon, nitrogen, phosphorus and surfactant sources. Our results show that supplementation of methanol in the culture medium, as carbon and energy source, enhance phytase liberation. Enhancement of phytase production in the presence of methanol could be due to the synthesis of compounds from methanol assimilation which stimulate the enzyme liberation (Dijkhuizen and Levering 1992). In the case of the present study, several organic and inorganic nitrogenous sources were tested and the highest phytase titer, in SF, was attained in the presence of 0.75% (w/v) YE. This result corroborates the findings of previous studies using Bacillus strains. Vuolanto et al. (2001) have, for instance, screened different organic and inorganic nitrogen sources for phytase production by a recombinant Bacillus subtilis BD170. After selecting and further exploring yeast extract and peptone, the authors concluded that the growth yields achieved by YE were better than those by peptone.
Concerning the effect of phosphorus supplementation, various studies, particularly those focusing on fungi, reported that the effect of this element is dose dependent (Kim et al. 1999; Vats and Banerjee 2004) and that phytase production was significantly enhanced at low phosphate levels (0.005–0.02%) and severely inhibited at higher ones (Kim et al. 1999). Some other studies, however, emphasized that P supplementation, even at low concentrations, repressed phytase secretion (Singh and Satyanarayana 2008). The present study showed that the supplementation of the wheat bran medium with inorganic P, even at low concentration (0.0005% w/v), repressed phytase liberation (Table 1). This result seems to be inconsistent with some reported studies. This can be explained by the fact that media compounds, particularly wheat bran, contain relatively high inorganic phosphorus (more than 0.1%) (Servi et al. 2008). Furthermore, Vuolanto et al. (2001), who assayed the production of the phytase PhyC of Bacillus subtilis VTTE-68013 in Bacillus subtilis BD170 using a phosphate depletion inducible pst-promoter, have explained that cells were probably able to liberate bound phosphate from YE, which resulted in a lower phytase expression of the recombinant phytase gene. Similarly, Arxula adeninivorans CBS 7377 and A. adeninivorans CBS 8335 have been previously reported to be able to secrete more phytase in the phosphate-depleted yeast extract medium (Jareonkitmongkol et al. 1997).
The surfactants have a stimulating effect on fungal enzyme excretion due to their action on cell permeability (Ebune et al. 1995). However, our result showed that 0.5 % Triton X-100 did not have any effect on phytase production of Bacillus subtilis US417. This finding is in accordance with the results previously reported by Lan et al. (2002), which indicated that optimum production could be achieved without any surfactant. The conflict in results may be related to the difference in cell wall structure of fungi and bacteria.
Furthermore, the current work showed that temperature, pH and agitation, over the ranges studied, have limited effects on phytase secretion. A temperature level ranging from 30 to 40°C can be applied for the strain presented in this study, which is in agreement with results previously reported on Bacillus species described to produce phytase at mesophilic temperature ranging from 37 to 39°C (Sunitha et al. 1999; Lan et al. 2002). However, higher temperatures have also been reported such as for Bacillus laevolacticus (Gulati et al. 2007) and Bacillus DS11 (Kim et al. 1998), which had an optimal temperature production of 50°C. A thorough review of the literature concerning phytase production by Bacillus sp. can lead to the conclusion that while temperature, in the mesophilic range, plays a crucial role in strain growth, it has a limited effect on phytase liberation.
The production of phytase by B. subtilis US417 was not greatly affected over a pH range of 6–7.5, with a maximal production at pH 6.5, which is in agreement with other previously reported results using Bacillus strains. For example, the production of Bacillus laevolacticus phytase in the pH range of 3.5–8.5 was not severely affected (Gulati et al. 2007).
The great importance of the inoculum size on phytase production by bacteria or fungi is also well emphasized in the literature. In the case of the present study, RSM helped demonstrate that production, in SF and SSF, is optimal at high inoculum sizes. The literature reported that phytase productions are, mostly cell growth-associated. This was the case with Bacillus sp., KHU-10 (Choi et al. 1999), Mucor racemosus NRRL 1994 (Roopesh et al. 2006) and Aspergillus niger (Krishna and Nokes 2001).
The synergistic effect of the most influential operating parameters, such as the nitrogen source, phytase production inducer, salt, inoculum size, on phytase production was reported in several statistical optimizations. The present study also showed a great interaction between YE and methanol (M), which can be considered as an inducer. In this respect, Sunitha et al. (1999) studied the production of Bacillus sp. DS11 phytase in E. coli BL21 and noted a substantial interaction between YE, NaCl, and tryptone, respectively. One can conclude that, for most Bacillus sp., phytase liberation was related to the interaction between elements in the culture media, particularly those including nitrogen sources such as YE.
While comparing the optimized medium with the initial SF conditions, the factorial experimental designs used in the present optimization study enabled improvements, in terms of phytase production by Bacillus subtilis US417 in SF and SSF by 5- and 4-fold, respectively (Table 5). The productivity of phytase by this bacterium in the case of SF [2.33 U/(g × h)] was higher than that of SSF [1.18 U/(g × h)]. In fact, reports on the comparative production of phytase by these two fermentation processes are very scarce, with only one study available in the literature in which a direct comparison was undertaken, namely that by Gunashree and Venkateswaran (2008).
Conclusions
The optimization of the factors (inoculum size, methanol and yeast extract) showed that phytase productivity by Bacillus subtilis US417 was higher in SF [2,330 U/(kg × h)] than in SSF [1,180 U/ (kg × h)].
References
Choi YM, Dong ON, Cho SH, Lee HK, Suh HJ, Chung SH (1999) Isolation of a phytase producing Bacillus sp. KHU-10 and its phytase production. J Microbiol Biotechnol 9(2):223–236
Dijkhuizen L, Levering PR (1992) De Vries GE. In: Murrell JC, Dalton H (eds) Methane and methanol utilizers. Plenum, New York, p 149
Ebune A, Al-Asheh S, Duvnjak Z (1995) Production of phytase during solid-state fermentation using Aspergillus ficuum NRRL 3135 in canola meal. Bioresour Technol 53:7–12
Engelen AJ, van der Heeft FC, Randsdorp PHG, Smit ELC (1994) Simple and rapid determination of phytase activity, J Assoc Off Anal Chem 77:760–764
Farhat A, Chouayekh H, Ben Farhat M, Bouchaala K, Bejar S (2008) Gene cloning and characterization of a thermostable phytase from Bacillus subtilis US417 and assessment of its potential as a feed additive in comparison with a commercial enzyme. Mol Biotechnol 40(2):127–135
Greiner R, Egli I (2003) Determination of the activity of acidic phytate-degrading enzymes in cereal seeds. J Agric Food Chem 51:847–850
Greiner R, Konietzny U (2006) Phytase for food application. Food Technol Biotechnol 44(2):125–140
Gulati HK, Chadha BS, Saini HS (2007) Production and characterization of thermostable alkaline phytase from Bacillus laevolacticus isolated from rhizosphere soil. J Ind Microbiol Biotechnol 34:91–98
Gunashree BS, Venkateswaran G (2008) Effect of different cultural conditions for phytase production by Aspergillus niger CFR 335 in submerged and solid-state fermentations. J Ind Microbiol Biotechnol 35(12):1587–1596
Haefner S, Knietsch A, Scholten E, Braun J, Lohscheidt M, Zelder O (2005) Biotechnological production and applications of phytases. Appl Microbiol Biotechnol 68(5):588–597
Heck JX, Flôres SH, Hertz PF, Ayub MAZ (2006) Statistical optimization of thermo-tolerant xylanase activity from Amazon isolated Bacillus circulans on solid-state cultivation. Bioresour Technol 97:1902–1906
Jareonkitmongkol S, Ohya M, Watanabe R, Takagi H, Nakamori S (1997) Partial purification of phytase from a soil isolate bacterium, Klebsiella oxytoca MO-3. J Ferment Bioeng 83(4):393–394
Kammoun R, Chouayekh H, Abid H, Naili B, Bejar S (2009) Purification of CBS 819.72 α-amylase by aqueous two-phase systems: modelling using response surface methodology. Biochem Eng J 46:306–312
Kerovuo J, Tynkkynen S (2000) Expression of Bacillus subtilis phytase in Lactobacillus plantarum 755. Lett Appl Microbiol 30(4):325–329
Kim YO, Kim HK, Bae KS, Yu JH, Oh TK (1998) Purification and properties of thermostable phytase from Bacillus sp. DS11. Enzyme Microb Technol 22:2–7
Kim DS, Godber JS, Kim HR (1999) Culture conditions for a new phytase-producing fungus. Biotechnol Lett 21:1077–1181
Krishna C, Nokes SE (2001) Predicting vegetative inoculum performance to maximize phytase production in solid-state fermentation using response surface methodology. J Ind Microbiol Biotechnol 26:161–170
Lan GQ, Abdullah N, Jalaludin S, Ho YW (2002) Culture conditions influencing phytase production of Mitsuokella jalaludinii, a new bacterial species from the rumen of cattle. J Appl Microbiol 93(4):668–674
Nampoothiri KM, Tomes GJ, Krishnan R, Szakacs G, Nagy V, Soccol CR, Pandey A (2004) Thermostable phytase production by Thermoascus aurantiacus in submerged fermentation. Appl Biochem Biotechnol 118(1–3):205–214
Ramachandran S, Roopesh K, Nampoothiri KM, Szakacs G, Pandey A (2005) Mixed substrate fermentation for the production of phytase by Rhizopus sp. using oilcakes as substrates. Process Biochem 40:1749–1754
Rao DECS, Rao KV, Reddy VD (2008) Cloning and expression of Bacillus phytase gene (phy) in ‘Escherichia coli’ and recovery of active enzyme from the inclusion bodies. J Appl Microbiol 105(4):1128–1137
Roopesh K, Ramachandran SI, Nampoothiri KM, Szakacs G, Pandey A (2006) Comparison of phytase production on wheat bran and oilcakes in solid-state fermentation by Mucor racemosus. Bioresour Technol 97:506–511
Servi S, Zkaya HO, Colakoglu AS (2008) Dephytinization of wheat bran by fermentation with bakers’ yeast, incubation with barley malt flour and autoclaving at different pH levels. J Cereal Sci 48:471–476
Singh B, Satyanarayana T (2008) Improved phytase production by a thermophilic mould Sporotrichum thermophile in submerged fermentation due to statistical optimization. Bioresour Technol 99:824–830
Sung HG, Shin HT, Ha JK, Lai HL, Cheng KJ, Lee JH (2005) Effect of germination temperature on characteristics of phytase production from barley. Bioresour Technol 96:1297–1303
Sunitha K, Lee JK, Oh TK (1999) Optimization of medium components for phytase production by E. coli using response surface methodology. Bioprocess Eng 21:477–481
Vats P, Banerjee UC (2004) Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzyme Microb Technol 35:3–14
Vuolanto A, Weymarn NV, Kerovuo J, Ojamo H, Leisola M (2001) Phytase production by high cell density culture of recombinant Bacillus subtilis. Biotechnol Lett 23:761–766
Xiong AS, Yao QH, Peng RH, Han PL, Cheng ZM, Li Y (2005a) High level expression of a recombinant acid phytase gene in Pichia pastoris. J Appl Microbiol 98:418–428
Xiong C, Shouwen C, Ming S, Ziniu Y (2005b) Medium optimization by response surface methodology for poly-γ-glutamic acid production using dairy manure as the basis of a solid substrate. Appl Microbiol Biotechnol 69(4):390–396
Acknowledgements
This work was funded by the Tunisian Government “Contrat Programme CBS-LMB” and the local Company “Nutrisud” through a research agreement. The authors wish to express their gratitude to Mr. Moncef Affes for his critical reading of the manuscript. Thanks are also due to Mr Anouar Smaoui from the Sfax Faculty of Sciences for carefully proofreading and editing the present paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kammoun, R., Farhat, A., Chouayekh, H. et al. Phytase production by Bacillus subtilis US417 in submerged and solid state fermentations. Ann Microbiol 62, 155–164 (2012). https://doi.org/10.1007/s13213-011-0240-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0240-7