Abstract
The survival of bacteria in natural environments is of practical importance. In this study, we investigated the effect of starvation on the survival and adhesion ability of Shigella in domestic treatment plant effluent microcosms after incubation at either room temperature or 4°C for 1 month. Our results showed that the number of cells decreased considerably after this period of stress. We also revealed some modifications to the biochemical and enzymatic profiles, and antibiotic susceptibility of stressed cells. Adherence assays to the human oral cavity epidermoid carcinoma (KB) cell line revealed an increase in the number of adherent cells from 0.7 to 1.85% after 1 month of incubation in domestic treatment plant effluent.
Similar content being viewed by others
Introduction
The genus Shigella is composed of Gram-negative facultative anaerobes of four species: S. dysentariae, S. boydii, S. sonnei and S. flexneri. All are human pathogens and are usually transmitted from person to person as well as by ingestion of contaminated water and food. The infective dose is very low, varying from 101 to 104 organisms (Rowe and Gross 1984). Virulent Shigella organisms cause the human illness known as bacillary dysentery, as do enteroinvasive Escherichia coli (EIEC) strains (Theron et al. 2001). Worldwide, shigelloses represents a considerable public health problem. Indeed, the frequency of infection is continually increasing. The annual number of fatal cases of diarrhoea due to Shigella is 650,000. Shigellosis is an infection classically very severe at onset (Teyssou et al. 1994), but it can also appear in a progressive way (Hyams et al. 1991). Several studies on the epidemiology of shigelloses have reported seasonal fluctuations closely related to climatic conditions and thus to hygiene and living conditions. For example, in Bangladesh, it has been shown that various water sources, e.g., ponds, lakes, wells, and rivers, can act as sources of infection (Islam et al. 1993). In the United States, outbreaks of shigellosis have also been attributed to swimming in contaminated water (Rosenberg et al. 1976). In South Africa, children under 5 years of age living in settlements with rudimentary access to water supply and sanitation are the most susceptible to diarrhoea, whereas adults often become symptomless carriers (Pergram et al. 1998).
The aim of this work was to study the survival of four Shigella strains incubated in a domestic treatment plant effluent microcosm for 1 month. Biochemical and enzymatic characterization was achieved using Api-20E and Api-ZYM systems. Adherence assays were performed with the human oral cavity epidermoid carcinoma (KB) cell line.
Materials and methods
Bacterial strains
Four Shigella strains, two S. sonnei (S1 + S2), S. boydii (S3) and S. flexneri (S4), were obtained from the Monastir hospital, Tunisia and maintained at −80°C in Luria-Bertani broth (LB) supplemented with glycerol (15%, v/v).
Growth conditions
Domestic treatment plant effluent (100 ml) from the National Sanitation Utility (ONAS) of Monastir, Tunisia (pH 7, N 1 mg/l; P 0.05 mg/l and COD 90 mg/l) was filtered through a membrane (pore size 0.22 µm; Millipore, Bedford, MA) and autoclaved (120°C for 20 min) in 250 ml Erlenmeyer flasks.
For the experiments, Shigella cells were grown at 37°C in Tryptic Soy broth (TSB, Difco, Detroit, MI) for 24 h. The strains were washed three times by centrifugation (13,000 rpm at 20°C for 10 min) with sterile domestic treatment plant effluent and then suspended in 10 ml of the same medium (Ben Abdallah et al. 2007). The domestic treatment plant effluent microcosms (100 ml) were inoculated with these suspensions (approximately 109 CFU/ml) and then incubated in a static state at room temperature (22–25°C) or at 4°C.
Enumeration techniques
Plate counts of culturable cells were determined by the drop plate method (Hoben and Somasegaran 1982), using Tryptic Soy agar (TSA, Difco). Microcosms were sampled for plate counts at time zero (inoculation time) and subsequently daily during the 1st week and weekly during the 1st month. The plates were incubated at 37°C, and the number of colonies was counted after 24 and 48 h.
Enzymatic characterization of starved cells
After 1 month of incubation in domestic treatment plant effluent microcosms at room temperature or 4°C, Shigella cells were characterized using the Api 20E system (bio-Mérieux, Marcy L'Etiole, France). Enzymatic profiles were determined with the Api-ZYM system (bio-Mérieux) according to the manufacturer’s instructions.
Antimicrobial susceptibility
Antibacterial assays were performed using the antibiogram method of the National Committee for Clinical Laboratory Standards (NCCLS 1999). The different antibiotic disks used were: ampicillin (Ap) 10 µg; cefotaxime (Ctx) 30 µg; kanamycin (K) 30 µg; tobramycin (Tm) 10 µg; neomycin (Nm) 30 IU; nalidixic acid (Na) 30 µg; oxolinic acid (Oa) 10 µg; flumequine (Ub) 30 µg; colistin (Cs) 50 µg; tetracycline (Tc) 30 µg; and chloramphenicol (Cm) 30 µg. The strains were classified after 24 h of incubation at 37°C on Muller Hinton agar diffusion plates (Bio-Rad, Marnes-la-Coquette, France) as sensitive, intermediate or resistant.
Cultured cell adherence assays
Quantitative adherence assays were performed with the KB cell line (Chatti et al. 2007). Shigella cells were grown overnight in TSB at 37°C, with shaking, subcultured at a 1:100 dilution in minimal essential medium (MEM; Biowest, Nuaillé, France), and grown for another 3 h with shaking. KB cells were infected with Shigella (108 CFU/ml) and the bacteria were allowed to adhere for 60 min at 37°C in 5% CO2. Then, bacterial suspension was removed to exclude unattached bacteria. The monolayers of KB cells were washed three times with MEM, and 1 ml Triton X-100 in phosphate-buffered saline (PBS, pH 7) was added for 5 min at room temperature to release the bacteria from the cells. The number of bacteria was estimated by plating serial dilutions. All experiments were performed in triplicate.
Molecular identification of stressed bacteria
Bacteria were cultured on TSA at 37°C for 24 h. One colony was cultured in TSB at 37°C for 24 h, and then 1.5 ml was centrifuged. The DNA was extracted by boiling for 5 min and centrifuging at 13,000 rpm for 8 min. The supernatant was used for amplification by PCR with Shigella primers of the target IpaH gene.
PCR was performed in 25 µl containing 50 ng extracted DNA, 5 µl green Go Taq buffer (5×), 0.25 µl dNTPs (10 mM), 0.5 µl MgCl2 (50 mM) , 1 µl paH forward primer 5′-GTTCCTTGACCGCCTTTCCGATAC-3′ (25 pM) and IpaH reverse primer 5′-CATTTC CTTCACGGCAGTGGA-3′ (25 pM; Hartman et al. 1990), 1 U GO Taq DNA polymerase (Promega, Charbonnieres, France). Amplification was conducted in a Thermocycler PTC 100 (Bio-Rad). The reaction mixtures were heated at 94°C for 5 min and were subjected to 35 cycles of denaturation at 94°C for 90 s, annealing at 57°C for 30 s, and elongation at 72°C for 90 s, followed by a 10-min final extension period at 72°C. PCR products (5 µl) were analyzed on 1% agarose gels stained with ethidium bromide (0.5 mg/ml) at 90 V for 1 h and visualized under ultraviolet transillumination. Amplification products were photographed and their sizes determined relative to a 100-bp molecular size marker (Promega).
Statistical analysis
Statistical analysis was performed using the SPSS 13.0 statistics package for Windows. The differences in the degree of adhesion assay were examined by the Friedman test, followed by the Wilcoxon signed ranks test. P-values < 0.05 were considered significant.
Results
Survival of Shigella in domestic treatment plant effluent
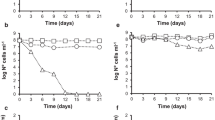
Figures 1, 2, 3 and 4 illustrate the quantitative variation in Shigella strains incubated in domestic treatment plant effluent microcosms at room temperature or 4°C. After 24 h incubation, cell numbers decreased by approximately 2–3 log units for all strains, depending on the temperature of incubation. This reduction continued gradually in both microcosms (room temperature and 4°C) during the period of incubation.
Enzymatic changes in starved Shigella cells (Figs. 1– 4)
Results from the API 20E system revealed some modifications in the biochemical profile of all Shigella strains incubated for 1 month in domestic treatment plant effluent microcosms at room temperature (Table 1). Indeed, strains S1, S2 and S3 lost the capacity to ferment mannitol, and strain S4 lost the capacity to ferment melibiose.
Analysis of enzymatic characters using Api ZYM galleries shows that all the stocks of Shigella are able to assimilate alkaline and acid phosphatase, esterase (C4), esterase lipase (C8), lipase (C14), α-galactosidase, β-galactosidase, α-glucosidase, β-glucosidase, α-mannosidase and α-fucosidase both before and after incubation in wastewater, whereas the two S. sonnei strains gained the ability to produce the naphthol-As-Bi-phosphohydrolase after incubation at room temperature (Table 2). Concerning exoenzymes, after 1 month of incubation in domestic treatment plant effluent we noted that S. sonnei (S1) incubated at room temperature becomes able to produce phospholipase.
Antimicrobial resistance
The results of antimicrobial susceptibility measurements (Table 3) showed that, after 1 month of incubation in domestic treatment plant effluent, all four strains of Shigella became sensitive to tobramycin and tetracycline.
Adherence to KB cells
We next assayed adherence of the four strains of Shigella to line KB cells. Indeed, before incubation, we noted that all the Shigella cells are slightly adherent to KB cells (0.8, 1.03, 0.7 and 1.1% for S1, S2, S3 and S4, respectively). After 1 month of incubation in domestic treatment plant effluent, we observed a significant increase in the percentage of adhesion for all strains. Adherence was much higher at room temperature than at 4°C (Table 4).
Molecular confirmation of Shigella cells
We used PCR to identify all tested strains of Shigella after 1 month of incubation in domestic treatment plant effluent microcosms (Figs. 5, 6). After amplification of the IpaH gene by PCR, we confirmed the identity of the investigated Shigella strains.
Agarose gel electrophoresis of PCR amplification of the ipaH gene. Lanes: 1 100 bp DNA molecular size marker, 2–8 PCR amplicons obtained with DNA amplification of Shigella strains (2 negative control, 3 S1, 4 S1′, 5 S1″, 6 S2, 7 S2′, 8 S2″. S Strain before incubation in domestic treatment plant effluent, S′ strain incubated for 1 month in domestic treatment plant effluent microcosms at room temperature, S″ strain incubated for 1 month in domestic treatment plant effluent at 4°C
Agarose gel electrophoresis of polymerase chain reaction (PCR) amplification of ipaH gene. Lanes: 1 100 bp DNA molecular size marker, 2–8 PCR amplicons obtained with DNA amplification of Shigella strains (2 negative control, 3 S3, 4 S3′, 5 S3″, 6 S4, 7 S4′, 8 S4″. S Strain before incubation in domestic treatment plant effluent, S″ strain incubated for 1 month in domestic treatment plant effluent microcosms at room temperature, S″ strain incubated for 1 month in domestic treatment plant effluent at 4°C
Discussion
The results developed in the present work show that Gram negative bacteria, such as Shigella, are able to adapt and survive in domestic treatment plant effluent. After 1 month in domestic treatment plant effluent, the reduction in cell number in all four strains tested was more rapid at room temperature than at 4°C. Several reports indicate that E. coli O157 exhibited a similar reduction in cell number after starvation in domestic treatment plant effluent (Ravva and Korn 2007). In addition, we reported previously that the same Shigella cells decline more rapidly in domestic treatment plant effluent compared to seawater (Ellafi et al. 2009). The reduction in Shigella cells numbers at the beginning of the experiment may have resulted from the death of bacterial cells caused by temperature stress (Monier and Lindow 2003) and/or the evolution of cells to viable but nonculturable (VBNC) state due to nutrient deficiency in this medium (Ben Abdallah et al. 2007).
The observed modification of the biochemical characters observed in the starved strains is probably due to the stressing factors of domestic treatment plant effluent. Indeed, the loss of the capacity to use certain sugars is probably caused by the nutrient deficiency of domestic treatment plant effluent. Similar results have been found with bacteria exposed to seawater (Ben Abdallah et al. 2007). The variable characteristics detected in bacteria facing nutrient starvation are as follows: low endogenous metabolism; broad variability of catabolic characters because of genetic complexity; strong production of catabolic enzymes with inducible synthesis; and catabolic repression to the minimal level, allowing the simultaneous use of several sources of energy (Van and Kuen 1984; Ben Abdallah et al. 2007). Moreover, the appearance of the activity of the enzyme naphthol-AS-BI phosphohydrolase for the two strains of S. sonnei and phospholipase for S. sonnei (S1) is the result of the expression of certain genes implied in the survival of these strains under stressful environmental conditions (Russell 1984). Indeed, microorganisms have large arsenals of tools to withstand a multitude of environmental insults. In most cases, exposure to harsh elements results in the production of specific enzymes that aid the alleviation of stresses and allow survival and/or growth (Siu 2002; Duguay and Silhavy 2004).
The modifications of resistance to antibiotics are probably due to structural modifications of the cellular envelope and/or with contraction of the periplasmic space in the stressed bacteria caused by domestic treatment plant effluent (Huisman et al. 1996). As a consequence, the cellular envelope becomes impermeable to certain antibiotics and lets diffuse others easily. This represents an additional strategy used by the bacterial cells to adapt to environmental changes (Russell 1984).
In this study, we also tested the effect of incubation in domestic treatment plant effluent on the adhesive power of Shigella cells. After 1 month in domestic treatment plant effluent, adhesion of Shigella to KB cells increased, showing that adhesive power increased under the effect of stress. Statistical analysis revealed a significant difference between the percentage of adherence to KB cells of stressed Shigella and control strains (P < 0.05). Similar results were found under variation of temperature. This increase could be explained by changes in the membrane structure of the bacteria as a result of stress. These changes in bacterial attachment characteristics can be related to changes in the hydrophobicity of the surface of the cell, and in the components of the external membrane involved in attachment, such as the pili and lipopolysaccharides (LPSs; Zhang and Normark 1996; Oliveira et al. 2007). The expression of these structures can change according to the environment to which the bacterium is exposed.
Conditioning microbial strains to nutrient scarcity and studying their response to starvation may allow screening for those that can adapt and survive for long periods under such conditions, and those strains in which starved cells can resume growth after environmental factors become favorable (Amy and Morita 1953). Shigella survival after introduction into domestic treatment plant effluent is affected by the physiological state of the cells. Indeed, in this environment, which is characterized by nutrient deficiency, Shigella has the capacity not only to survive but also to increase in virulence.
References
Amy PS, Morita RY (1953) Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol 45:1109–1115
Ben Abdallah F, Chaieb K, Snoussi M, Bakhrouf A, Gadour K (2007) Phenotypic variations and molecular identification of Salmonella enterica Serovar Typhimurium cells under starvation in seawater. Curr Microbiol 55:485–491
Chatti A, Daghfous D, Landoulsi A (2007) Effect of seqA mutation on Salmonella typhimurium virulence. J Infect 54:241–245
Duguay AR, Silhavy TJ (2004) Quality control in the bacterial periplasm. Biochim Biophys Acta 1694:121–134
Ellafi A, Denden I, Ben AF, Souissi I, Bakhrouf A (2009) Survival and adhesion ability of Shigella spp. strains after their incubation in seawater microcosms. World. J Microbiol Biotechnol 25:1161–1168
Hartman AB, Venkatesan MM, Oaks EV, Buysse JM (1990) Sequence and molecular characterization of multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol 172(4):1905–1915
Hoben HJ, Somasegaran P (1982) Comparaison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol 44:1246–1247
Huisman GW, Siegele DA, Zambrano MM, Kolter R (1996) Morphological and physiological changes during stationary phase. In: Neidhardt FC et al (eds) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, pp 1672–1682
Hyams KC, Merrell BR, Bourgeois AL (1991) Diarrheal disease during operation desert shield. N Engl J Med 325(20):1423–1428
Islam MS, Hasan MK, Miah MA, Sur GC, Felsenstein M, Venkatesan M, Sack RB, Albert MJ (1993) Use of the polymerase chain reaction and fluorescent-antibody methods for detecting viable and nonculturable Shigella dysenteriae Type 1 in laboratory microcosms. Appl Environ Microbiol 59(2):536–540
Monier JM, Lindow SE (2003) Differential survival of solitary and aggregated, bacterial cells promotes aggregate formation on leaf surfaces. Proc Natl Acad Sci USA 100:15977–15982
NCCLS (1999) Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement, vol. 19, No. 1. National Committee for Clinical Laboratory Standards document M100-S9. Villanova, PA
Oliveira K, Oliveira T, Teixeira P, Azeredo J, Oliveira R (2007) Adhesion of Salmonella enteritidis to stainless steel surfaces. Braz J Microbiol 38:318–323
Pergram GC, Rollins N, Esprey Q (1998) Estimating the costs of diarrhoea and epidemic dysentery in KwaZulu-Natal and South Africa. Water SA 24(1):11–20
Ravva S, Korn A (2007) Extractable organic components and nutrients in wastewater from dairy lagoons influence the growth and survival of Escherichia coli O157:H7. Appl Environ Microbiol 73:2191–2198
Rosenberg ML, Hazlet KK, Schaefer J, Wells JG, Pruneda RC (1976) Shigellosis from swimming. J Am Med Assoc 236(16):1849–1852
Rowe B, Gross RJ (1984) Facultatively anaerobic Gram negative rods. Genus II. Shigella. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 423–427
Russell NJ (1984) Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci 9(3):108–112
Siu LK (2002) Antibiotics: action and resistance in Gram negative bacteria. J Microbiol Immunol Infect 35:1–11
Teyssou R, Gerome P, Buisson Y (1994) Triple shigellose chez un même malade contractée pendant l'opération "Turquoise" au Ruande. Bull Soc Pathol Exot 87:228–230
Theron J, Morar D, Preez MDU, Brozel VS, Venter SN (2001) A sensitive seminested pcr method for the detection of Shigella in spiked environmental water samples. Water Res 35(4):869–874
Van GH, Kuen JG (1984) Strategies for growth and evolution of microorganisms in oligotrophic habitats. In: Hobbie JE, Williams PJB (eds) Heterotrophic activity in the sea. Nato Conference series IV. Plenum, New York, pp 25–54
Zhang JP, Normark S (1996) Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science 273:1234–1236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellafi, A., Abdallah, F.B. & Bakhrouf, A. Effect of starvation on survival and adhesion ability of Shigella spp. in domestic treatment plant effluent microcosms. Ann Microbiol 60, 383–389 (2010). https://doi.org/10.1007/s13213-010-0053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-010-0053-0