Abstract
Flow lithography in a conventional rectangular microchannel is limited to fabrication of particles for which only the shapes of top perimeters are controlled. We present a flow lithography technique for fabrication of microparticles of diverse 3D shapes and multiple layers using non-rectangular microchannels with designed cross sections that allow the creation of complex shapes and diverse cross-sectional shapes. Variations in cross-sectional shape allow high-throughput, on-demand production of microparticles in unconventional shapes such as tetrahedrons and pyramids. Multilayered 3D particles were generated in an enlarging triangular channel combined with on-chip PDMS valves, which allow particle alignment and fluid exchange. These 3D microparticles are expected to further expand the wide variety of applications of microparticles, especially in drug delivery and tissue engineering fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the utilization of functional polymer particles has increased in the past century, many methods for manufacturing and applications of various types of polymeric microparticles have been newly developed1-8. Polymeric particles as new functional materials have grown in demand for various fields including, but not limited to, tissue engineering9–12 and drug delivery13–18. The manufacturing methods of polymeric particles have also been studied extensively such as microfluidic-based processes, photolithography, emulsification, and sol-gel-based processes3,4,19–22. In particular, widely used methods including droplet-generation, flow lithography, and particle assembly were based on microfluidic-based processes. Droplet generation is one of the representative microfluidic-based production techniques for polymeric microparticles. It provides highly uniform size distribution and high throughput23. However, the shape of the generated particles has been limited to spheres and simple variations from the sphere such as conjoined spherical particles or pressed spherical particles1,24–26. On the other hand, various techniques for production of non-spherical microparticles have been developed due to the advantages including controllable packing density, surface-to-volume ratio, and variations in optical characteristics27,28. The characteristics of microparticles with different shapes beyond spherical shape have been studied because the physical factors of such particles are intimately related to studies of biological or medical applications such as cell growth and drug release9,29. In particular, there has been an increasing trend to report on the fabrication and influence of particle geometry such as size, shape, and surface morphology5,30. For example, it was possible to overcome the inherent limitations of existing spherical particles such as the influence of migration and adsorption in blood vessels, gastrointestinal tract, and respiratory tract depending on the size of the non-spherical particles31–33.
Flow lithography technology has offered an efficient method for generating non-spherical microparticles in a microfluidic channel of rectangular cross section3,4. Although people have recognized the need for diversity in microparticle characteristics, the available particle shapes are mostly limited to extruded 2D shapes5,20,33–36. Past attempts at the variation of microparticle shape in 3D using stop or continuous flow lithography included the use of light interference, grey-scale lithography, or pneumatic valve membrane control systems2,8,20,26,30,37. However, in the end, most attempts to further diversify 3D microparticle shapes were limited by the rectangular cross section and resulted in slight variations from the original extruded 2D shapes. Grey-scale lithography using pneumatic valve membranes were able to 3-D print microparti-cles in complex shapes, but resolution and throughput still needed to be improved. There have been other attempts at changing the microparticle cross section using methods such as structural hydrodynamics, but the mixing of the monomer stream resulted in a lower resolution, and large particle sizes due to the Reynold number region where proper hydrodynamics could occur.
Here, we used non-rectangular cross section micro-fluidic channels for flow lithography, which allows production of diverse 3D shaped microparticles, such as tetrahedral or pyramidal shapes. The tetrahedral particles are known to have a high disordered packing density larger than that of an FCC (Face Centered Cubic) crystal structure38, which is difficult to make with currently available approaches. We also developed a fabrication method for producing multilayered 3D microparticles, which could be partially or fully engulfed in other layers with any number of particles placed in the inner layer surrounded by outer layers. Non-spherical multilayered particles can be mass-produced with desired characteristics such as shapes, material, or packing density. The particular type of polymeric particle to be introduced in this paper would prove useful in the field of drug delivery and particle self-assembly, where time and phase sensitive actions along with structural uniqueness are required.
Results and Discussion
3D Microparticle Fabrication Using Non-rectangular Channels
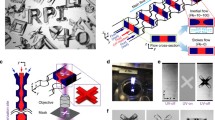
Channels with various non-rectangular cross sections were fabricated for flow-lithographic generation of microparticles with different shapes and sizes by the process shown in Figure 1a. The non-rectangular channels’ molds were prepared by the conventional microfabrication techniques including photoresist (PR) reflow, anisotropic wet etching of Si, and tilted-exposure lithography. The cross sections of PDMS channels are shown in Figure 1b, and the particles fabricated from the channel molds are shown in Figure 1c. A PR reflow method was used to produce the semicircular shape of the cross section. Anisotropic wet etching of Si allows fabrication of two cross-sectional shapes of isosceles triangles with vertex angles of 70.6° and 90°39,40. Trapezoidal cross sections can also be achieved by stopping the Si etching before the triangular shape is achieved. After creating the trenches on a SiN wafer, the channel molds were replicated from the trenches. A tilted-exposure lithography method can provide a versatile platform for fabricating microchannels in diverse cross sections. Practically any sidewall angle for triangular or trapezoidal cross section channels can be achieved with tilted-exposure. Limitations of the tilt-exposure method include channel size limited by the thickness of the PR and the inability of conventional equipment to tilt the stage.
(a) Schematics of an Optofluidic Maskless Lithography (OFML) system and stop-flow procedure. (b) Cross sections of non-rectangular channels. Semi-circular, triangular, and trapezoidal channel cross sections are shown (Scale bar 50 μm). (c) Optical microscopy image of particles produced with non-rectangular cross section channels (Scale bar 200 μm). (d) SEM images of the particles generated in triangular and trapezoidal channels. Interesting shapes such as tetrahedrals, half-pyramids, and boomerangs could be fabricated (Scale bar 50 μm).
The various channels were then used in the Optofluidic Maskless Lithography (OFML) procedure (Figure 1a) to produce polymeric microparticles as shown in Figure 1c (See method section for the detail). The particles with triangular and trapezoidal cross sections are shown in Figure 1d. Non-rectangular cross section has allowed flow lithography to produce polymeric particles in interesting shapes that were not possible with conventional rectangular cross sections such as tetrahedrals and half-pyramids. Unlike the traditional rectangular microfluidic channels producing mere extrusions of 2-dimensional shapes, non-rectangular channels produced particles with variations in all three spatial dimensions.
Control of Side Wall Angles with Simple PDMS Mold Stretching and Capillary Molding
It is possible to achieve further diversification of the particle shapes by a simple Polydimethylsiloxane (PDMS) channel stretching (Figure 2). The elasticity and durability of PDMS allow adjustment of sidewall angles and leads to creation of assorted cross-sectional shapes branched from original non-rectangular cross-sectional molds produced by microfabrication. Stiffness, or Young’s modulus, of PDMS can be easily controlled with the mixture ratio of curing agent to base elastomer41.
We demonstrate PDMS channel stretching up to 100% of its original length. PDMS made with the 1:20 ratio of the curing agent to the base ration was found to provide enough elasticity. First, a soft PDMS block was made and detached from the original non-rectangular channel mold (Figure 2a). After being stretched, the PDMS block was held in place over a glass slide, and the microfluidic channel was then filled with UV-curing adhesive (NOA 81, Norland Products). The UV adhesive was hardened using a UV source with a wavelength of 365 nm (power 6 W, 5 minutes) after which the PDMS was removed from the UV adhesive. Then the UV adhesive structure was used as a new channel mold to fabricate non-rectangular PDMS channels.
During the process of curing the PDMS using the UV adhesive mold, it was observed that some PDMS at the perimeter of the mold remains uncured even after being in the 65 °C oven for extensive lengths of time. Unknown components of UV adhesive were suspected to be acting as inhibitors of the PDMS cross-linking. In order to solve this problem, the channel was washed with various organic solvents. Among which, the widely used organic solvent, acetone damaged UV adhesive surfaces while ethanol and isopropyl alcohol (IPA) failed to rinse the inhibitor away. Fortunately, rinsing with SU-8 developer (propylene glycol monomethyl ether acetate) was found to be effective. SU-8 developer is used when constructing inlets and outlets (I/Os) with additional SU-8 photolithography on top of the UV adhesive structure. The rinsing of the inhibitor can be completed during the SU-8 development step.
The PDMS stretching technique resulted in the various channels shapes shown in (Figure 2b). Cross sections of the channels made with the PDMS stretching are shown in Figure 2b, and the particles made from those channels are shown below the corresponding cross sections. The sidewall angle as small as 30° (90° defined as vertical) could be achieved with a relatively simple method. Tilted exposure lithography could also have provided the method to diversify the triangular and trapezoidal channels. However, the tilted-exposure method required a complex process including anti-reflection coating and has a limited in the tilt angle. Without a special index matching liquid bath, the tilt angle is typically smaller than 45°. The PDMS stretching method was not applied to the semicircular cross-sectioned channels since stretched semicircular channels could be easily fabricated by simply patterning a wider channel during photolithography and reflow.
Generation of Multilayered Particles Using Triangular Channels
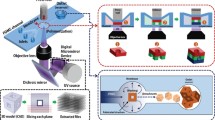
A novel method for fabricating non-spherical multilayered microparticles was developed using a triangular channel with increasing channel cross section where particles were generated and sequentially encapsulated with different materials (Figure 3). The multilayer on-chip PDMS valves were used to control fluid and particle flows42. The main triangular channel was constructed using KOH etching of Si. It is well known that a proper alignment of the etching mask to the Si wafer allows a self-limiting effect which can lead to a triangular trench with <111> surfaces exposed. An etching mask that had long rectangles with three different widths allowed an enlarging triangular trench with three different sizes in a single etching step. The triangular trench was replicated to a PDMS trench after consecutive PDMS replica molding steps. This PDMS trench was then used for replicating the triangular channel mold on a bare Si substrate by capillary molding of UV adhesive. On top of the main triangular channel mold, the branch channel molds including I/Os for fluid exchange were added. Using the mold, we have duplicated the flow channel of the PDMS multilayer device. The flow layer was aligned and bonded to a control layer to form push-up PDMS valves after which the combined channels were plasma-bonded to a glass substrate (Figures. 3a and 3b).
(a) Image of multilayered particle fabrication device with flow channels filled with red dye and pneumatic control valves filled with blue dye (Scale bar 10 mm). (b) Schematic of the multilayered particle procedure. Orange channels represent control valves for exchange inlets/outlets. Purple channels are leaky valves for triangular main flow channels. (c) Schematic of the multi-layered particle fabrication procedure.
Multilayered particles were fabricated through medium replacement using control valves, and micro-particle displacement controlled by leaky valves. In this study, three-layered microparticles of triangular prism shapes were manufactured by using three polymers in total. Poly(ethylene glycol) diacrylate (PEGDA), a photocurable material, was used for microparticle preparation by photopolymerization. Red and green fluorescent dyes were added to the PEGDA to show that each layer has been fabricated through a polymer replacement. The three input channels were injection passages for three photocurable materials. Injection and blocking of liquid were controlled through open and closed control valve actuation. The leaky valves in the middle and final stages served to confine the microparticles inside the channel during the polymer exchange. The leaky valves were designed to incompletely close the channel, allowing liquid to flow properly while stopping the microparticles. The process of fabricating the three-layered 3D microparticles is as follows (Figure 3c1–3c4). With the first control valve and the first output valve open, the polymer of the innermost layer is injected. The fluid is stopped and the particle with triangular cross-section is generated through photo-polymerization in the first stage of the channel (Figure 3c1). The fabricated particle is then moved along with the fluid to the middle leaky valve in the second stage of the channel. Before the particle reaches the center of the second stage, the leaky valve was closed so that the particle gets trapped in the desired location. After the particle is held at the second stage of the channel (Figure 3c2), the second medium is injected with the second control valve and the second output valve open. At this time, the control valves in the first stage remain closed to prevent the second medium from flowing back to the first input channel. When the medium is completely replaced, the middle leaky valve is reopened, and the second layer is cured around the first particle. The particle density is larger than that of uncured medium and the particle settles down to the bottom. Timed exposure after the leaky valve opening allows the outside of the first particle completely wrapped in another material (Figure 3c3). The layered particle is then moved to the final stage of the channel, and the photocurable medium exchange and the photopolymerization reaction is repeated to form the outermost layer (Figure 3c4).
The leaky valves for stopping and holding the particles during the solution exchange and UV-exposure is one of the key element for multilayered particle generations. A push-up type PDMS valve system was adapted to form leaky valves along the main triangular channel. In addition, the push-up valve structure provides bottom PDMS layers which are necessary to form oxygen-aided polymerization inhibition layer as seen thinly surrounding the first fabricated particle cross section in Figure 3b. When pressure is applied to the control channel, the semi-circular I/O parts of the flow channel are completely cut off from all flow. However, the main triangular channel cannot completely close off since the triangular cross section forms leaky valves (Figure 3b). As seen in Figure 4a, the main flow channel with the triangular cross section is placed perpendicular to the set of control valves with widths of 100 μm and 300 μm. As pressure is applied (0.1 Mpa) to the control valves, the overlaying PDMS membranes are pushed up at different heights into the main flow channel due to the differences in membrane area. Both valves do not seal the flow channel and form leaky valves although their functions are different. The larger valve was designed for stopping the flowing particle and the smaller valve was designed for pushing the particle up to the top of the channel.
(a) A set of leaky valves perpendicularly aligned over the main flow channel in open and closed states. (Scale bar 200 μm). The wider valve shows the lager deflection. (b) The simulated maximum deflection of the PDMS membrane at the closed state. Inset: the side-view of the channel with 0.1 MPa applied on the bottom surface of the membrane. (c) 3D aerial view of the membrane deflection with a trapped particle (red).
A finite element method (FEM) simulation of the PDMS channel with 0.1 MPa applied to the control channel membrane is shown in Figures 4b and 4c (COMSOL Multiphysics). As shown in Figure 4b, the small membrane can only deflect 26 μm up to the main flow channel. However, the larger membrane deflects 43 μm into the main flow channel. The valves at rounded I/O channels were completely closed with similar pressure ranges. Figure 4(c) shows a particle pushed against the top channel walls. The triangular cross section of the channel naturally forces the particles to be aligned at the center of the channel (in width direction). Since the particle along with the leaky valves still cannot cover and block the entire cross section of the main flow channel, the photocurable solution around the particle can flow through and be exchanged while the particle is being held in place. Once the particles were positioned at the designated location and the solution had been exchanged, the leaky valves were released and the particle was allowed to settle. Since the density of the cross-linked particle is larger than the surrounding photopolymer solution, the particle slowly moves down to the bottom of the channel. A timed polymerization of the exchanged solution 10 seconds after valve release can allow the exchanged photocurable solution to polymerize around the initial particle to completely envelop the particle with another polymer.
The production time of the three-layered particles is determined by four factors: fluid exchange, photo-polymerization, particle movement, and position alignment. There have been attempts to fabricate multi-material microparticles using laminar flow in a microfluidic channel. In these works, photocuring time and microparticle treatment time were general factors affecting the efficiency. However, in this study, the production time was increased because fluid exchange systems were added to produce microparticles with multiple layers. We installed a leaky valve in the intermediate and output stages of the channel to exchange fluids at the same time as fixing the microparticles. When the microparticle was placed in the leaky valve, the fluid flow was decreased, resulting in an increase of liquid exchange time. As a result, it took up to ~ 30 seconds for fluid exchange in both stages of the channel. Fluid exchange time was relatively long considering that the time required for photocuring, particle movement, and position alignment was several tens to several hundreds of milliseconds. Therefore, the total time needed for three-layered particle production was ~ 2 min/particle. Parallelization of the microfluidic device would easily help increase the throughput. A new design of the leaky valve for an acceleration of the fluid exchange is expected to solve the issue of low throughput.
A typical method for producing multilayered microparticles is the evaporation solvent method. While the technique has the advantage of being capable of mass production, it is difficult to form an entirely uniform size, and its shape is restricted to a spherical shape. On the other hand, our method showed that it is possible to fabricate non-spherical multilayered microparticle by changing channel dimension and UV pattern. As a result, one microfluidic channel device can be used to fabricate single-, two-, or three-layered microparticles, and the results have uniform size and shape. We expect to be able to fabricate microparticles with more than three layers by adding the input channel of the device. The final fabricated polymeric multilayered particle, as shown in Figure 5, contains three layers of polymerized materials each layer containing different types of fluorescent dyes (red, clear, green in order from outer to inner). The width of the particle was designed to be 90 μm for the inner layer, 115 μm for the middle, and 140 μm for the outer layer as the schematics show in Figure 5a. Since the geometry of the triangular cross section was fixed at an isosceles triangle with 54.7°, 54.7°, 70.6° angles, increasing the designated width of the main flow channel produced a predictable shape of triangular cross sections that could envelop each other. The vertical position of the particle can be controlled by adjusting the moment of the UV exposure during particle settling down. For better accuracy of the inner particle position, it would be preferred to reduce the rate at which the particle sediments to the floor, which could be achieved by controlling the density differences between monomer solution and the cured particle. With the proper desired polymers, the process of multilayered particle generation would enable polymeric particles to have many different numbers of solutions inside its structure as shown in Figures 5b–5c. The composition of the particles is only limited by their monomer/polymer compatibility meaning any two materials which do not chemically react each other can be used for layers of the multilayer particles.
(a) 3D rendering schematic of a multilayered particle fabricated by the multilayered particle device. (b) Bright field image of a fabricated particle from top view (xy plane) with the boundaries between different parts of the particle clearly visible. (c) The fluorescent imaging of the particle coupled with an orthogonal image taken from confocal imaging. (Scale bar: 100 μm).
Conclusion
We developed a novel flow lithography technique combined with non-rectangular cross section microchannels that can result in microparticles with unique and diverse shapes. Utilizing widely used micro- fabrication techniques, we have fabricated channels of semi-circular, triangular, and trapezoidal cross-sections. Particle shapes generated by conventional flow lithography techniques are limited to 2D extrusion of the shape defined by a photomask. Non-rectangular cross section channel allows a set of interesting particle shapes such as half-pyramids or tetrahedrals. The geometry of previously fabricated channels was also changed by stretching the PDMS channel up to ~100% of the original size, which allowed further diversified the range of possible shapes. Most importantly, flow lithography in enlarging triangular channel allows generation of the multilayered particle, which has not been possible with conventional flow lithography methods. On-chip PDMS valves controlled particle movements and solution exchanges. The leaky valves were designed for particle capture and alignment while allowing flow exchange around the captured particle. With the self-alignment added by triangular cross section and timed UV-exposure, complete enveloping of the captured particles within the outer layer was achieved. The current device only demonstrated three layers but the number of layers can be easily increased. The non-spherical multilayered particles are expected to provide unique solutions in the field of drug delivery and tissue engineering. Since the sizes of independent compartments within the multilayered particle could be designed and controlled to desired numbers, a particle with set distances and amount of materials in between compartments could be synthesized to be used in timed drug-delivery. The other 3D microparticles could be used in studies such as effects of different surface topology on cell growth or culturing. The variety of packing densities that 3D microparticles offer could also provide unique structures and interesting insight on tissue engineering or cell culture scaffolds.
Materials and Methods
Fabrications of Non-rectangular Channel Molds
The mold with semi-circular cross section was made by reflow of a positive PR (AZ 9260, MicroChemicals) at 130 °C after lithographic patterning of rectangular channels.
KOH etching of SiN wafers (at 80 °C with 30% (w/w) KOH solution) was used to achieve isosceles triangular cross section trenches with apex angle 70.6°. A PDMS block molded from the Si trench can be served as a PDMS channel mold directly. In this case, coating with surfactant (2% Micro-90, Micro-90) is required for easy detachment of the two PDMS layers. The PDMS mold can be easily torn at the sharp edge regions. Alternatively, a hard, UV adhesive (NOA 81, Norland Products) mold can be replicated on a Si wafer or a glass slide by capillary molding with the PDMS channel. A UV-mask aligner with a customized photomask stage with tilt function was used to expose PR (AZ50XT, AZ electronic materials) at tilted angles. An anti-reflection coating (AZ Barli II, Microchemicals) was used to minimize the UV-light reflected from the wafer surface.
Fabrication of PDMS Molds of a Device for Multilayered Microparticle
The main triangular channel was first constructed with UV adhesive. An enlarging triangular trench with three different sizes was patterned on Si using KOH etching. Then a triangular PDMS trench was made by PDMS-PDMS replica molding. This PDMS trench was then used for replicating the triangular channel shape on a bare Si substrate by capillary molding of UV adhesive. After the fabrication of the main flow channel, branch channels working as I/Os for solution exchange and particle collection outlet were patterned using positive PR (AZ 9260, MicroChemicals). These branch channels were then rounded at 130 °C to allow on-chip PDMS valves to close off completely. The control layer was patterned using SU-8 at a height of 50 μm. PDMS mixture of ratio 1:20 was spin-coated onto the control layer wafer at 1250 rpm for 45 seconds to achieve a height of 70 μm. Meanwhile, a PDMS mixture of ratio 1:5 was poured onto the main flow channel wafer and degassed for 10 minutes. Then both layers were cured in an 80 °C oven for 30 minutes. Then the flow layer PDMS block was cut out and I/O holes were punched. The flow layer was aligned onto the control layer and the combined flow and control layers were then further cured for >1 hour in 80 °C for stronger bonding. The multilayer PDMS channels were then cut out of the wafer and control channel inlet holes were made. The entire device was then plasma bonded onto a glass slide.
Flow Lithographic Generation of 3D Microparticles
The microparticles were produced by a stop-flow process using an OFML system. The OFML system is composed of a Digital Micromirror Device (DMD, W4100 0.7" XGA, Wintech, USA), an Ultraviolet (UV) source (LC-8, Hamamatsu, Japan), an inverted microscope (IX-73, Olympus, Japan), and a Microfluidic Flow Control System (MFCS-EZ, Fluigent, France) (Figure 3a). The DMD reflects UV rays and forms a pattern. In this case, the UV pattern is determined by the digital image (1024 × 728 pixels) entered in the DMD. By changing the digital image entered in the DMD, the shape of the UV pattern can be changed. The structure of the microparticle changes with the shape of the UV pattern irradiated on the PDMS microfluidic channel. The UV pattern induces the crosslinking of a photocurable solution. In this research, a photocurable solution which combined polyethylene glycol diacrylate (PEGDA, Sigma-Aldrich, USA) and 2-Hydroxy-2-methylpropiophenone (irgacure 1173, Sigma-Aldrich, USA) in the volume ratio of 10% are used. A resulting particle has a molecular weight of 575 g/mol. The UV pattern passes the X10 objective lens of the inverted microscope and irradiated on the surface of the PDMS microfluidic channel. The maximum energy of the UV pattern which passes the objective lens is 900 mW/cm2. The irradiation time of the UV pattern is controlled in the unit of ms following the on/off time interval of the digital image entered in the DMD. A microparticle is produced by the stop-flow process (Figure 3(b)). The stop-flow is realized using the MFCS controlling the air pressure. If the photocurable solution stops inside the PDMS microfluidic channel due to the MFCS, a microparticle is produced by irradiating the UV pattern. The produced microparticle flows to the direction of the outlet due to the flow formed again and is collected. The MFCS stops the flow again to produce the next microparticle. This process is repeated with the stop (1000 ms), the microparticle fabrication (100 ms), and the flow (500 ms) and microparticles are produced consecutively.
References
Jang, J.H., Dendukuri, D., Hatton, T.A., Thomas, E.L. & Doyle, P.S. A route to three-dimensional structures in a microfluidic device: Stop-flow interference lithography. Angew. Chem., Int. Ed. Engl. 46, 9027–9031 (2007).
Dendukuri, D. & Doyle, P.S. The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater. 21, 4071–4086 (2009).
Dendukuri, D., Pregibon, D.C., Collins, J., Hatton, T. A. & Doyle, P.S. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 5, 365–369 (2006).
Dendukuri, D., Gu, S.S., Pregibon, D.C., Hatton, T. A. & Doyle, P.S. Stop-flow lithography in a microfluidic device. Lab Chip. 7, 818–828 (2007).
Champion, J.A., Katare, Y.K. & Mitragotri, S. Particle shape: A new design parameter for microand nanoscale drug delivery carriers. J. Controlled Release. 121, 3–9 (2007).
Paulsen, K.S., Di Carlo, D. & Chung, A.J. Optofluidic fabrication for 3D-shaped particles. Nat. Commun. 6, 6976 (2015).
Walther, A. & Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 113, 5194–5261 (2013).
Bong, K.W., Pregibon, D.C. & Doyle, P.S. Lock release lithography for 3D and composite microparticles. Lab Chip. 9, 863–866 (2009).
Peppas, N.A., Hilt, J.Z., Khademhosseini, A. & Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360 (2006).
Parekh, N., Hushye, C., Warunkar, S., Sen Gupta, S. & Nisal, A. In vitro study of novel microparticle based silk fibroin scaffold with osteoblast-like cells for load- bearing osteo-regenerative applications. RSC Adv. 7, 26551–26558 (2017).
Chen, Q., Dong, C., Jing, W. & Jin-Ming, L. Flexible control of cellular encapsulation, permeability, and release in a droplet- templated bifunctional copolymer scaffold. Biomicrofluidics 10, 064115 (2016).
Murthy, B.R.S., Ramanathan, G. & Sivagnanam, U.T. Fabrication of chitosan microparticles loaded in chitosan and poly (vinyl alcohol) scaffolds for tissue engineering application. Bull. Mater. Sci. 40, 645–653 (2017).
LaVan, D.A, McGuire, T. & Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 21, 1184–1191 (2003).
Yu, X., Khalil, A., Dang, P.N., Alsberg, E. & Murphy, W.L. Multilayered Inorganic Microparticles for Tunable Dual Growth Factor Delivery. Adv. Funct. Mater. 24, 3082–3093 (2014).
Lee, W.L. & Loo, S.C.J. Revolutionizing drug delivery through biodegradable multilayered particles evolutionizing drug delivery through biodegradable multilayered particles. J. Drug Targeting 2330, 632–647 (2017).
Li, W.L., Yu, P., Hong, M., Widjaja, E. & Loo, S.C.J. Designing multilayered particulate systems for tunable drug release profile. Acta Biomater. 8, 2271–2278 (2012).
Santhosh, K.T., Alizadeh, A. & Karimi-abdolrezaee, S. Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury. J. Controlled Release 261, 147–162 (2017).
Kim, M.A., Yoon, S.D. & Lee, C.-M. A drug release system induced by near infrared laser using alginate microparticles containing melanin. Int. J. Biol. Macromol. 103, 839–844 (2017).
Wang, W., Zhang, M.J. & Chu, L.Y. Functional polymeric microparticles engineered from controllable microfluidic emulsions. Acc. Chem. Res. 47, 373–384 (2014).
Nisisako, T. Recent advances in microfluidic production of Janus droplets and particles. Curr. Opin. Colloid Interface Sci. 25, 1–12 (2016).
Utada, A.S., Fernandez-Nieves, A., Stone, H.A. & Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 99, 1–4 (2007).
Choi, N.W., Kim, J., Chapin, S.C., Duong, T., Donohue, E., Pandey, P., Broom, W., Hill, W.A. & Doyle, P.S. Multiplexed detection of mRNA using porosity-tuned hydrogel microparticles. Anal. Chem. 84, 9370–9378 (2012).
Teh, S.-Y., Lin, R., Hung, L.-H. & Lee, A.P. Droplet microfluidics. Lab Chip. 8, 198–220 (2008).
Shum, H.C., Abate, A.R., Lee, D., Studart, A.R., Wang, B., Chen, C.H., Thiele, J., Shah, R.K., Krummel, A. & Weitz, D.A. Droplet microfluidics for fabrication of non-spherical particles. Macromol. Rapid Commun. 31, 108–118 (2010).
Wurm, F. & Kilbinger, A.F.M. Polymeric janus particles. Angew. Chem. Int. Ed. 48, 8412–8421 (2009).
Lee, S.A., Chung, S.E., Park, W., Lee, S.H. & Kwon, S. Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography. Lab Chip. 9, 1670–1675 (2009).
Donev, A., Cisse, I., Sachs, D., Variano, E.A., Stillinger, F.H., Connelly, R., Torquato, S. & Chaikin, P.M. Improving the Density of Jammed Disordered Packings Using Ellipsoids. Science 303, 990–993 (2004).
Vukusic, P. & Sambles, J.R. Photonic structures in biology. Nature. 424, 852–855 (2003).
Champion, J.A, Katare, Y.K. & Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. U. S. A. 104, 11901–11904 (2007).
Song, S.-H., Kim, K., Choi, S.-E, Han, S., Lee, H.-S., Kwon, S. & Park, W. Fine-tuned grayscale optofluidic maskless lithography for three-dimensional freeform shape microstructure fabrication. Opt. Lett. 39, 5162–5165 (2014).
Haghgooie, R., Toner, M. & Doyle, P.S. Squishy Non-Spherical Hydrogel Microparticlesa. Macromol. Rapid Commun. 31, 128–134 (2010).
El-Sherbiny, I.M., El-baz, N.M. & Yacoub, M.H. Inhaled nano- and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 1–14 (2015).
Chen, J., Clay, N. & Kong, H. Non-Spherical Particles for Targeted Drug Delivery. Chem. Eng. Sci. 125, 20–24 (2015).
Shepherd, R.F., Panda, P., Bao, Z., Sandhage, K.H., Hatton, T.A., Lewis, J.A. & Doyle, P.S. Stop-flow lithography of colloidal, glass, and silicon microcomponents. Adv. Mater. 20, 4734–4739 (2008).
Baah, D., Donnell, T., Srinivasan, S. & Floyd-smith, T. Stop Flow Lithography Synthesis and Characterization of Structured Microparticles. J. Nanomater. 2014, 142929 (2014).
Lu, Y., Yin, Y. & Xia, Y. Three-dimensional photonic crystals with non-spherical colloids as building blocks. Adv. Mater. 13, 415–420 (2001).
Pregibon, D.C., Toner, M. & Doyle, P.S. Multifunctional encoded particles for high-throughput biomolecule analysis. Science. 315, 1393–1396 (2007).
Baule, A. & Makse, H.A. Fundamental challenges in packing problems: From spherical to non-spherical particles. Soft Matter 10, 4423–4429 (2014).
Kim, J., Lee, J., Wu, C., Nam, S., Di Carlo, D. & Lee, W. Inertial focusing in non-rectangular crosssection microchannels and manipulation of accessible focusing positions. Lab Chip. 16, 992–1001 (2016).
Koh, J., Kim, J., Shin, J.H. & Lee, W. Fabrication and integration of microprism mirrors for high-speed three-dimensional measurement in inertial microfluidic system. Appl. Phys. Lett. 105, 114103, (2017).
Wang, Z., Volinsky, A.A. & Gallant, N.D. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J. Appl. Polym. Sci. 131, 41050, 1–4 (2014).
Studer, V., Hang, G., Pandolfi, A., Ortiz, M., Anderson, W.F. & Quake, S.R. Scaling properties of a low-actuation pressure microfluidic valve. J. Appl. Phys. 95, 393–398 (2004).
Acknowledgements
This work was supported by the Radiation Technology R&D program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2015M2A2A4A02044826) as well as the Advanced Research Program of National Research Foundation of Korea (NRF-2017R1A2B4005933).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interests
The authors declare no competing financial interests.
These authors contrilbuted equally.
Rights and permissions
About this article
Cite this article
Nam, S.M., Kim, K., Kang, IS. et al. Generation of 3D Microparticles in Microchannels with Non-rectangular Cross Sections. BioChip J 13, 226–235 (2019). https://doi.org/10.1007/s13206-019-3308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-019-3308-2