Abstract
The production of second-generation bioethanol has several challenges, among them finding cheap and efficient enzymes for a sustainable process. In this work, we analyzed two native fungi, Cladosporium cladosporioides and Penicillium funiculosum, as a source of cellulolytic enzyme production, and corn stover, wheat bran, chickpeas, and bean straw as a carbon source in two fermentation systems: submerged and solid fermentation. Corn stover was selected for cellulase production in both fermentation systems, because we found the highest enzymatic activities when carboxymethyl cellulase activity (CMCase) was assessed using CMC as substrate. C. cladosporioides showed the highest CMCase activity (1.6 U/mL), while P. funiculosum had the highest filter paper activity (Fpase) (0.39 U/mL). The ß-glucosidase activities produced by both fungi were similar in submerged fermentation using corn stover as substrate. Through in-gel zymography, three polypeptides with cellulolytic activities were identified in each fungus: with molecular weights of ~ 38, 45 and 70 kDa in C. cladosporioides and ~ 21, 63 and 100 kDa in P. funiculosum. The best results for saccharification (10.11 g/L of reducing sugars) of diluted acid pretreated corn stover were obtained after 36 h of the hydrolytic process at pH 5 and 50 °C using the enzyme extract of P. funiculosum. This is the first report of cellulase identification in C. cladosporioides and the saccharification of corn stover using enzymes of this fungus. Enzymatic extracts of C. cladosporioides and P. funiculosum obtained from low-cost lignocellulosic biomass have great potential for use in the production of second-generation bioethanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of lignocellulosic biomass, found mainly as agricultural residue, is one of the most promising alternatives for the sustainable development of bioenergy production (Niju et al. 2020). Cellulose, present in lignocellulosic biomass, is the most abundant polymer in nature. However, its transformation into bioethanol is an arduous process that involves three main steps: biomass pretreatment, saccharification, and fermentation. So far, the phase of enzymatic saccharification is the one that presents the most critical challenges: the low efficiency of hydrolysis and the high cost of enzyme production are the main obstacles in this process (Obeng et al. 2017).
Cellulases are the enzymes responsible for the hydrolysis of cellulose into soluble sugars; these can be classified into three different types according to their reaction mechanism: endoglucanases (endo-1,4-β-D-glucanase, EC 3.2.1.4), exoglucanases (exo-1,4-β-d-glucanase EC 3.2.1.91), and β-glucosidases (1,4-β- d-glucosidase EC 3.2.1.74) (Liu et al. 2018). In addition to its use to produce biofuels, cellulases have many applications in different areas of industry such as food, detergents, textiles, and paper (Sharma et al. 2016).
The fungi have been used for the industrial production of commercial cellulases—among other metabolites—because they produce a broad spectrum of these enzymes, highlighting the genera Trichoderma, Penicillium, Fusarium, and Aspergillus (Liu et al. 2018; Ferreira et al. 2016). The synthesis of cellulases by microorganisms is affected by various factors, including the components of the culture medium and the conditions of the process. These enzymes are inducible by lignocellulosic, as well as by non-lignocellulosic (e.g., lactose) substrates (Li et al. 2017). It has been reported that the use of non-lignocellulosic substrates leads to obtaining enzymatic preparations with low specific activity, in addition to the high substrate cost, resulting in an economically unfeasible production process on a large scale (Capolupo and Faraco 2016). However, insoluble cellulose materials are considered as the most effective inducers for obtaining cellulases (Jiang et al. 2017).
Agroindustrial waste, lignocellulosic biomass such as sugarcane bagasse, corn cob, and rice bran, has been extensively researched as substrates to induce these enzymes through different fermentation strategies. Due to certain inherent advantages of the cultivation system, submerged fermentation has been the most used process for large-scale production of enzymes (Ravindran and Jaiswal 2016). However, solid-state fermentation has demonstrated great potential for enzyme productions (such as α-amylase, protease, cellulase, pectinase, and xylanase activities). The origin and the physicochemical characteristics of the lignocellulosic substrate affect the microorganism growth as well enzyme production. Among the main limitations of this bioprocess, we could detect heat and mass transfer. This is caused by the physical properties of the medium, such as resilience, cohesiveness, and springiness, constituting the mechanical property index (Imp), As well as the substrate morphology (pore size distribution) (Zhang et al. 2017; Ravindran et al. 2018; Rohrbach and Luterbacher 2021).
Recent reports indicate the potential benefits of the integration of cellulase production with ethanol conversion (Farinas et al. 2018; Lee et al. 2017; Olofsson et al. 2017). The authors propose that the use of cheap lignocellulosic raw materials to produce cellulolytic enzymes and obtaining bioethanol is the most feasible option considering the environmental and economic impact. The integration of the raw material for saccharification and obtaining enzymes could halve greenhouse gas emissions and reduce the cost of second-generation bioethanol, when compared with a system where the cellulase enzymes are purchased (Olofsson et al. 2017).
Therefore, in this research work, two fermentation strategies to produce cellulases were evaluated, from the filamentous fungi Cladosporium cladosporioides and Penicillium funiculosum. Four agricultural residues: corn stover, wheat bran, chickpea, and bean straw were analyzed as substrates to produce cellulases. In 2021, in the Mexican state of Sinaloa, 682,203 ha was used to produce these crops, producing 1–8 tons/ha of residue after harvest as lignocellulosic biomass. Therefore, these are considered highly available and low-cost substrates in this project (data obtained from the Secretariat of Agriculture and Rural Development and Agri-food and Fisheries Information Service (SIAP) on the website https://www.gob.mx/siap). In addition, the characterization of the enzymatic extracts obtained is presented and their use in the bioconversion of corn stover to fermentable sugars. To our knowledge, this is the first report in the literature that evaluated cellulase production using two native fungi of P. funiculosum and C. cladosporioides on chickpea straw and bean straw as substrate. Characterization of the enzymatic extracts and saccharification process using corn stover as a carbon source using C. cladosporioides.
Materials and methods
Microorganisms and maintenance
Penicillium funiculosum (GenBank accession number: KR185322) and Cladosporium cladosporioides (GenBank accession number: KR185324) were obtained from the microorganism collection of the Bioenergetics Laboratory of the Department of Agricultural Biotechnology at IPN, CIIDIR Sinaloa (Mexico). Strains were isolated from moringa straw (Vázquez-Montoya et al. 2020). For their preservation, the fungi were maintained on potato dextrose agar (PDA) at 4 °C and transferred to a new medium every 2 months.
Inoculum preparation
P. funiculosum: The fungus was grown on PDA plates and incubated at 30 ºC for 7 days. C. cladosporioides. The fungus was grown on PDA plates and incubated at 25 °C for 7 days. In submerged fermentation, the inoculum consisted of 5 mm diameter discs cut from grown fungus. In the other case, a suspension of spores (1 × 106 spores/mL) prepared in sterile 0.1% (v/v) Tween-80 solution was used as inoculum in the solid-state fermentation.
Culture media and substrates
CM-1 medium for P. funiculosum Composition of the mineral medium per liter proposed by Gomaa (2013): 1 g KH2PO4, 0.7 g MgSO4, 0.5 g NaCl, 0.7 g FeSO4.H2O, 0.3 g NH4NO3, 0.3 g MnS04.H2O. This culture medium was used for the growth of P. funiculosum.
CM-2 medium for C. cladosporioides Prepared according to Mandels and Weber (1969), composition per liter: 1 g KH2PO4, 0.2 g CaCl2.2H2O, 0.15 g urea, 0.125 g peptone, 0.125 g yeast extract, 0.7 g (NH4)2SO4, 0.0025 g FeSO4.H2O, 0.008 g MnS04.H2O, 0.007 g ZnSO4.7H2O, 0.01 g CoCl2.6H2O, 0.15 g MgSO4.7H20.
Substrates for cellulase production
Agroindustrial waste
The residues of wheat bran, corn stover, bean, and chickpea straw were collected in local agricultural fields in Guasave, Mexico. These were washed with water and then placed in a drying oven at 60 °C overnight to remove moisture. Subsequently, the material was milled and sieved to obtain 1 mm particle size. Each sample of the agricultural residue was individually placed in a plastic bag and stored under suitable conditions until later use. The mineral media were supplemented with agricultural powder residue as lignocellulosic substrate.
Submerged fermentation (SmF)
SmF was carried out in 250 mL Erlenmeyer flasks, containing 25 mL culture medium and the pH was adjusted to 5 before sterilization (121 °C for 15 min). The flasks were inoculated under aseptic conditions and incubated for 5 days. The microorganisms were incubated as follows: P. funiculosum at 30 °C, 200 rpm, and C. cladosporioides at 25 °C, 150 rpm. An aliquot was taken every 24 h, the cultures were filtered using filter paper (Whatman No. 1) to separate the cells. The filtrates were used as a crude enzymatic extract for the analysis of enzyme activity.
Solid-state fermentation (SSF)
SSF was performed in Erlenmeyer flasks (250 mL) containing 1 g untreated agricultural residue as the substrate and 10 mL mineral medium. The flasks were sterilized in an autoclave and, after cooling, inoculated under aseptic conditions. The microorganisms were incubated at 30 °C (P. funiculosum) and 25 °C (C. cladosporioides) for 5 days under static conditions. Samples were taken every 24 h and enzyme extraction was used for enzymatic activity analysis. Phosphate buffer (50 mM, pH 5.0) was added to the fermented substrate up to a total volume of 25 mL and mixed for 1 h on a rotary shaker at 200 rpm. The suspension was centrifuged at 4000g, 20 min at RT, and the supernatant was used as a crude enzymatic extract for enzymatic activity analysis.
Determination of enzymatic activity
The detection of cellulase activity in crude extracts were assessed using the agar-CMC diffusion method (Begum and Alimon 2011). The agar plate was prepared with 1% (w/v) carboxymethylcellulose and 2% (w/v) agar. After solidification, wells (5 mm diameter) were aseptically formed. The wells were filled with 100 μL of the crude extract sample or the corresponding negative control and incubated at 30 °C for 2 days. The plates were then stained with 1% (w/v) Congo red solution for 15 min and decolorized with 1 M NaCl for 15 min.
The endoglucanase (CMCase) activity was determined following the protocol previously described (Miller 1959) with some modifications. Briefly, 0.2 mL of 1% (w/v) carboxymethyl cellulose (CMC) in acetate buffer (50 mM, pH5.0) was mixed with 0.05 mL of the enzyme extract. The reaction mixture was incubated at 50 °C for 10 min and subsequently, to quantify released sugars, 0.25 mL dinitrosalicylic acid (DNS) reagent was added, and the mixture was placed in a heating block at 100 °C for 10 min. After cooling, the absorbance was measured at 540 nm (Miller 1959). Enzyme and substrate blanks were analyzed simultaneously with the samples. One unit of CMCase activity was defined as the amount of enzyme required to release 1 μmol of reducing sugar per minute of reaction under the assay conditions.
Filter paper activity (FPase) was determined by the modified method of Ghose (1987). First, 0.2 mL 0.1 M acetate buffer (pH 5.0) and 12.5 mg of filter paper strips (Whatman No.1) were placed in the test tube. Then, 0.05 mL of cellulase extract was added. The mixture was incubated at 50 °C for 50 min. The reducing sugars released were determined by the DNS method (Miller, 1969).
β-Glucosidase activity was quantified by a spectrophotometric assay described by Joo et al. (2010) using p-nitrophenyl-glucopyranoside (pNPG) as a substrate. Briefly, the reaction mixture consisted of 0.05 mL enzyme extract and 0.2 mL 0.01 M pNPG in 0.1 M acetate buffer (pH 5.0). The hydrolytic reaction was carried out at 50 °C for 10 min, and then 0.25 mL 0.1 M Na2CO3 was added. The absorbance of the released p-nitrophenol was measured at 420 nm. One unit of β-glucosidase activity was defined as the amount of enzyme that releases 1 μmol p-nitrophenol per minute under the assay conditions.
Determination of protein concentration
Protein concentration was determined using the Bradford method (1976), with a commercial protein quantification kit (Bio-Rad Laboratories, Richmond, CA), according to the manufacturer's instructions. Bovine serum albumin was used as a protein standard to construct the standard curve. Each activity and protein determination were carried out in three independent trials.
Electrophoresis and zymography characterization
The proteins in the crude enzyme extracts were precipitated by the addition of ammonium sulfate to obtain 90% of saturation. An appropriate amount of ammonium sulfate was added to the supernatant and kept overnight at 4 °C. The precipitates were separated from the supernatant after centrifugation at 12,000 rpm for 20 min at 4 °C. The proteins were dissolved in 0.1 M acetate buffer, pH 5.0. The separation of the precipitated proteins was carried out using 12% acrylamide gels under denaturing conditions (SDS-PAGE) as described by Laemmli (1970). The relative molecular weights were determined by comparing their mobility with two molecular weight markers: PageRuler unstained broad range protein ladder (size range 5–250 kDa) from Thermo Scientific, and broad molecular weight standards (size range 6.5–200 kDa) from Bio-Rad. The relative position of the bands was analyzed using a Bio-Rad gel documentation system.
The cellulolytic activity of extract was determined in the acrylamide gel (SDS-PAGE 12%) copolymerized with 0.2% (w/v) CMC (de Almeida et al. 2013). After electrophoresis, part of the gel was stained with Coomassie blue, and the rest was used in a zymogram for the detection of enzymatic activity in situ. For zymography, at the end of the electrophoresis, the enzymes were renatured by washing the acrylamide gel with 0.1 M phosphate buffer (pH 5.0) for 1 h with constant agitation at room temperature. A second wash was performed on the same phosphate buffer containing 5% (v/v) Triton X-100 under the same conditions and the initial wash step was repeated. Substrate hydrolysis was performed by incubating the gel in a sodium acetate buffer at 30 °C overnight. After hydrolysis, the gel was stained with 0.1% (w/v) Congo red and destained by washing with 1 M NaCl. The gel was washed with 5% (w/v) acetic acid to increase the contrast with the background. Endoglucanase activity was visible as a clear band.
Effect of pH and temperature on CMCase activity and stability
The optimum temperature of CMCase activity was determined using CMC as a substrate at different temperatures (25, 30, 40, 50, 60 and 70 °C) at pH 5.0. The stability of the enzyme extract was assayed by incubation of 0.05 mL enzyme solution for 1 h in a 50 mM phosphate buffer, pH 5.0, at the corresponding temperatures. The residual CMCase activity was measured by the previously described assay. The optimum pH for the CMCase activity was determined at 50 °C in buffer solutions with pH values of 3–10 (interval of 1 unit): 0.05 M sodium acetate for pH 3, 4 and 5, 0.05 M potassium phosphate for pH 6 and 7, 0.05 M Tris–HCl for pH 8, and 0.05 M glycine–NaOH for pH 9 and 10. The effect of pH on the stability of the enzyme was analyzed by preincubation of the enzyme extract for 24 h at 25 °C in different buffer solutions (pH 3–9). In the end, the residual CMCase activity was evaluated as described above.
Corn stover as a substrate for saccharification
The pretreatment of lignocellulosic biomass is essential for the saccharification process. The corn stover was washed with water and then dried at 60 °C on a tray at 0.25 g/cm2 overnight, then the sample was ground to reduce its size to 1 mm. Pretreatment was carried out through an autoclave process in screw cap bottles with screw caps using a 1:10 solid–liquid ratio, and H2SO4 was diluted at 2% (v/v) for 40 min at 121 °C. The diluted acid pretreatment shows better results compared to alkaline pretreatment (unpublished results). Upon completion, the pretreated biomass was washed with water until neutralized.
For the saccharification step, the crude enzymatic extracts of each fungus were subjected to a lyophilization process. The enzymatic hydrolysis was carried out in a 50 mL Erlenmeyer flask, containing 1 g pretreated stover, 10 mL 50 mM acetate buffer (pH 5.0), and 300 mg lyophilized enzyme extract (protein base). The saccharification reaction was incubated at 50° C and stirred at 150 rpm for 48 h. Samples were periodically taken to analyze the release of reducing sugars using the DNS method (Miller 1959). The percentage of saccharification was calculated with the following equation (Meena et al. 2018):
Statistical analysis
All results are presented as the mean with standard deviations of three independent experiments performed in triplicate. Statistical analysis data from the degradation of cellulose substrates and enzymatic assays were subjected to analysis of variance (ANOVA) using the Design Expert v 13 program. Tukey’s test was used for the post hoc comparison of means (p ≤ 0.05).
Results and discussion
CMCase activity production under SmF and SSF and valorization of agricultural residues
The use of different human activity residues to produce energy is one of the greatest challenges for a sustainable development. The use of waste materials is of great importance, either for reducing the production cost of industrial products already available or for the establishment of new processes. Several examples of waste usage for energy production can be found, i.e., in Indonesia, animal waste for biogas production, can be potentially utilized to generate electric power (Khalil et al. 2019); food waste from municipal solid was evaluated as a substrate for a bioelectrochemical technology (microbial fuel cells), and the authors state that the energy generated by this system could be harvested, demonstrating the potential of this strategy (Khalil et al. 2019). In Bangladesh, a study states that the promotion of biogas generation incorporated into a sustainable circular economy strategy could result in a drop of 37.5% of greenhouse gas emission compared to current business situations (Islam et al. 2021). In this context, the integral use of agricultural waste for the production of biofuels and metabolites of interest is a sustainable alternative to the use of non-renewable resources of fossil origin. Lignocellulosic technology for bioethanol production is characterized by the low cost of the raw material and also avoiding direct conflict with food and feed markets (Al-Azkawi et al. 2019).
The production of cellulolytic enzymes and their application in cellulose degradation from lignocellulosic biomass of agricultural residues is a focus of interest for researchers around the world. Considering the high impact that the cost of enzyme production has over the economic feasibility of the production process of second-generation bioethanol (Maitan-Alfenas et al. 2015; Bertacchi et al. 2022), we evaluated two methods and four alternative low-cost sources as substrates for the production of cellulases from filamentous fungi.
The cultivation of both P. funiculosum and C. cladosporioides were analyzed in mineral media CM-1 and CM-2, respectively, supplemented with natural cellulose from different agricultural residues as the sole source of carbon. The fungi were grown on solid-state fermentation (SSF) and submerged fermentation (SmF) for 7 days, taking samples every 24 h. Figure 1a shows the qualitative determination of cellulolytic activity by staining with Congo red. The characteristic hydrolysis halos of CMC were observed as a result of the cellulolytic enzymatic activity produced by the fungi culture in both SFF and SmF.
The cellulolytic enzymatic activity was determined by culture volume to compare the two fermentation processes (SmF vs SSF). After quantification of endoglucanase (CMase) activity by spectrophotometric assays using CMC as the substrate, a differing influence of agricultural residues on the production of CMCase activity was found for C. cladosporioides (Fig. 2 a,b). Among the carbon sources evaluated, the highest CMCase activity was obtained when the fungi were cultivated in a medium supplemented with corn stover in the two types of fermentation process evaluate. In SmF, the maximum CMCase activity (1.6 U/mL) was observed on the 4th day of fermentation, and after that, there was a slow decrease in cellulolytic activity (Fig. 2a). It could be due to degradative processes or to diminishing nutrient components in the culture medium. When the fungus was grown in a culture medium containing chickpea straw, wheat bran or bean straw as a sole carbon source, CMCase activity was also detected, although in a much less extent. In the case of SSF (Fig. 2b), the higher cellulolytic activity (1.5 U/mL) was on day 5 with corn stover as the carbon source, followed by chickpea straw (0.9 U/mL). Similar to C. cladosporioides, the best substrate for CMCase activity production from P. funiculosum was corn stover. With both SmF and SSF, maximum activity (1.5 U/mL) was achieved at 5 days of culture (Fig. 2c,d). Regarding the other carbon sources, in both cultures the enzyme production was similar than with C. cladosporioides. Recently, our group reported the CMCase activity for both fungi, 0.48 U/mL for P. funiculosum and 0.41 U/mL C. cladosporioides, using 1% CMC as substrate in the media (Vazquez-Montoya et al. 2020). It was also reported that the result of CMCase activity of P. funiculosum using moringa biomass as sole carbon source (1.6 U/mL) was similar to that reported in the present work.
CMCase activity in submerged and solid-state fermentation by Cladosporium cladosporioides (a, b, respectively) and Penicillium funiculosum (c, d, respectively) using agricultural waste as the sole carbon source. Data represent the mean (± standard deviation, SD) of three independent experiments, each performed in triplicate. Error bars indicate SD
Media formulation based on agricultural residues are heterogeneous by nature, and nutrient availability varies depending on the raw material used. For each microorganism, the use of certain residues favors the synthesis of specific cellulolytic enzymes that release sugars necessary for development and maintenance of the cell. The production of different enzymes is susceptible to the presence of specific substrates in the media composition (Ravindran and Jaiswal 2016). The obtained results using corn stover as a substrate could be attributed to the particular lignocellulosic composition, its favorable degradability and the presence of certain nutrients in corn stover (Isaac and Abu-Tahon 2015).
There are several reports regarding extracellular CMCase production by fungi such as Aspergillus, Penicillium and Fusarium on corn stover substrate (Panagiotou et al. 2003; Gao et al. 2008; Liu et al. 2011; Jung et al. 2015; Isaac and Abu-Tahon 2015; Sun et al. 2018) under SSF and SmF systems (Table 1). However, no reported data were found in literature about the filamentous fungi C. cladosporioides for corn stover. Mushimiyimana and Tallapragada (2013) reported the optimization of extracellular CMCase production using agricultural wastes (potato peel, onion peel, carrot peel and sugar beet peel). Our results are within the CMCase activity (1.56–2.45 U/ml). Sun et al. (2018) compared cellulase production by Penicillium oxalicum in growing media using different carbon sources. The fungus was cultivated in a liquid medium, exhibiting a higher endoglucanase activity at 5 days of culture; among nature, lignocellulosic material (corn stover, rice straw, wheat bran) and commercial polymeric material (CMC–Na, Avicel) corn stover were found to be the most effective carbon source. Medium optimization was investigated for cellulase production by Penicillium brasilianum KUEB15. The optimal composition of the variable for maximum CMCase activity (1.18 U/mL) was 12.39 g/L of corn stover (Jung et al. 2015).
The different results observed on cellulase productions among both fermentation methods could be due to several factors, such as substrate accessibility and growing rate of the microorganism. The metabolism exhibited by microorganisms is different in SSF and SmF, and the influx of nutrients and efflux of waste materials need to be carried out based on these metabolic parameters. Therefore, the fermentation technique must be decided based on the microorganism that is being used for production (Subramaniyam, R., and Vimala, R., 2012).
The results obtained show the feasibility of the production of cellulolytic enzymes by C. cladosporioides and P. funiculosum, with either of the fermentation methods, submerged and solid. However, considering that the measurement of process and scaling-up parameters are more accessible in submerged than in solid fermentation, we chose SmF to continue the study.
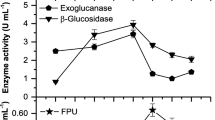
Protein secretion, FPase and β-glucosidase activity
Synergic action of the three enzymes is required to achieve the hydrolysis of the cellulose. To identify FPase and β-glucosidase activity by the fungi, these activities were quantified during fermentation using corn stover as a sole carbon source. Figure 3 shows the kinetic profiles of the extracts produced by the fungi, as well as protein secretion during their 7 days of culture in submerged fermentation. FPase activity in P. funiculosum (0.39 U/mL, day 5, Fig. 3b) reached the highest values than those obtained by C. cladosporioides (0.22 U/mL, day 4,, Fig. 3a); on the other hand, ß-glucosidase activity showed similar values, 0.10 U/mL and 0.13 U/mL, for C. cladosporioides and P. funiculosum, respectively (Fig. 3a,b). The FPase activity for both fungi, P. funiculosum and C. cladosporioides, using 1% CMC as substrate in the media was 0.11 u/mL in both cases (Vazquez-Montoya et al. 2019). It was also reported that the result of FPase activity of P. funiculosum using moringa biomass as the sole carbon source (1.7 U/mL) was higher than that reported in the present work.
Protein secretion, FPase and B-glucosidase activity by (a) Cladosporium cladosporioides and (b) Penicillium funiculosum in submerged fermentation using corn stover as the sole carbon source. C. cladosporioides was grown in CM-2 and P. funiculosum in CM-1. Results represent the mean (± standard deviation SD) of three independent experiments, each performed in triplicate. Error bars indicate SD
The production of FPase and β-glucosidase activities, using corn stover as a substrate by different mutants of Aspergillus terreus AUMC was reported by Issac and Abu-Tahon (2015). FPase activity obtained for the strains ranged from 0.46 to 1.12 U/mL and ß-glucosidase from 0.23 to 0.54 U/mL. Filamentous fungi have a naturally high protein secretion capacity. Proteins are mainly secreted through the growing hyphal tip, making growth and protein secretion intimately linked (Nevalainen and Peterson 2014). In C. cladosporioides, secreted proteins were found since day 1 in the culture medium and this increased until it reached a maximum of 211 μg/mL after 6 days of fermentation (Fig. 3a). In P. funiculosum, a lag phase lasted up to the 2nd day, then the protein content increased up to a maximum on the 6th day (178 μg/mL) (Fig. 3b).
Characterization of the enzymatic extract
Enzymes have pH and a temperature at which their catalytic activity on given substrates is optimal; outside this range, they lose stability due to the breaking of forces that maintain the correct folding of these proteins (affecting the active site or substrate binding sites). The establishment of the best conditions of the enzymatic exposure is essential for its possible application in industry. The enzymatic extract of C. cladosporioides and P. funiculosum was subjected to different pH and temperature conditions, evaluating their effect on CMCase activity and stability. The results obtained are shown in Fig. 4. The optimum temperature of CMCase activity in C. cladosporioides was between 50 and 60 °C (Fig. 4a) and for P. funiculosum 50 °C (Fig. 4c). Thermal stability was found to be more than 70% initial CMCase activity in the temperature range of 25–50 °C for both enzymatic extracts. Above these temperatures, the enzyme activity decreases.
Effect of temperature and pH on relative CMCase activity and stability of Cladosporium cladosporioides (a, b, respectively) and Penicillium funiculosum (c, d, respectively). Results are presented as a percentage of the initial activity. Data represent the mean (± standard deviation, SD) of three independent experiments, each performed in triplicate. Error bars indicate SD
The effect of pH on the CMCase activity of crude extracts was investigated in a pH range of 3.0–10.0. The optimal pH for C. cladosporioides CMCase activity was between pH 4.0–6.0 (Fig. 4b), while in the P. funiculosum extract, the highest activity was observed at pH 4.0 (Fig. 4d). pH stability in both extracts was found to be greater, maintaining more than 80% initial activity in the 4–6 pH range.
Our results coincide with those obtained for most fungal cellulase. Silva et al. (2018) reported that the Botrytis ricini URM 5627 endoglucanase showed the optimum temperature of 50 °C at a pH of 5.0. Characterization of crude cellulases from Aspergillus niger BK01 showed that highest CMCase activity was obtained at pH 4.8 and temperature 40 °C. The CMCase was stable at a pH range of 4.8–5.5 and temperature from 35 to 50 °C (Aggarwal et al. 2017). In another study, a CMCase from Penicillium pinophilum MS 20 had an optimal activity in a pH range of 4.0–7.0 with maximum activity at pH 5 and showed the optimum temperature of 50 °C (Pol et al. 2012).
Enzyme identification by SDS-PAGE
The proteins present in the enzymatic extract of corn stover cultures of C. cladosporioides and P. funiculosum were concentrated by ammonium sulfate precipitation. Subsequently, the proteins were subjected to separation by SDS-PAGE and "in situ" activity analysis using CMC as a substrate. In both extracts, different protein bands were observed; however when analyzing the zymogram, only three bands were detected with cellulolytic activity. In C. cladosporioides, clear bands were visualized in the gel, with molecular weights of ~ 38, 45 and 70 kDa (Fig. 5a), whereas in P. funiculosim they were ~ 21, 63 and 100 kDa (Fig. 5b). Cellulases produced by different species of fungi of the genus Penicillium have been widely studied. Jørgensen et al. (2003) identified five cellulases in Penicillium brasilianum IBT 20,888, two of which had a size comparable to those detected in this work (21 and 63 kDa); the remaining enzymes showed molecular weights of 53, 54, and 70 kDa respectively. Identification analysis showed that the 21 kDa protein is similar to another endoglucanase belonging to the 12 family of glycosyl hydrolases, which is also similar in molecular weight (24 kDa) to the Cel12A cellulase of T. reesei, known for its activity on CMC and Avicel substrates, while the 63 kDa cellulase was identified as a cellobiohydrolase (an exoglucanase). In another work, Zorov et al. (2001) isolated a cellobiase (ß-d-glucosidase) with a molecular weight of 100 kDa from the cellulolytic system of Penicillium verruculosum. This seems to be the first report of the identification of cellulases produced by fungi of the genus Cladosporium using zymogram analysis.
Analysis of secreted protein profile and enzymatic activities of (a) Cladosporium cladosporioides and (b) Penicillium funiculosum grown in medium containing corn stover as the carbon source. SDS-PAGE separated proteins are visualized with Coomassie blue staining (lane 1) and the cellulase activity shown by zymography using CMC visualized using Congo red (lane 2). MWM, molecular weight marker
Corn stover saccharification
Due to the interest in the development of a second-generation bioethanol production process, different agricultural residues have been evaluated to obtain fermentable sugars. In this work, hydrolysis of corn stover pretreated with 2% H2SO4 (v/v), using the cell-free supernatants of previously lyophilized C. cladosporioides and P. funiculosum was analyzed. The kinetic profile of the formation of reducing sugars is shown in Fig. 6. We found the highest production of reducing sugars after 48 h saccharification at 50 °C in both cellulolytic extracts. However, in the reaction with P. funiculosum enzymatic extract (Fig. 6a), the content of reducing sugars released (10.11 g/L) was more than 1.6 times higher than that obtained with C. cladosporium enzymatic extract (Fig. 6b). The results presented here are comparable to those obtained by Begun and Alimon (2011) who evaluated the capacity of different substrates for cellulase induction by A. oryzae ITCC 4857.01, as well as the saccharification thereof by the enzymes obtained. Reducing sugar yields obtained varied from 1.12 g/L using pre-treated sawdust at 48 h saccharification to 7.53 g/L in sugarcane bagasse pretreated with alkali obtained at 96 h. In another work of enzymatic hydrolysis of corn stover pretreated with deep eutectic solvents (consisted of quaternary ammonium salts and hydrogen donors), the authors obtained 17.0 g/L glucose using a commercial enzymatic preparation (ACCELLERASE® 1500) (Xu et al. 2016).
Saccharification of corn stover biomass pretreated with 2% H2S04 (v/v), using enzymatic extracts from (a) Penicillium funiculosum and (b) Cladosporium cladosporioides. Results are presented as reducing sugar yields (circle) and saccharification percentage (triangle). Data represent the mean (± standard deviation SD) of three independent experiments, each performed in triplicate. Error bars indicate SD
Conclusions
In this work, cellulase activity production by C. cladosporioides and P. funiculosum was obtained in both submerged and solid-state fermentation. Under the conditions studies, corn stover was the best lignocellulosic biomass for cellulases production compared to wheat bran, chickpea and bean straw. Characterization of the crude enzymes verified the thermal stability in the temperature range of 25–50 ºC. Three bands with cellulolytic enzymes were identified by SDS-PAGE zymogram analysis after fermentation using corn stover as the only carbon source in the two fungi evaluated. For the fungus C. cladosporioides, it is the first time that this characterization study has been carried out. Also, the ability of the cellulases to hydrolyze corn stover pretreated with sulfuric acid and to obtain fermentable sugars was confirmed in the saccharification process. Finally, corn stover is one of the most abundant agricultural residues in the world and it can be used as raw material of low cost for second-generation bioethanol production (bioethanol 2G) and cellulases production. However, it is necessary to continue with process optimization to increase yields in the processes evaluated.
Data Availability
The raw data of the manuscript will be available up request.
References
Begum M, Alimon AR (2011) Bioconversion and saccharification of some lignocellulosic waste by Aspergillus oryzae ITCC-4857.01 for fermentable sugar production. Elect J Biotechnol 14:1–9
Bertacchi S, Jayaprakash P, Morrissey JP, Branduardi P (2022) Interdependence between lignocellulosic biomasses, enzymatic hydrolysis and yeast cell factories in biorefineries. Microb Biotechnol 15:985–995
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Capolupo L, Faraco V (2016) Green methods of lignocellulose pretreatment for biorefinery development. Appl Microbiol Biotechnol 100:9451–9467
de Almeida MN, Falkoski DL, Guimarães VM, Ramos HJ, Visser EM, Maitan-Alfenas GP, de Rezende ST (2013) Characteristics of free endoglucanase and glycosidase multienzyme complex from Fusarium verticillioides. Bioresour Technol. https://doi.org/10.1016/0003-2697(76)90527-3
Ferreira JA, Mahboubi A, Lennartsoon PR, Taherzadeh MJ (2016) Waste biorefineries using filamentous ascomycetes fungi: present status and future prospects. Bioresour Technol 215:334–345. https://doi.org/10.1016/j.biortech.2016.03.018
Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y (2008) Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terrreus M11 under solid-state cultivation of corn stover. Bioresour Technol 99:7623–7629. https://doi.org/10.1016/j.biortech.2008.02.005
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Gomaa EZ (2013) Optimization and characterization of alkaline protease and carboxymethyl-cellulase produced by Bacillus pumillus grown on Ficus nitida wastes. Braz J Microbiol 44:529–537. https://doi.org/10.1590/S1517-83822013005000048
Isaac GS, Abu-Tahon MA (2015) Enhanced alkaline cellulase production by the thermohalophilic Aspergillus terreus AUMC 10138 mutated by physical and chemical mutagens using corn stover as substrate. Braz J Microbiol 46:1269–1277. https://doi.org/10.1590/S1517-838246420140958
Islam KN, Sarker T, Taghizadeh-Hesary F, Atri AC, Alam MS (2021) Renewable energy generation from livestock waste for a sustainable circular economy in Bangladesh. Renew Sustain Energy Rev 139:110695
Joo A, Jeya M, Lee K, Lee K, Moon H, Kim Y, Lee J (2010) Production and characterization of β-1,4-glucosidase from a strain of Penicillium pinophilum. Process Biochem 45:851–858. https://doi.org/10.1016/j.procbio.2010.02.005
Jørgensen H, Errikson T, Børjesson J, Tjerneld F, Olsson L (2003) Purification and characterization of five cellulases and one xylanase from Penicillium brasilianum IBT 20888. Enzyme Microb Technol 32:851–861. https://doi.org/10.1016/S0141-0229(03)00056-5
Jung DU, Yoo HY, Kim SB, Lee JH, Park C, Kim SW (2015) Optimization of medium composition for enhanced cellulase production by mutant Penicillium brasilianum KUEB15 using the statistical method. J Ind Eng Chem 25:145–150. https://doi.org/10.1016/j.jiec.2014.10.026
Khalil M, Berawi MA, Heryanto R, Rizalie A (2019) Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia. Renew Sustain Energy Rev 105:323–331
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lee H, Lee YM, Heo YM, Lee J, Kim JS, Kang KY, Kim J (2017) Utilization of agricultural residues for enhanced of cellulolytic enzyme production and enzymatic saccharification by Trichoderma harzianum KUC1716. Ind Crop Prod 109:185–191. https://doi.org/10.1016/j.indcrop.2017.08.042
Li C, Lin F, Zhou L, Qin L, Li B, Zhou Z, Jin M, Chen Z (2017) Cellulase hyper-production by Trichoderma reesei mutant SEU-7 on lactose. Biotechnol Biofuels 10:228. https://doi.org/10.1186/s13068-017-0915-9
Liu D, Zhang R, Yang x, Xu H, Xu D, Tang Z, Shen Q, (2011) Thermostable cellulase production of Aspergillus fumigatus Z5 under solid-state fermentation and its applications in degradation of agricultural wastes. Int Biodeterior Biodegradation 56:717–725. https://doi.org/10.1016/j.ibiod.2011.04.005
Liu H, Sun J, Chang JS, Shukla P (2018) Engineering microbes for direct fermentation of cellulose to bioethanol. Crit Rev Biotechnol 38:1089–1105. https://doi.org/10.1080/07388551.2018.1452891
Maitan-Alfenas GP, Visser EM, Guimarães VM (2015) Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr Opin Food Sci 1:44–49
Mandels M, Weber J (1969) The Production of Cellulases Advan Chem 95:391–414. https://doi.org/10.1021/ba-1969-0095.ch023
Meena SS, Sharma V, Gupta S (2018) In vitro optimization of fungal cellulase production from fruit waste for handmade paper industries. Biotechnol 17:35–43. https://doi.org/10.3923/biotech.2018.35.43
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Mushimiyimana I, Tallapragada P (2013) Optimization of process parameters for biosynthesis of cellulase by Cladosporium Cladosporioides using agro wastes. Int J Pharma Bio Sci 4:B1129–B1138
Nevalainen H, Peterson R (2014) Making recombinant proteins in filamentous fungi- are we expecting much? Front Microbiol 5:75. https://doi.org/10.3389/fmicb.2014.00075
Niju S, Swathika M, Balajii M (2020) Pretreatment of lignocellulosic sugarcane leaves and tops for bioethanol production. Lignocellulosic Biomass to Liquid Biofuels 2020:301–324
Obeng EM, Siti NNA, Budiman C, Ongkudon CM, Mass R, Jose J (2017) Lignocellulases: a review of emerging and developing enzymes, systems, and practices. Bioresour Bioprocess 4:16. https://doi.org/10.1186/s40643-017-0146-8
Olofsson J, Barta Z, Börjesson P, Wallberg O (2017) Integrating enzyme fermentation in lignocellulosic ethanol production; life-cycle assessment and techno-economic analysis. Biotechnol Biofuels 10:51. https://doi.org/10.1186/s13068-017-0733-0
Panagiotou G, Kekos D, Macris BJ, Chistakopoulus P (2003) Production of cellulolytic and xylanolytic enzymes by Fusarium oxysporum grown on corn stover in solid state fermentation. Ind Crop Prod 18:37–45. https://doi.org/10.1016/S0926-6690(03)00018-9
Pol D, Laxman RS, Rao M (2012) Purification and biochemical characterization of endoglucanase from Penicillium pinophilum MS 20. Indian J Biochem Biophys 49:189–194
Rohrbach JC, Luterbacher JS (2021) Investigating the Effects of Substrate Morphology and Experimental Conditions on the Enzymatic Hydrolysis of Lignocellulosic Biomass through Modeling. Biotechnol Biofuels 14(1):1–14. https://doi.org/10.1186/s13068-021-01920-2
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179:1346–1380. https://doi.org/10.1007/s12010-016-2070-3
Silva TP, de Albuquerque FS, Dos Santos CWV, Franco M, Caetano LC, Pereira HJV (2018) Production, purification, characterization and applications of a new halotolerant and thermostable endoglucanase of Botrytis ricini URM 5627. Bioresour Technol 270:263–269. https://doi.org/10.1016/j.biortech.2018.09.022
Subramaniyam R, Vimala R (2012) Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat 3(3):480–486
Sun X, Shen BB, Han HY, Lu Y, Zhang BX, Gao YF, Hu BZ, Hu XM (2018) Screening of potential IL-tolerant cellulases and their efficient saccharification of IL-pretreated lignocelluloses. RSC Adv 8:30957–30965. https://doi.org/10.1039/c8ra05729j
Vázquez-Montoya EL, Castro-Ochoa LD, Maldonado-Mendoza IE, Luna-Suárez S, Castro-Martínez C (2020) Moringa straw as cellulase production inducer and cellulolytic fungi source. Rev Argent Microbiol 52:4–12. https://doi.org/10.1016/j.ram.2019.02.005
Xu GC, Ding JC, Han RZ, Dong JJ, Ni Y (2016) Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour Technol 203:364–369. https://doi.org/10.1016/j.biortech.2015.11.002
Zhang Y, Wang L, Chen H (2017) Correlations of Medium Physical Properties and Process Performance in Solid-State Fermentation. Chem Eng Sci 165:65–73. https://doi.org/10.1016/j.ces.2017.02.039
Zorov IN, Gusakov AV, Baraznenok VA, Bekkarevich AO, Okunev ON, Sinitsyn AP, Kondrat’eva EG (2001) Isolation and properties of cellobiase from Penicillium verruculosum. Appl Biochem Micro 37:587–593. https://doi.org/10.1023/A:1012351017032
Aggarwal NK, Goyal V, Saini A, Yadav A, Gupta R (2017) Enzymatic saccharification of pretreated rice straw by cellulases from Aspergillus niger BK01. 3 Biotech 7:158. https://doi.org/10.1007/s13205-017-0755-0
Al‐Azkawi A, Elliston A, Al‐Bahry S, Sivakumar N. (2019) Waste paper to bioethanol: Current and future prospective. Biofuels, Bioprod. Biorefining 1–13
Farinas CS, Florencio C, Badino AC (2018) One-site production of cellulolytic enzymes by the sequential cultivation method. In: Lübeck M (ed) Cellulases. Methods in Molecular Biology, vol 1796. Humana Press, New York, pp273–282. https://doi.org/10.1007/978-1-4939-7877-9_19
Jiang F, Ma L, Cai R. Ma Q, Guo G, Du L, Xiao D (2017) Efficient crude multi-enzyme produced by Trichoderma reesei using corncob for hydrolysis of lignocellulose. 3 Biotech 7:339. https://doi.org/10.1007/s13205-017-0982-4
Ravindran R, Hassab SS, Williams GA, Jaiswal AK (2018) A review on bioconversion of agro-industrial wastes to industrially important enzymes, Bioengineering (Basel) 5:93. https//doi:https://doi.org/10.3390/bioengineering5040093
Ravindran R, Jaiswal AK (2016) Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering (Basel) 3:30. https//doi:https://doi.org/10.3390/bioengineering3040030
Secretariat of Agriculture and Rural Development and Agri-food and Fishing Information Service (SIAP) at the website https://www.gob.mx/siap. Accessed 08 July 2022
Acknowledgements
The authors thank the Mexican National Council for Science and Technology and the Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food (CONACYT-SAGARPA Grant No. 291143) and IPN-SIP (Grant No. 20182335) for support in conducting the research, and thanks also go to Patricia Margaret Hayward-Jones MSc and Dulce María Barradas-Dermitz MSc for their critical reading of the manuscript. Castro-Ochoa L.D. thanks CONACYT for the grant awarded for the completion of the postdoctoral program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castro-Ochoa, L.D., Hernández-Leyva, S.R., Medina-Godoy, S. et al. Integration of agricultural residues as biomass source to saccharification bioprocess and for the production of cellulases from filamentous fungi. 3 Biotech 13, 43 (2023). https://doi.org/10.1007/s13205-022-03444-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03444-4