Abstract
The present study describes the ex-situ production of a biosurfactant by Pseudomonas sp. TMB2 for its potential application in enhancing oil recovery. The physicochemical parameters such as temperature and pH were optimized as 30 °C and 7.2, respectively, for their maximum laboratory scale production in mineral salt medium containing glucose and sodium nitrate as best carbon and nitrogen sources. The surface activity of the resulting culture broth was declined from 71.9 to 33.4 mN/m having the highest emulsification activity against kerosene oil. The extracted biosurfactant was characterized chemically as glycolipid by Fourier-transform infrared spectroscopy and 1H and 13C nuclear magnetic resonance spectroscopy analyses. The presence of mono-rhamnolipids (Rha-C8:2, Rha-C10, Rha-C10-C10, and Rha-C10-C12:1) and di-rhamnolipids (Rha-Rha-C12-C10, Rha-Rha-C10-C10, and Rha-Rha-C10-C12:1) congeners were determined by liquid chromatography-mass spectroscopy analysis. The thermostability and degradation pattern of the candidate biosurfactant were tested by thermogravimetry assay and differential scanning calorimetry studies for its suitability in ex-situ oil recovery technology. The rhamnolipid based slug, prepared in 4000 ppm brine solution reduced the interfacial tension between liquid paraffin oil and aqueous solution to 0.8 mN/m from 39.1 mN/m at critical micelle concentration of 120 mg/L. The flooding test was performed using conventional core plugs belonging to oil producing horizons of Upper Assam Basin and recovered 16.7% of original oil in place after secondary brine flooding with microscopic displacement efficiency of 27.11%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recovery of crude oil from the depleted reservoir is one of the promising areas and stands as major challenges in petroleum industry research due to the rising demand of crude oil in daily life. Conventional primary and secondary oil recovery methods produce nearly 30–40% of oil from the reservoirs (Al-Sulaimani et al. 2011). Initially, natural reservoir pressure is sufficient to produce oil from the oil reservoir as in primary recovery method. As the reservoir pressure declines, secondary methods like water flooding become necessary to enhance recovery efficiency. An anticipated amount of nearly 7 trillion barrels of conventional and heavy oil still remains in the reservoir porous media worldwide after using these conventional technologies (Thomas 2008). Enhanced oil recovery (EOR) is a tertiary oil recovery method and receives more attention as it enables further recovery of trapped oil with the use of external pressure (used CO2, CH4), heat (hot water) and some synthetic chemicals such as surfactants, polymers, etc. But such methods and technologies have come with some difficulties such as high toxicity and production cost to commercialize them. Microbial enhanced oil recovery (MEOR) as an alternative of EOR technologies bears advantages of low toxicity, bioequivalence, biodegradability and economic feasibility for field implementation. In the MEOR technology, microbes and their metabolites including biosurfactants, biopolymers, bio-acids, biosolvents and biogas are used for enhancement of oil recovery.
Because of the amphipathic nature, biosurfactants have gained substantial interest in biotechnological research for developing an efficient MEOR technology. Biosurfactants (BS) are categorized into glycolipid, phospholipids, polymeric biosurfactants and lipopeptides, etc. based on their nature and origin (Banat et al. 2010). Out of them, rhamnolipid (glycolipids) and surfactin (lipopeptide) are the most studied BSs produced by Pseudomonas aeruginosa (Eskandari et al. 2009; Amani et al. 2013) and Bacillus subtilis (Pereira et al. 2013; Joshi et al. 2016) to use in MEOR technology, respectively.
Generally, the hydrophilic head of rhamnolipids is comprised of mono- or di-rhamnose groups, while the hydrophobic tail consists of a long fatty acid chain (Desai and Banat 1997). Therefore, these rhamnolipids can act as an intermediate between oil and water or oil and rock plug to reduce the interfacial tension (IFT) and thereby, tends to reduce the capillary force for the significant recovery of trapped oil (Santos et al. 2016). Because of low toxicity, high biodegradable property, bioavailability and high stability at extreme pH, temperature and salinity, these BSs have gained lots of attention in the oil recovery process (Desai and Banat 1997).
Based on the use of biosurfactant, MEOR technology is divided into two categories: ex-situ and in-situ. In ex-situ MEOR technology, BSs are produced externally in shake-flask medium or bioreactor to inject into the reservoir through a separate well for the reduction of IFT. In other cases, indigenous microorganisms inherent or infused to the reservoirs are induced for augmented production of their metabolites by supplying required nutrients (Al-Sulaimani et al. 2011). Numerous investigations have been reported for the recovery of residual oil from the reservoir using rhamnolipids (Eskandari et al. 2009; Zhao et al. 2016). Amani et al. recovered nearly 27% of residual oil using 120 mg/L of rhamnolipid in sand pack column (Amani et al. 2013), whereas Pseudomonas aeruginosa PBS produced rhamnolipid recovered approximately 56.18 ± 1.59% of additional oil from sand pack column (Sharma et al. 2018). But, very fewer investigations have been reported for the oil recovery study with special reference to the first oil reservoir of India, which belongs to the Upper Assam Basin. Therefore, it is worth reporting a systematic investigation for the recovery of residual oil from Upper Assam based reservoir plugs using a new bacterial strain, isolated from crude oil contaminated soil of Assam.
In this report, the crude rhamnolipids extracted from liquid culture of Pseudomonas sp. TMB2 was used for oil recovery study in Upper Assam (India) reservoir based rock plug. Based on the above literature survey, an optimized strategy was used to produce biosurfactant from a new bacterial strain, Pseudomonas sp. TMB2 and characterized physicochemically to examine its suitability for ex-situ application in MEOR technology. Two fundamental mechanisms, interfacial tension reduction and wettability alteration, were also validated before using the biosurfactant in oil recovery technology.
Materials and methods
Screening of biosurfactant producing bacteria for MEOR
Hydrocarbon-degrading bacterial colonies were isolated from crude oil contaminant soil of local petrol pumps situated at Tezpur, Assam. One gram of collected soil samples was added to n-hexadecane containing mineral salt medium (Salehizadeh and Mohammadizad 2009) and incubated at 37 °C and 150 rpm for 4 days. After that, 1 mL of incubated culture medium was separated aseptically and transferred to a fresh n-hexadecane containing mineral salt medium (MSM) for another 4 days of incubation at the same conditions. These cycles were repeated for another three more times before isolation of the bacterial samples in MSM agar medium (Haloi and Medhi 2019).
Conventional tests such as surface tension (ST) reduction, emulsification index (E24%), haemolysis and oil spreading were performed for the screening of BS producing bacterial strains (Walter et al. 2010). For the screening test, 2% glucose was used as a carbon source in 50 mL MSM containing Erlenmeyer flask for the growth of the bacterial strain. A semi-quantitative method, cetyltrimethylammonium bromide (CTAB) agar test was also performed to determine the anionic nature of produced BS (Walter et al. 2010).
Identification of biosurfactant producing bacteria for MEOR
After screening, the bacterial strain was identified by 16 s rDNA sequencing. Two universal primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-GGTTACCTTGTTACGACTT-3′) were used for the amplification of approximately 1.5 kb desired product (Jiang et al. 2006). The phylogenetic tree was constructed by CLC sequence viewer after retrieving the closely related sequences from NCBI-BLAST (National Centre for Biotechnology Information–Basic Local Alignment Search Tool).
Optimization of biosurfactant production in laboratory scale
Physicochemical (carbon and nitrogen sources) and environmental (pH and temperature) parameters of production medium were optimized for maximum yield of the metabolites using one factor at a time (OFAT) method. Carbon source was optimized among hydrophilic (glucose and sucrose) and hydrophobic (hydrocarbons such as n-pentadecane, n-hexadecane, n-tridecane and kerosene oil) substrates, whereas the optimum nitrogen source was selected among sodium nitrate (NaNO3), urea (CH4N2O), ammonium chloride (NH4Cl), ammonium sulphate (N2H8SO4), ammonium acetate (C2H3O2NH4) and yeast extract. The effects of environmental parameters such as temperatures (23 °C, 30 °C, 37 °C and 45 °C) and pH (4, 5.5, 7.2, 8.5 and 10) on BS production were also evaluated by measuring BS activity of growth medium. BS activities of the cell-free supernatant were evaluated by tensiometer in triplicates (Govindammal 2014).
Production of biosurfactant
The selected bacterial isolate was incubated at optimum conditions in 50 mL of MSM medium, where the carbon source concentration was 2%. After 96 h of incubation, cell-free supernatant (CFS) was collected by centrifugation at 7000 rpm in 4 °C for 20 min and maintained the pH at 2 using 6 N HCl followed by keeping overnight at 4 °C. After that, the precipitate was collected by centrifugation at 7000 rpm in 4 °C for 20 min and extracted the BS by dissolving in ethyl acetate for two to three times. Honey colour BS was collected from ethyl acetate after drying in a rotary evaporator (Bharali et al. 2011; Haloi and Medhi 2019).
Characterization of biosurfactant
FTIR analysis
Crude BS was chemically characterized using FTIR (Perkin Elmer Spectrum 1000, USA) analysis. The BS was mixed with pure KBr powder to make pallet and scan in the range of 400–4000 cm−1 at the resolution of 4 cm−1 to determine the presence of various chemical bonds and functional groups in the investigated BS (Singh and Tiwary 2016).
1H and 13C NMR analyses
Partially purified BS structure was characterized by 1H and 13C NMR spectroscopy (JNM, ECS-400 MHz NMR, version 4.3.6, Japan) analysis. The spectra were recorded at 293.95 K on 400 MHz spectrophotometers after dissolving approximately 50 mg of BS in 100% chloroform. Tetramethylsilane (TMS) was used as an internal standard for this experiment.
HR-LCMS study
The BS was dissolved in methanol (HPLC grade, Sigma) followed by filtering through a 0.22 μm syringe filter to analyse in HR-LCMS (Model 1290, Agilent Technologies, USA). 5 μL injected volume was used for this analysis in the m/z range of 50–1000 Da and scan rate of 1 spectrum/min (Tavares et al. 2013).
TGA–DSC study
The decomposition temperature of the BS was evaluated by the TGA (Shimadzu TGA-50H, Japan) study under nitrogen flow rate of 30 mL/min and the heating rate of 10 °C/min in the temperature range of 25–600 °C (Sharma et al. 2015).
Different transition stages such as glass transition (Tg), crystallization (Tc) and melting temperature (Tm) were determined by DSC (DSC-60, Shimadzu, Japan) study under nitrogen flow rate of 30 mL/min in the temperature range of 25–300 °C (Gnanamani et al. 2010).
Characterization of crude oil and rock plug
Crude oil characterization
The characterization of crude oil, collected from ONGC-Borholla, Jorhat, Assam was done by following ASTM (American society for testing and materials) methods: for API (American Petroleum Institute) gravity and kinematic viscosity determinations, ASTM D1298 and ASTM D445 were used, respectively. ASTM D97 and ASTM D664 methods were used for the determination of pour point and acid number of the investigated crude oil. The dynamic viscosity was calculated from kinematic viscosity after dividing by the density of the crude oil, while the characterization factor (CF) and correlation index (CI) were determined using Rao (2002). The empirical formulas are as follows:
where TBP(R) is the average boiling point (oR).
where TB is the boiling point (moral) °K.
Rock plug
The porosity was measured in TPI-219 teaching Helium Porosimeter, while the ratio of air volume of rock to the total volume of the rock i.e. pore volume (PV) was calculated using the following formula:
where D and L represent the diameter and length in cm of the core plug, respectively, and ϕ is the porosity of the core plug.
IFT reduction and wettability test
ST and IFT
The ST reduction ability of the investigated BS was measured in 4000 ppm brine solutions with varying BS concentrations, whereas the static IFT between the oleic phase and aqueous phase in varying BS concentrations were measured by Du Nouy ring method (Kruss Easy Dyne Tensiometer-K20, Germany) at room temperature for determination of critical micelle concentration (CMC). CMC is the concentration of BS at which the ST or IFT is least and after which the ST and IFT remain almost constant even though the BS concentrations in the aqueous phase increases.
Wettability test
Wettability study was performed as per Nwidee et al. (2017) with minor modification by measuring the contact angle between the core chips and the CMC valued BS containing aqueous phase. The core chips were cut in the core-cutting machine (Veenedyt Instruments, India) into 2.5 cm diameter and 0.8 cm length. Then, the chips were cleaned with a mixer of methanol and toluene (1:1) in soxhlet apparatus at 40 °C for 24 h followed by saturating with the crude oil in a vacuum pump. Consequently, the chips were immersed for a period of 40 days in 4000 ppm brine and cleaned by hand and dried. After that, a drop of 4000 ppm brine and an aqueous solution containing CMC value of BS in 4000 ppm brine were dropped carefully on the surface of the chips using a stainless steel needle containing syringe to measure the contact angles. The angles of contact between the brine and BS containing aqueous solution, on the core plugs were recorded simultaneously in the contact angle measurement setup (Dataphysics OCA 15 EC, GmbH, Germany).
Flooding experiments
Determination of absolute permeability
Core plugs of size 3.74 cm diameter and 8.9 cm length were prepared using the core cutting, core plugging (Bharat Bijlee Limited, India) and end facing (Veenedyt Instruments, India) machines. The plugs were cleaned in a soxhlet apparatus for 24 h, followed by ultrasonic cleaning for 30 min in an ultrasonic instrument and dehumidified in a humidity control oven for 72 h. The plugs were then saturated with 4000 ppm brine in a vacuum pump till no bubbles appear from the surface of the plugs. 4000 ppm brine solution was prepared in distilled water using NaCl and the brine salinity was selected with reference to the previous literate where it was found that the salinity of reservoir brine of Upper Assam was approximately 3800 ppm (Phukan et al. 2019). The saturated core samples were fixed in the Husserl core holder of the core flooding instrument (Fig. 1) and flooded with 4000 ppm brine solution for the determination of absolute permeability (Kabs). The flooding experiments were performed at 70 °C, as recommended in the previous report of Phukan et al. (2019). The calculation of Kabs was done for steady state one phase flow using Darcy’s low as in the following equation:
where K is the permeability, q is the flow rate in cm3/s, µ is the dynamic viscosity in cp, L is the length of the core in cm, ΔP is the pressure difference between inlet and outlet to the plug in dyne/cm, and A is the area of the plug in cm2.
Schematic diagram of Core flooding apparatus. 1. Core, 2. Silicon sleeve, 3. Hot air oven, 4. Burden pressure, 5. Gas/air cylinders, 6 and 7. Hydraulic hand pump, 8. Slug container, 9. Oil container, 10. Brine container, 11. Stainless steel solid rods, 12. Hydraulic pusher (10 ton capacity), 13. Scanner with data acquisitions system, and 14. Outlet
Drainage process
In the drainage process, a brine saturated rock plug is flooded with the oil (Gogoi 2014). The light paraffin oil is so selected that it matches the crude oil characteristics. During the injection of oil, the effective permeability of brine (Kw) is obtained till the point of breakthrough of oil and after the breakthrough both oil and water will be produced, this is the relative permeability of oil and water w.r.t. water and oil (Kro and Krw), respectively. This process is continued until oil relative permeability (Kro) at initial water saturation or connate water saturation (Swi). In this process, initial oil saturation (Soi) and initial brine saturation (Swi) were calculated using Eqs. 6 and 7, respectively. Swi was used for the calculation of relative permeability’s by Johnson, Bossler and Naumann (JBN) method (Johnson et al. 1959):
First imbibition process
In the first imbibition process or secondary brine flooding, oil saturated plugs are flooded with 4000 ppm brine (Gogoi and Das 2012). During the injection of brine, the effective permeability of oil (Ko) is obtained till the point of breakthrough of brine and after the breakthrough both oil and brine will be produced, this is Kro and Krw w.r.t. water and oil, respectively. This process is continued until Sor (residual oil saturation) at Swi. Sor was calculated in this process as follows:
Both the drainage and imbibition’s processes are immiscible, incompressible two phase unsteady processes. Therefore, JBN method was used to derive the relative permeability neglecting the capillary pressure. The graph of Kro and Krw was plotted against Sw (water saturation), from the finding of the JBN method (Johnson et al. 1959).
Second imbibition process
The plugs after the first imbibition process were saturated with Sor and Sw. Recovery efficiency is defined as an amount of oil that can be recovered from Sor by the 4000 ppm brine solution comprising of BS at CMC. This process is known as second imbibition process or tertiary oil recovery process (Gogoi 2014).
Oil recovery calculations
Literature in chemical EOR says that slug comprising of polymers improve the microscopic volumetric sweep efficiency, whereas the surfactant based slug enhances the microscopic displacement efficiency of the reservoir (Nelson et al. 1984; Liu 2008). So, microscopic displacement efficiency (ED) of the investigated rhamnolipid based slug was calculated according to the following equation (Olajire 2014):
Results and discussion
Screening of biosurfactant producing bacteria for MEOR
Out of 13 hydrocarbons degrading bacteria isolated from crude oil contaminated soil, an efficient biosurfactant producing bacterium was selected based on conventional screening tests such as ST reduction, E24%, haemolysis and oil spreading test.
The isolated strain, initially coined as TMB2 has reduced the ST of inoculated medium from 71.9 to 33.4 mN/m. Aparna et al. reported Pseudomonas sp. 2B with ST reduction capacity of 30.14 mN/m (Aparna et al. 2012), which is quite closer to our reporting strain. E24% capacity of the reporting stain was investigated against different hydrocarbons such as n-hexadecane, n-pentadecane, olive oil and kerosene oil and observed maximum emulsification capacity against kerosene oil (78.6%) followed by olive oil (62.5%), n-pentadecane (50%) and n-hexadecane (25%). The emulsion layers were observed to be stable for more than 3 months in all the cases. Previously, a maximum E24% activity of 68.75% was reported for BS producing Achromobacter sp. TMB1 with olive oil, n-hexadecane and Kerosene oil (Haloi and Medhi 2019). On the other hand, the maximum activity was reported to be 69% for Pseudomonas sp. BUP6 with kerosene oil followed by groundnut oil, diesel, n-decane and petrol, respectively (Priji et al. 2017). A clear zone in haemolysis test indicated the production of BS from the isolated strain. Similarly, oil spreading test also showed a positive indication for BS production. The semi-quantitative method, CTAB agar test showed a blue colour hole around the bacterial colony in CTAB and ethylene blue containing agar plate, which indicated the production of anionic BSs by the isolated bacterial strain.
Identification of the isolated biosurfactant producing bacteria for MEOR
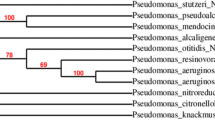
The selected strain was identified as Pseudomonas sp. TMB2 (Fig. 2) after partial 16 s rDNA sequencing and phylogenetic tree analysis. Further, the partial sequence was uploaded into NCBI-gene bank database with accession number KX661384.
Optimization of biosurfactant production in laboratory scale
Carbon and nitrogen sources have significant effect on BS production. Among all hydrophilic and hydrophobic substrates used in this investigation, glucose was found more appropriate for maximum ST reduction (33.4 ± 0.77 dyne/cm) followed by n-tridecane, n-pentadecane, n-hexadecane, kerosene oil and sucrose (Fig. 3a). Earlier, El-Sheshtawy and Doheim (2014) have also found glucose as the best carbon source for maximum production by Pseudomonas aeruginosa. Although the reporting Pseudomonas sp. TMB2 showed growth in hydrophobic carbon sources, the corresponding increase in BS production was not found significant enough compared to water-soluble counterparts especially, in glucose. This finding is in contradiction with the report of Wei et al. (2005) where hydrophobic substrates or hydrocarbons were reported as an inducer for the production of BS. Alkanes are very insoluble in water and the solubility decreases with increasing molecular weight (Eastcott et al. 1988). In case of long-chain n-alkanes, microorganisms may gain access to them either by adhering to hydrocarbon droplets or by a surfactant-facilitated process. Surfactants have been reported to increase the uptake and assimilation of alkanes such as n-hexadecane in liquid cultures (Beal and Betts 2000; Noordman and Janssen 2002). Microbial surfactants are known to have other roles as well, such as facilitating cell motility on solid surfaces (Köhler et al. 2000; Caiazza et al. 2005) or the adhesion/detachment to surfaces or biofilms (Neu 1996; Boles et al. 2005). Moreover, the CH activation and functionalization of alkanes are a relatively slower and energy-intensive reaction involving mainly oxygenases (Crabtree 2004). The reporting bacterial strain may metabolize glucose easily through the glycolysis pathway to generate energy for growth and production of BS as like as other reported Pseudomonas sp. (Rashedi et al. 2015). Pseudomonas sp. strain LP1 (Obayori et al. 2009) and Pseudomonas aeruginosa PG1 (Patowary et al. 2017) were also reported for BSs production by utilizing hydrocarbons as carbon sources and these organisms might be switched to the lipogenic pathway and gluconeogenesis for BS production as reported by Nurfarahin et al. (2018). Moreover, Kumar et al. (2015) reported another Pseudomonas sp., which produced maximum amount of BS in presence of glucose and n-hexadecane as carbon sources in the growth medium.
NaNO3 was evaluated as the best nitrogen source for maximum ST reduction and BS production followed by CH4N2O and C2H3O2NH4 (Fig. 3b). Yeast extract was used as a micronutrient in this study, whereas Pseudomonas otitidis P4 utilized yeast extract as the best nitrogen source for maximum BS production (Singh and Tiwary 2016). However, it was interesting to found that solitary use of NaNO3 or yeast extract in the production medium was not found sufficient for BS yield. As like as different carbon sources, nitrogen sources such as NH4Cl, (NH4)2SO4 and yeast extract were also utilized by the isolate for growth, not for BS production. NaNO3 was already reported to be the best nitrogen source for BS production by other Pseudomonas sp. like Pseudomonas aeruginosa LBM10 (Prieto et al. 2008) and Pseudomonas nitroreducens (Onwosi and Odibo 2012).
Temperature and pH are the two most important environmental parameters with a significant effect on BS production. Here, the temperature 30 °C (Fig. 4a) and pH 7.2 (Fig. 4b) were observed as the optimum conditions for maximum production. Maximum ST reduction in optimum growth medium was observed after 24 h of incubation and remained constant till 120 h. Generally, BS-producing strain required an optimum condition for maximum production of BS (Perfumo et al. 2006; Govindammal 2014). A BS-producing strain identified as Pseudomonas aeruginosa S2 also required 4% glucose as carbon source, 50 mM NH4NO3 as nitrogen source, temperature of 37 °C, agitation rate of 200 rpm and incubation time of 100 h for maximum production in shake-flask conditions (Chen et al. 2007). Pseudomonas aeruginosa 2297 (Kumar et al. 2015), Pseudomonas aeruginosa MM1011 (Rashedi et al. 2015), Pseudomonas aeruginosa (Câmara et al. 2019), etc. were other organisms reported in the recent past with optimum conditions for maximum BS production. The investigated Pseudomonas sp. TMB2 produced approximately 2.8 ± 0.5 g/L of crude BS in shake-flask method after 72 h of incubation and the production rate was also found comparable to another Pseudomonas sp., as reported earlier (Haba et al. 2003; Saikia et al. 2012; Patowary et al. 2017). The ST reduction capacity and BS production abilities of different Pseudomonas sp. are summarized in Table 1.
Characterization of biosurfactant
FTIR analysis
The glycolipid nature of partially purified BS was characterized by the presence of different functional groups and chemical bonds visualized in FTIR spectrum (Fig. 5). Two sharp peaks at 3599.81 cm−1 and 3347.93 cm−1 indicated the presence of –OH group of H-bonding of polysaccharides and –OH stretching of carboxylic acids, respectively. Other sharp peaks at 2924.49 cm−1 and 2856.92 cm−1 have shown the stretching variation of –CH bond of sugar, whereas the absorption peaks at 1732.04 cm−1 and 1648.03 cm−1 suggested the presence of ester group in the fatty acids side chain of glycolipids due to C=O stretching variation. The sharp peaks at 1458.42 cm−1 and 1166.19 cm−1 indicated the presence of –C–O– deformation variation due to carbonyl group of glycolipids extracted from the bacterial strain Pseudomonas sp. TMB2. These adsorption bands together confirm the presence of hydrophobic tails and rhamnose group as components of rhamnolipids which agrees with the previous compositional analyses done in case of rhamnolipids produced by other Pseudomonas sp. (Abbasi et al. 2012; Singh and Tiwary 2016; Sharma et al. 2018).
1H and 13C NMR analyses
Rhamnolipids are categorized into mono- and di-rhamnolipids based on the number of rhamnose group links with the fatty acid tails. The presence of two types of rhamnolipid in the extracted BS was confirmed by 1H and 13C NMR analyses. Different chemical shifts obtained in the 1H and 13C NMR spectra indicated the presence of two rhamnolipids mixer in the BS. The characteristic chemical shifts for mono- and di-rhamnolipids were observed at 0.86 and 0.89 ppm for –CH3 on β hydroxyl fatty acids, respectively. Similarly, other chemical shifts for rhamnolipid characterization were observed at 1.2612 ppm for –CH3 (ring), 1.5384 ppm and 2.5396 ppm for –CH(O)–CH2COO, 4.1318 ppm for –(CH2)–CH(–O–C=O)–CH2COO due to the β-hydroxyl fatty acid chain. The proton chemical shift at 4.8912 ppm was found for –CH–OH–, due to the rhamnose moiety of rhamnolipid (Fig. 6a). The 1H NMR spectrum profile was found comparable with other rhamnolipids produced by different Pseudomonas sp. (Monteiro et al. 2007; Sharma et al. 2015).
13C NMR spectrum shows the presence of lipid in rhamnolipid due to the signal at 14.1792 ppm for –CH3 and at 22.7032–31.8277 ppm for CH2. Moreover, ester and carboxylic group signals were generated at 171.5475 and 173.8072 ppm, respectively (Fig. 6b). The presence of lipid, ester and carboxylic groups as reflected in 13C NMR spectrum clearly indicated the chemical nature of BSs produced by Pseudomonas sp. TMB2 to be rhamnolipid as like as the ones reported previously for other Pseudomonas sp. (Lotfabad et al. 2010; Christova et al. 2013; Sharma et al. 2015).
HR-LCMS analysis
The presence of different mono- and di-rhamnolipids congeners in the investigated rhamnolipid were further determined by HR-LCMS analysis. On comparison of the LC–MS data of other reports, total seven numbers of mono- and di-rhamnolipids congeners (Table 2) were identified in the rhamnolipid produced by Pseudomonas sp. TMB2 based on the m/z ratio (Yin et al. 2009; Patowary et al. 2017; Chen et al. 2018). Predominant peaks were observed at m/z 302.1731, 331.1627, 499.2849, 527.3161, 645.3422 and 673.3733, corresponding to [M+H]+ of rhamnolipid congeners Rha-C8:2, Rha-C10, Rha-C10-C10, Rha-C10-C12:1, Rha-Rha-C10-C10 and Rha-Rha-C10-C12:1, respectively. Moreover, another peak at m/z 507.1893 was corresponding to [M+Na]+ of Rha-Rha-C12-C10. In this case, mono-rhamnolipid congener's presence was dominant to di-rhamnolipids (Fig. 7). However, P. aeruginosa CPCL (Arutchelvi and Doble 2010) and Burkholderia kururiensis (Tavares et al. 2013) produced BS contained total 19th and 23rd numbers of rhamnolipids congeners, respectively.
TGA–DSC analyses
The thermostability of the rhamnolipid is a very important criterion for its use in MEOR technology. TGA study revealed two steps of degradation at 102.41 °C and 218.57 °C (Fig. 8a). At 102.41 °C, only 5.05% of the total BS weight was lost, which might occur due to the loss of water and solvent present in the crude sample. It was reported previously that the constant slope of BSs in TGA study till 200 °C indicated the absence of moisture in BS, followed by maximum weight loss in the temperature range of 240–340 °C represented the decomposition of thermolabile rhamnose and lipids components (Singh and Tiwary 2016; Khademolhosseini et al. 2019). Figure 8a clearly shows that about 76.73% of total BS weight is lost between 218 and 299 °C, which attributes to the degradation of thermolabile components present in the investigated rhamnolipid. Moreover, only 10% weight was lost in the temperature range of 299–599.8 °C, which corresponded to the degradation of non-labile components. A similar observation was also reported from another Pseudomonas aeruginosa produced rhamnolipid (Abbasi et al. 2012), where approximately 80% of the total BS weight was lost at around 300 °C.
DSC (Fig. 8b) study was used to investigate different transition states of the isolated BS. The glass transition, crystallization and melting temperature were found to be 83.61 °C, 174.56 °C and 253.48 °C, respectively. This pattern was also found quite similar with another rhamnolipid produced by Pseudomonas aeruginosa (Gogoi et al. 2016), where also the extracted rhamnolipid was used to recover trapped oil from sand packed column.
Moreover, the stability of the investigated rhamnolipid at different temperature (30–100°C), pH (2–12) and salinity (0–10%) on ST reduction as well as E24% ability also suggests its applicability in MEOR technology [Supplementary information S.1: Fig. S (a), S (b) and S (c)].
Characterization of crude oil and rock plug
The specific gravity, API gravity (Fig. 9) at 15.6 °C, kinematic viscosity and dynamic viscosity of the investigated crude oil were found to be 0.853 g/cm3, 34.2°, 17.75 cP and 12.425 cP, respectively. From the above results, crude oil could be inferred as light crude oil; since API gravity of the crude oil was greater than 31.1 °C. The crude oil will be heavy or medium oil, if the API gravity at 15.6 °C is less than 22.3° or between 22.3 and 31.1°, respectively. Also, the CF and CI were found to be 12.48 and 44.55, respectively indicating mixed base crude inclined towards paraffinic nature. So, light paraffin oil (API gravity = 34.97°) resembling investigated crude oil characteristics was selected for IFT and flooding experiments.
The porosity of the collected rock plugs from Upper Assam oil field, named as NH1, NH2 and NH3 were determined as 22%, 21.3% and 19.7%, respectively, while the pore volumes of the respected rock plugs were found to be 21.5 cc, 20.81 cc and 19.25 cc.
Interfacial tension (IFT) reduction and wettability test
IFT reduction and wettability alteration have significant roles in MEOR technology (Rabiei et al. 2013). Figure 10 shows the ST and IFT reduction capacity of the investigated rhamnolipid in different concentrations. The rhamnolipids containing slug reduced maximum IFT between oleic and aqueous phase from 39.1 to 0.8 mN/m at the concentration of 120 mg/L and remained same above all rhamnolipid concentrations. So, CMC of the Pseudomonas sp. TMB2 produced rhamnolipid was observed as 120 mg/L, which was comparable to BSs produced by other Pseudomonas sp. as reported by Haba et al. (2003) and Câmara et al. (2019) having CMC values of 108.8 mg/L and 127 mg/L, respectively. The investigated rhamnolipid reduced maximum ST 60.62% (from 71.1 to 28 mN/m) of 4000 ppm saline water at CMC value 120 mg/L as like as B. subtilis MTCC 242 (Makkar and Cameotra 1999) produced BS. Also, these BSs could be one of the best alternatives to chemical surfactants due to its ultra-low ST and IFT reduction abilities. The CMC value of this investigated rhamnolipid was also much lesser than chemical surfactants, sodium dodecyl sulphate (2100 mg/L) (Chen et al. 2006) and Tween-80 (3200 mg/L) (Mandal et al. 1988). The IFT reduction capacity of the extracted BS was found to be even lesser than the BS produced from Bacillus licheniformis W16 with IFT reduction capacity 2.47 ± 0.32 mN/m (Joshi et al. 2016). Drop in IFT indicates the increase of capillary numbers with Sor reduction.
The effect of BS based solution on wettability alteration was evaluated by measuring the contact angles, as shown in Fig. 11. The rock plugs are divided into water wet (< 75°), neutral wet (75° < to < 105°) and oil wet (> 105°) based on the angle formed between the solid substrate and aqueous phase (Anderson 1986). The investigated plugs were water wet as the angles between the oil saturated plugs and the 4000 ppm saline water were recorded below 75°. But, the angles were reduced to 42°, 45.3° and 46°, respectively when a drop of rhamnolipid containing 4000 ppm brine at CMC was placed in oil saturated NH1, NH2 and NH3 plugs. It indicates the wettability alteration property of the BS containing solution. IFT and wettability alteration effects on MEOR technology have already reported by different investigations (Rabiei et al. 2013; Darvishi et al. 2015). E. cloacae and E. hormaechei produced BSs reduced IFT to 3.2 mN/m from 29 mN/m (Rabiei et al. 2013), whereas the investigated rhamnolipids reduced IFT to 0.8 mN/m from 39.1 mN/m.
Flooding experiment
The core flooding experiment was conducted at 70 °C and at a pressure difference between inlet and outlet of 40.7 atm. During the 1st imbibition process, the irreducible water saturation and initial oil saturation were observed as 17.21% and 82.8% for NH1; 17.68% and 82.32% for NH2, and 19.4% and 80.6% for NH3 rock plugs, respectively. Figure 12 shows the graph of Kro and Krw vs. Sw in the rock plug NH1. Moreover, approximately 38.4%, 32.5% and 28.6% of oil from original oil in place were recovered in 1st imbibition process from the rock plugs NH1, NH2 and NH3, respectively. So, systematic investigations have been done for recovering remaining 61.6%, 67.5% and 71.4% (Sor) of oil from the respective rock plugs using rhamnolipid based aqueous flooding (4000 ppm salinity) at CMC value 120 mg/L, respectively.
Rhamnolipid containing brine solution (4000 ppm) at CMC 120 mg/L recovered overall 55.1%, 45.7% and 39.3% of residual oil from initial oil saturation in core flooding test i.e. 16.7%, 13.2% and 10.7% of the additional oil has been recovered by reported rhamnolipid based aqueous solution from NH1, NH2 and NH3 plugs, respectively. Figure 13 shows the relationship between the brine and rhamnolipids based slugs flooding, with respect to the pore volume injected in the flooding process in the injected flow rates of 0.8 mL/min. Figure 13 clearly shows that the injection of saline solution up to 10 PV resulted in a sufficiently higher recovery of oil. However, the oil displacement was observed minimum, after injection of saline in between the PV 10–12. Therefore, the injection of saline solution was abandoned and the formulated BS based slug was injected, which resulted in a rapid increase in oil recovery as shown in Fig. 13. The microscopic displacement efficiency (ED) for core plugs NH1, NH2 and NH3 of the rhamnolipid containing 4000 ppm brine solution was found to be 27.11%, 19.55% and 14.98%, respectively and these efficiencies were increased due to the reduction of IFT between oil and displacing rhamnolipid based aqueous liquid (Satter et al. 2008). This again suggests its effectiveness over the reported Pseudomonas aeruginosa SGD produced rhamnolipid for ex-situ MEOR technology (Zhao et al. 2015), where additionally 8.33% of original crude oil from core flooding test was recovered with IFT reduction capacity of 91.8% (26.1–2.14 mN/m). However, another rhamnolipids produced by P. aeruginosa HATH recovered only 5% of additional oil from homogeneous glass micro model (Amani et al. 2013). In this investigation, the rhamnolipids produced by Pseudomonas sp. TMB2 was found more efficient in oil recovery process displaying the highest 16.7% of additional oil from Upper Assam based reservoir rock. Therefore, the reported rhamnolipid seems as an effective BS extracted from Pseudomonas sp. TMB2 for use in Upper Assam oil reserves as ex-situ MEOR agents over a wide range of temperatures, pH and salinity.
Conclusion
The biosurfactant reported in this investigation was extracted and partially purified from hydrocarbon degrading bacterial strain Pseudomonas sp. TMB2 (KX661384), with an approximate yield of 2.8 ± 0.5 g/L glycolipid-type biosurfactant under optimized condition of carbon (glucose), nitrogen (NaNO3), pH (7.2) and temperature (30 °C). FTIR and NMR spectroscopy analyses revealed the chemical nature of the isolated biosurfactant as rhamnolipid (glycolipid) with further detection of both mono- and di-rhamnolipid in the HR-LCMC analysis. The observed thermostability and degradation pattern of the rhamnolipids (TGA–DSC) were suggestive of superior adaptability to extreme environmental conditions vis a vis ex-situ MEOR application since the complete weight loss of the biosurfactant has occurred only after temperature rise as high as 599.80 °C. There was also marked IFT reduction for the rhamnolipids from 39.1 to 0.8 mN/m (97.95%) at the CMC value of 120 mg/L. The wettability of the rock plugs also showed significant changes from water wet to more water wet (\(\theta = 75^{ \circ } ,{\text{ reduced }}\;{\text{to}} \;42^{ \circ }\)) after the biosurfactant treatment. The core flooding experiments predicted maximum additional recovery of 16.7% from rock plug NH1 after secondary brine flooding, with the overall efficiency of 27.11%, which demonstrated the effective use of the investigated rhamnolipids for efficient and environment friendly oil recovery technology in Upper Assam oil reservoir.
References
Abbasi H, Hamedi MM, Lotfabad TB, Zahiri HS, Sharafi H, Masoomi F, Moosavi-Movahedi AA, Ortiz A, Amanlou M, Noghabi KA (2012) Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: physicochemical and structural characteristics of isolated biosurfactant. J Biosci Bioeng 113(2):211–219
Al-Sulaimani H, Joshi S, Al-Wahaibi Y, Al-Bahry S, Elshafie A, Al-Bemani A (2011) Microbial biotechnology for enhancing oil recovery: current developments and future prospects. Biotechnol Bioinf Bioeng 1(2):147–158
Amani H, Müller MM, Syldatk C, Hausmann R (2013) Production of microbial rhamnolipid by Pseudomonas aeruginosa MM1011 for ex-situ enhanced oil recovery. Biotechnol Appl Biochem 170(5):1080–1093
Anderson WG (1986) Wettability literature survey-part 2: wettability measurement. J Petrol Technol 38(11):1246–1262
Aparna A, Srinikethan G, Smitha H (2012) Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B Biointerfaces 95:23–29
Arutchelvi J, Doble M (2010) Characterization of glycolipid biosurfactant from Pseudomonas aeruginosa CPCL isolated from petroleum contaminated soil. Lett Appl Microbiol 51:75–82
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87(2):427–444
Beal R, Betts WB (2000) Role of rhamnolipid biosurfactants in the uptake and mineralization of hexadecane in Pseudomonas aeruginosa. J Appl Microbiol 89:158–168
Bharali P, Das S, Konwar BK, Thakur AJ (2011) Crude biosurfactant from thermophilic Alcaligenes faecalis: feasibility in petro-spill bioremediation. Int Biodeterior Biodegrad 65(5):682–690
Boles BR, Thoendel M, Singh PK (2005) Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57:1210–1223
Caiazza NC, Shanks RM, O'Toole GA (2005) Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 187:7351–7361
Câmara JM, Sousa MA, Barros Neto EL (2019) Optimization and characterization of biosurfactant rhamnolipid production by Pseudomonas aeruginosa isolated from an artificially contaminated soil. J Surfactants Deterg. https://doi.org/10.1002/jsde.12287
Chen J, Wang XJ, Hu JD, Tao S (2006) Effect of surfactants on biodegradation of PAHs by white-rot fungi. Huanjingkexue 27(1):154–159
Chen SY, Lu WB, Wei YH, Chen WM, Chang JS (2007) Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol Prog 23(3):661–666
Chen C, Sun N, Li D, Long S (2018) Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ Sci Pollut R 25:14934–14943
Christova N, Tuleva B, Kril A, Georgieva M, Konstantinov S, Terziyski I, Nikolova B, Stoineva I (2013) Chemical structure and in vitro antitumor activity of rhamnolipids from Pseudomonas aeruginosa BN10. Appl Biochem Biotechnol 170(3):676–689
Crabtree RH (2004) Organometallic alkane CH activation. J Organomet Chem 689(24):4083–4091
Darvishi P, Ayatollahi S, Roostaei AR (2015) Microbial enhanced oil recovery, wettability alteration and interfacial tension reduction by an efficient bacterial consortium, ERCPPI-2. J Oil Gas Petrochem Technol 2:27–42
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(1):47–64
Eastcott L, Shiu WY, Mackay D (1988) Environmentally relevant physical-chemical properties of hydrocarbons: a review of data and development of simple correlations. Oil Chem Pollut 4:191–216
El-Sheshtawy HS, Doheim MM (2014) Selection of Pseudomonas aeruginosa for biosurfactant production and studies of its antimicrobial activity. Egypt J Petrol 23(1):1–6
Eskandari S, Rashedi H, Ziaie-Shirkolaee Y, Mazaheri-Assadi M, Jamshidi E, Bonakdarpour B (2009) Evaluation of oil recovery by rhamnolipid produced with isolated strain from Iranian oil wells. Ann Microbiol 59(3):573
Gnanamani A, Kavitha V, Radhakrishnan N, Rajakumar GS, Sekaran G, Mandal AB (2010) Microbial products (biosurfactant and extracellular chromate reductase) of marine microorganism are the potential agents reduce the oxidative stress induced by toxic heavy metals. Colloids Surf B Biointerfaces 79(2):334–339
Gogoi SB (2014) Effluent as surfactant for enhanced oil recovery. Innov Energy Policies 3:46–54
Gogoi SB, Das BM (2012) Use of an effluent for enhance oil recovery. Indian J Chem 19:366–370
Gogoi D, Bhagowati P, Gogoi P, Bordoloi NK, Rafay A, Dolui SK, Mukherjee AK (2016) Structural and physico-chemical characterization of a di-rhamnolipid biosurfactant purified from Pseudomonas aeruginosa: application of crude biosurfactant in enhanced oil recovery. RSC Adv 6(74):70669–70681
Govindammal M (2014) Effect of carbon and nitrogen sources on the production of biosurfactant by Pseudomonas fluorescens isolated from mangrove ecosystem. Int J Pharm Biol Sci Arch 5:2
Haba E, Pinazo A, Jauregui O, Espuny MJ, Infante MR, Manresa A (2003) Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol Bioeng 81(3):316–322
Haloi S, Medhi T (2019) Optimization and characterization of a glycolipid produced by Achromobacter sp. to use in petroleum industries. J Basic Microbiol 59(3):238–248
Jiang H, Dong H, Zhang G, Yu B (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in north western Chinan. Appl Environ Microbiol 72:3832–3845
Johnson EF, Bossler DP, Naumann VO (1959) Calculation of relative permeability from displacement experiments. Petrol Trans 216:370–372
Joshi SJ, Al-Wahaibi YM, Al-Bahry SN, Elshafie AE, Al-Bemani AS, Al-Bahri A, Al-Mandhari MS (2016) Production, characterization, and application of Bacillus licheniformis W16 biosurfactant in enhancing oil recovery. Front Microbiol 7:1853
Khademolhosseini R, Jafari A, Mousavi SM, Hajfarajollah H, Noghabi KA, Manteghian M (2019) Physicochemical characterization and optimization of glycolipid biosurfactant production by a native strain of Pseudomonas aeruginosa HAK01 and its performance evaluation for the MEOR process. RSC Adv 9(14):7932–7947
Köhler T, Curty LK, Barja F, Van Delden C, Péchère JC (2000) Swarming of Pseudomonas aeruginosa is dependent on cell to cell signaling and requires flagella and pili. J Bacteriol 182:5990–5996
Kumar AP, Janardhan A, Radha S, Viswanath B, Narasimha G (2015) Statistical approach to optimize production of biosurfactant by Pseudomonas aeruginosa 2297. 3 Biotech 5(1):71–79
Liu S (2008) Alkaline surfactant polymer enhanced oil recovery process. Ph.D. thesis. Rice University, Houston
Lotfabad TB, Abassi H, Ahmadkhaniha R, Roostaazad R, Masoomi F, Zahiri HS, Ahmadian G, Vali H, Noghabi KA (2010) Structural characterization of a rhamnolipid-type biosurfactant produced by Pseudomonas Aeruginosa MR01: enhancement of di-rhamnolipid proportion using gamma irradiation. Colloids Surf B Biointerfaces 81(2):397–405
Makkar RS, Cameotra SS (1999) Structural characterization of biosurfactant produced by Bacillus subtilis at 45 C. J Surfactants Deterg 2(3):367–372
Mandal AB, Nair BU, Ramaswamy D (1988) Determination of the critical micelle concentration of surfactants and the partition coefficient of an electrochemical probe by using cyclic voltammetry. Langmuir 4(3):736–739
Monteiro SA, Sassaki GL, de Souza LM, Meira JA, de Araújo JM, Mitchell DA, Ramos LP, Krieger N (2007) Molecular and structural characterization of the biosurfactant produced by Pseudomonas aeruginosa DAUPE 614. Chem Phys Lipids 147:1–13
Nelson, RC, Lawson JB, Thigpen DR, Stegemeier GL (1984) Cosurfactant-enhanced alkaline flooding. In: SPE enhanced oil recovery symposium. Society of Petroleum Engineers.
Neu TR (1996) Significance of bacterial surface–active compounds in interaction of bacteria with interfaces. Microbiol Rev 60:151–166
Noordman WH, Janssen DB (2002) Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa. Appl Environ Microbiol 68:4502–4508
Nurfarahin AH, Mohamed MS, Phang LY (2018) Culture medium development for microbial-derived surfactants production—an overview. Molecules 23(5):1049
Nwidee LN, Lebedev M, Barifcani A, Sarmadivaleh M, Iglauer S (2017) Wettability alteration of oil-wet limestone using surfactant-nanoparticle formulation. J Colloid Interface Sci 504:334–345
Obayori OS, Ilori MO, Adebusoye SA, Oyetibo GO, Omotayo AE, Amund OO (2009) Degradation of hydrocarbons and biosurfactant production by Pseudomonas sp. strain LP1. World J Microbiol Biotechnol 25(9):1615–1623
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges. Energy 77:963–982
Onwosi CO, Odibo FJC (2012) Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J Microbiol Biotechnol 28(3):937–942
Patowary K, Patowary R, Kalita MC, Deka S (2017) Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front Microbiol 8:279
Pereira JF, Gudiña EJ, Costa R, Vitorino R, Teixeira JA, Coutinho JA, Rodrigues LR (2013) Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 111:259–268
Perfumo A, Banat IM, Canganella F, Marchant R (2006) Rhamnolipid production by a novel thermophilic hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. Appl Microbiol Biotechnol 72(1):132
Phukan R, Gogoi SB, Tiwari P (2019) Enhanced oil recovery by alkaline-surfactant-alternated-gas/CO2 flooding. J Pet Explor Prod Technol 9(1):247–260
Prieto LM, Michelon M, Burkert JFM, Kalil SJ, Burkert CAV (2008) The production of rhamnolipid by a Pseudomonas aeruginosa strain isolated from a southern coastal zone in Brazil. Chemosphere 71(9):1781–1785
Priji P, Sajith S, Unni KN, Anderson RC, Benjamin S (2017) Pseudomonas sp. BUP6, a novel isolate from Malabari goat produces an efficient rhamnolipid type biosurfactant. J Basic Microbiol 57(1):21–33
Rabiei A, Sharifinik M, Niazi A, Hashemi A, Ayatollahi S (2013) Core flooding tests to investigate the effects of IFT reduction and wettability alteration on oil recovery during MEOR process in an Iranian oil reservoir. Appl Microbiol Biotechnol 97(13):5979–5991
Rao BKB (2002) Modern petroleum refining processes, 4th edn. Oxford & IBH Publishing Co. Pvt. Ltd, New Delhi, pp 58–59
Rashedi H, Izadi A, Bidhendi ME (2015) Optimization of operational parameters in rhamnolipid production by Pseudomonas aeruginosa MM1011 in a miniaturized shaken bioreactor. J Appl Biotechnol Rep 2(3):271–278
Saikia RR, Deka S, Deka M, Banat IM (2012) Isolation of biosurfactant-producing Pseudomonas aeruginosa RS29 from oil-contaminated soil and evaluation of different nitrogen sources in biosurfactant production. Ann Microbiol 62(2):753–763
Salehizadeh H, Mohammadizad S (2009) Microbial enhanced oil recovery using biosurfactant produced by Alcaligenes faecalis. Iran J Biotechnol 7(4):216–223
Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci 17(3):401
Satter A, Iqbal G, Buchwalter J (2008) Practical enhanced reservoir engineering: assisted with simulation software. Practical enhanced reservoir engineering. PennWell, Oklahoma
Sharma D, Ansari MJ, Al-Ghamdi A, Adgaba N, Khan KA, Pruthi V, Al-Waili N (2015) Biosurfactant production by Pseudomonas aeruginosa DSVP20 isolated from petroleum hydrocarbon-contaminated soil andit’s physicochemical characterization. Environ Sci Pollut Res 22(22):17636–17643
Sharma R, Singh J, Verma N (2018) Optimization of rhamnolipid production from Pseudomonas aeruginosa PBS towards application for microbial enhanced oil recovery. 3 Biotech 8(1):20
Singh P, Tiwary BN (2016) Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines. India Bioresour Bioprocess 3(1):42
Tavares LF, Silva PM, Junqueira M, Mariano DC (2013) Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl Microbiol Biotechnol 97:1909–1921
Thomas S (2008) Enhanced oil recovery-an overview. Oil Gas Sci Technol Revue de l'IFP 63(1):9–19
Walter V, Syldatk C, Hausmann R (2010) Screening concepts for the isolation of biosurfactant producing microorganisms. In: Biosurfactants. Adv Exp Med Biol, vol 672, pp 1–13
Wei QF, Mather RR, Fotheringham AF (2005) Oil removal from used sorbents using a biosurfactant. Bioresour Technol 96(3):331–334
Wongsa P, Tanaka M, Ueno A, Hasanuzzaman M, Yumoto I, Okuyama H (2004) Isolation and characterization of novel strains of Pseudomonas aeruginosa and Serratia marcescens possessing high efficiency to degrade gasoline, kerosene, diesel oil, and lubricating oil. Curr Microbiol 49:415–422
Yin H, Qiang J, Jia Y, Ye J (2009) Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil containing wastewater. Process Biochem 44:302–308
Zhao F, Zhang J, Shi R, Han S, Ma F, Zhang Y (2015) Production of biosurfactant by a Pseudomonas aeruginosa isolate and its applicability to in situ microbial enhanced oil recovery under anoxic conditions. RSC Adv 5(45):36044–36050
Zhao F, Zhou JD, Ma F, Shi RJ, Han SQ, Zhang J, Zhang Y (2016) Simultaneous inhibition of sulfate-reducing bacteria, removal of H2S and production of rhamnolipid by recombinant Pseudomonas stutzeri Rhl: applications for microbial enhanced oil recovery. Bioresour Technol 207:24–30
Acknowledgements
The authors are gratefully acknowledged the financial assistance extended in the form of a project Junior Research Fellowship (entitled “Strengthening of Biotechnology Teaching, Training and Research in Universities and Colleges in the North-East”) by the Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India to Saurav Haloi. The authors are also grateful to Institute Of Biotechnology & Geotectonics Studies (INBIGS)-Oil and Natural Gas Corporation (ONGC), Assam & Assam-Arakan Basin Eastern Region, Cinnamara, Jorhat, Assam, India for providing us crude oil from Borhola oil field, Jorhat, Assam and ONGC-Centre for Petroleum Biotechnology, Department of Molecular Biology and Biotechnology, Tezpur University for providing us laboratory facilities. The authors are also grateful to SAIC, IIT Mumbai (for HR-LCMS), Department of Chemical Sciences (for NMR and TGA–DSC) and Material Research Laboratory, Department of Physics, Tezpur University (for Contact angle measurement) for their support. We also like to thank Mr. Rituraj Das, RS of the Department of Chemical Sciences, Tezpur University for the help.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haloi, S., Sarmah, S., Gogoi, S.B. et al. Characterization of Pseudomonas sp. TMB2 produced rhamnolipids for ex-situ microbial enhanced oil recovery. 3 Biotech 10, 120 (2020). https://doi.org/10.1007/s13205-020-2094-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2094-9