Abstract
The purpose of the present study was to discover antimicrobial endophytic fungi from Astragalus chinensis. Three fungal endophytes with antibacterial activity were isolated and determined as Chaetomium sp. HQ-1, Fusarium sp. HQ-7 and Fusarium sp. HQ-9 based on the neighbor-joining phylogenetic tree. Chaetomium sp. HQ-1 showed the best antibiotic potential and was thus selected for large-scale fermentation. Bioactivity-directed separation of ME fermentation of strain HQ-1 led to the discovery of three compounds, which were identified as differanisole A (1), 2,6-dichloro-4-propylphenol (2) and 4,5-dimethylresorcinol (3), from the HR–ESI–MS and NMR data analysis. All three compounds exhibited moderate antibacterial activity against Listeria monocytogenes, Staphylococcus aureus, and methicillin-resistant S. aureus, with MIC values ranging from 16 to 128 μg/mL. Compounds 1 and 3 also displayed promising antifungal activity against Selerotium rolfsii with IC50 values of less than 16 and 32 μg/mL, respectively, which were comparable to that of actidione (8 μg/mL). The findings of the present study suggest that the endophytic fungi from A. chinensis have the potential to be used as bactericides and fungicides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The constant emergence of antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and carbapenem-resistant Acinetobacter baumannii, has shown that drug resistance is spreading faster and becoming a serious global public health problem (Basak et al. 2016). It has been reported that approximately 700,000 people are killed by antibiotic-resistant bacteria every year, and this number will continue to increase if efforts are not made against this problem (Willyard 2017). However, in recent decades, the types of antibacterial drugs approved by the FDA have been continuously reduced (Boucher et al. 2009; Lewis 2012). Fluoroquinolone, an antibiotic discovered 40 years ago, is the latest anti-Gram-negative bacteria drug that has been introduced to the market (Spellberg 2012). Due to the emerging new diseases and serious bacterial drug resistance, the need for novel antibiotics is ever increasing.
In general, there are three pathways for discovering new pharmaceutical molecules: rational drug design, chemical synthesis and natural product discovery. Due to unique structures, low costs and potent bioactivities, natural product drug discovery has regained the interest of chemists, biologists, and pharmacologists (Newman and Cragg 2016). Natural products from plants have been shown to be one of the most promising. However, the quantity of many bioactive compounds in plant tissues is not sufficient. To address this issue, endophytes were discovered and exploited. Endophytes, which are microorganisms (mostly bacteria and fungi) that reside in plants during a period of their life cycle (Strobel 2003), may produce similar bioactive compounds as their plant hosts. In addition, to adapt to the special internal environment of organisms, they usually have unique physiological and metabolic mechanisms, increasing the possibility of producing new active substances (Tan and Zou 2001). Recently, endophytes have become a hot spot and been proven to be underexplored resources for the discovery of natural products (Martinez-Klimova et al. 2017; Alvin et al. 2014).
Astragalus, a kind of traditional Chinese medicinal plant, is a prolific producer of structurally diverse molecules with interesting biological activities (Ibrahim et al. 2013; Li et al. 2010; Liu et al. 2018; Wu et al. 2017; Li et al. 2014). A number of bioactive metabolites from the endophytes of Astragalus have also been reported (Bashyal et al. 2017; Xu et al. 2013). However, in the case of endophytes from the Chinese Astragalus chinensis, the antimicrobial potential has never been studied. Here, three endophytic fungi isolated from A. chinensis exhibited an ability to produce bioactive agents with antibiotic potential. One of the strains, Chaetomium sp. HQ-1, contained three compounds with antifungal and antibacterial activity that were further isolated. These results provided a scientific basis for the exploitation of endophytes of A. chinensis as biological sources of antimicrobial activity.
Materials and methods
Isolation and identification of endophytic fungi
Astragalus chinensis was collected in Tai’an, Shandong Province, China. According to methods detailed previously (Schulz et al. 1993) with some modifications, the fungi were isolated from the tissue of healthy A. chinensis. The tissues were washed sufficiently with distilled water and sterilized as follows. Tissue samples were soaked in 75% ethanol for 2 min, 5% hypochlorite for 3 min, 75% ethanol for 2 min, and finally rinsed with sterile distilled water for 5 min. Subsequently, samples were cut into pieces (1 cm2 of the leaf and 1 cm length of the stem) and placed onto potato dextrose agar (PDA) supplemented with 50 µg/mL ampicillin to inhibit bacterial growth. These samples were incubated aerobically for 7 days at 28 °C. The pure colonies were transferred to fresh medium (PDA) and preserved at 4 °C for further use.
Fungal genomic DNA was extracted with a Fungal DNA Kit (OMEGA) according to the manufacturer’s recommendations. ITS gene amplification and sequencing were performed by BioSune Inc. (Shanghai). The ITS gene sequence of each isolate obtained from A. chinensis was subjected to a BLAST search in GenBank. The closely related strains were obtained to establish a neighbor-joining distance tree using MEGA 7.0 with 1000 bootstrap replicates (Visser et al. 2012).
Fermentation of the endophytic fungi
The fungal mycelia of each isolate were inoculated into two 250 mL Erlenmeyer flasks, one containing 100 mL of potato dextrose (PD) broth and the other containing 100 mL of malt extract (ME) liquid medium (Tian et al. 2015), and cultured for 10 days at 28 °C on a rotary shaker (150 rpm). The broth culture was filtered through two layers of muslin cloth and refiltered through a 0.22 µm bacterial filter (Zhao et al. 2017). The sterile fermentation broth was preserved at 4 °C for further bioactivity testing.
Antibacterial activity of the fungal fermentation broth
The antibacterial activity of the fermentation broth was screened against 3 gram-positive pathogens, S. aureus (ATCC25923), MRSA (local isolate) and Listeria monocytogenes (CGMCC1.10753), as well as 2 gram-negative bacteria, E. coli (local isolate) and Salmonella enterica subsp. enterica serovar (ATCC10708) using the agar well diffusion method (Sharma et al. 2016). The bacteria were spread on Luria–Bertani (LB) agar plates (1% peptone, 0.5% yeast extract, 1% NaCl, 1.5% agar). Then, the wells were bore on the plates, and 100 µL of fermentation broth was poured into the wells using ampicillin (50 μg/mL) as a positive control and fresh LB medium as a negative control. After an incubation for 24 h at 37 °C, the inhibition zone diameters were determined. Three replicates were carried out for each antibacterial activity test. The fungal strain that showed the best bioactivity, was selected for upscale fermentation using the medium with better performance (ME).
Extraction, purification and characterization of an antibacterial compound
The upscale fermentation was performed in a 1000 mL Erlenmeyer flask containing 400 mL of ME broth. After 10 days of cultivation at 28 °C on a rotary shaker at 150 rpm, the fermentation broth (10 L) was filtered and extracted with ethyl acetate. The solvent was removed by rotary evaporation to yield a weight of 5.2 g of crude extract.
The crude extract was fractionated by column chromatography over silica gel (200–300 mesh) eluting with petroleum ether/acetone mixtures of increasing polarity (100:1, 50:1, 25:1, 10:1, 5:1, v/v) to obtain five fractions (F1 ~ F5). The eluted fractions were tested for antibacterial activity using the agar well diffusion method (as described above). Fractions that inhibited the growth of bacteria were then purified on a Sephadex LH-20 and semipreparative reversed-phase HPLC to afford compounds 1 (51 mg), 2 (9 mg) and 3 (12 mg).
The structures of the bioactive compounds were identified using an Agilent G6230 TOF mass spectrometer and a nuclear magnetic resonance (NMR) spectrometer (Bruker Avance 600, Germany). 1H NMR and 13C NMR spectra were measured in acetone-d6 using tetramethylsilane (TMS) as an internal standard.
Determination of the minimum inhibitory concentration
The minimum inhibitory concentration (MIC) was determined according to the method described by Sharma et al. (Sharma et al. 2016) with some modifications. First, stock solutions of the compounds or ampicillin were prepared (256 μg/mL in LB broth). Then, the solutions were diluted using a twofold serial dilution method in sterile 5 mL tubes with LB broth (1 mL/tube). Subsequently, an equal volume of overnight-grown bacterial culture was added to each tube. The final concentrations of the compounds in the eight tubes were 128, 64, 32, 16, 8, 4, 2 and 1 μg/mL. LB broth was used as a negative control. The tubes were all incubated at 37 °C for 24 h on a rotary shaker at 200 rpm. The minimum inhibitory concentration (MIC) was determined as the lowest concentration that inhibited the growth of the tested microorganism.
Antifungal activity of the compound
The four plant pathogens, Selerotium rolfsii, Fusarium moniliforme, Fusarium stratum and Fusarium oxysporum, used for antifungal activity determination were all isolated and identified in our laboratory. The assay was performed as described previously (Zhang et al. 2013) with minor modifications. Different concentrations of compounds were produced by a twofold serial dilution method and mixed completely with preheated PDA in Petri dishes (9 cm diameter). The final concentrations of the compounds ranged from 128 to 1 μg/mL. Actidione was used as a positive control. The mycelia of the test fungi were transferred onto the center of solidified medium and incubated at 28 °C for 6 days. The percent inhibition was calculated according to the following formula (1 − Da/Db) × 100, where Da is the colony diameter in the experimental plates (mm) and Db is the colony diameter in the control plates (mm).
Results
Identification of endophytic fungi
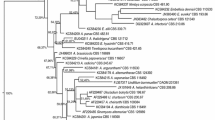
Three fungi with different morphotypes were isolated from A. chinensis (one from the leaf and two from the stem) (Fig. 1). Their ITS genes were amplified and sequenced (GenBank accession number MK597925–MK597927). Phylogenetic analysis of the ITS gene sequences revealed that the endophytic fungi from A. chinensis did not form a monophyletic group. As evident from the neighbor-joining tree (Fig. 2), the most closely related species of strain HQ-1 was Chaetomium rectangulare IRAN1641C (99.44%) (Asgari and Zare 2011). Thus, strain HQ-1 is a member of the genus Chaetomium. Similarly, isolates HQ-7 and HQ-9 were both identified as members of the genus Fusarium.
Neighbor-joining phylogenetic tree based on ITS sequences obtained from the 3 fungal isolates. The endophytic strains are highlighted in bold. Numbers at the branch points are the bootstrap values based on 1000 resamplings. Bootstrap values of above 35% are shown at branch points. The scale bar represents 0.020 nucleotide changes per position
Antibacterial activity of the fermentation broth by the agar well diffusion method
The ME and PD fermentation broth of the three fungi were tested against gram-positive and gram-negative bacteria using the agar well diffusion method. As shown in Table 1, the ME fermentation broth exhibited more effective antibacterial activity than the PD broth. Among the tested endophytic fungi, the ME broth of Chaetomium sp. HQ-1 exhibited the highest zones of inhibition (17 mm against S. aureus, 18 mm against MRSA, 16 mm against L. monocytogenes, 15 mm against E. coli and 11 mm against S. enterica subsp. enterica serovar). Thus, Chaetomium sp. HQ-1 was selected for further research.
Structural characterization of bioactive compounds
Compound 1, obtained as an amorphous white powder, was determined to have a molecular formula of C11H12Cl2O4 according to the Na+-ligand molecular ion at m/z 301.0034 (calcd for C11H12Cl2O4Na, 301.0036) from its high-resolution electrospray ionization mass spectrometry (HR–ESI–MS) analysis (Fig. S1). 1H NMR (Fig. S2) (600 MHz; acetone-d6; δ, ppm; J, Hz): 3.91 (3H, s), 3.09 (2H, t, J = 8.4), 1.63 (2H, dt, J = 7.8, 8.4), 1.00 (3H, t, J = 7.8). 13C NMR (Fig. S3) (150 MHz; acetone-d6; δ, ppm): 171.9, 157.8, 157.1, 142.8, 121.5, 115.8, 113.3, 60.9, 34.7, 23.8, 14.5. These data were identical to those of differanisole A (Oka et al. 1985) (Fig. 3).
Compound 2, an amorphous white powder, was shown to have a molecular formula of C9H10Cl2O from its protonated molecular ion at m/z 205.0802 in its HR–ESI–MS (Fig. S4) (C9H11Cl2O requires 205.0799). 1H NMR (Fig. S5) (600 MHz; acetone-d6; δ, ppm; J, Hz): 8.01 (1H, s), 6.17 (2H, s), 2.41 (2H, t, J = 8.4), 1.56 (2H, dt, J = 7.8, 8.4), 0.90 (3H, t, J = 7.8). 13C NMR (Fig. S6) (150 MHz; acetone-d6; δ, ppm): 159.3, 145.6, 107.7, 107.6, 100.9, 100.8, 38.7, 25.1, 14.1. These data were identical to those of 2,6-dichloro-4-propylphenol (Christie et al. 1977) (Fig. 3).
Compound 3, isolated as an amorphous white powder, was shown to have a molecular formula of C8H10O2 by the Na+-ligand molecular ion at m/z 161.0716 in its HR–ESI–MS spectrum (Fig. S7) (C8H10O2Na requires 161.0712). 1H NMR (Fig. S8) (600 MHz; acetone-d6; δ, ppm; J, Hz): 7.96 (1H, s), 7.82 (1H, s), 6.24 (1H, s), 6.18 (1H, s), 2.12 (3H, s), 2.00 (3H, s). 13C NMR (Fig. S9) (150 MHz; acetone-d6; δ, ppm): 156.5, 156.2, 138.9, 114.1, 108.2, 100.7, 20.3, 11.0. These data were identical to those of 4,5-dimethylresorcinol (Ogata and Shibata 2004) (Fig. 3).
MIC of the bioactive compounds
The MIC values of the three compounds were determined by a tube dilution technique against 3 gram-positive and 2 gram-negative bacteria. As reported in Table 2, compound 1 showed an MIC of 16 μg/mL for L. monocytogenes and an MIC of 128 μg/mL for S. aureus and MRSA. When compared to differanisole A, the antibiotic ampicillin exhibited higher antibacterial activity against L. monocytogenes and S. aureus (MIC of 2 μg/mL), but lower activity against the resistant pathogen MRSA (MIC > 128 μg/mL). Compounds 2 and 3 could inhibit the growth of L. monocytogenes with MICs of 64 and 32 μg/mL, respectively.
Antifungal activity of bioactive compounds
Four plant pathogenic fungi were used for the antifungal activity determination of the three compounds, and actidione was used as a positive control. As shown in Table 3, all the pathogenic fungi in the test were inhibited by Compound 1. The inhibition rates increased with increasing concentration, and the inhibition rates against the different organisms ranged from 10.3 to 91.2% at a concentration of 128 μg/mL of 1. Particularly, differanisole A showed pronounced inhibitory activity against S. rolfsii with a 50% inhibitory concentration (IC50 value) of less than 16 μg/mL, which was comparable to that of actidione (< 8 μg/mL, Table 4). Compound 3 also displayed promising antifungal activity (Table 5). The inhibition rates of 3 against the four plant pathogens ranged from 19.4 to 75.7% at 128 μg/mL, and the IC50 value against S. rolfsii was less than 32 μg/mL. Compound 2 exhibited no activity against the four plant pathogens (data not shown).
Discussion
Staphylococcus aureus is a major human pathogen that causes bacteremia, infective endocarditis and other clinical infections. These infections depend to a large extent on the dissemination of antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA) (Vuong et al. 2016). Listeria monocytogenes is widespread in the environment and is the second leading cause of death due to food-borne bacterial outbreaks in the USA, with a mortality rate of 20–25% (Hamon et al. 2006; Hoffmann et al. 2012). The first leading cause of food-borne infection is Salmonella. As estimated by the Centers for Disease Control and Prevention (CDC), each year in the USA, S. enterica causes 1.2 million infections, 24,000 hospitalizations, and 450 deaths (Scallan et al. 2011). In view of this, four pathogenic strains, S. aureus, MRSA, L. monocytogenes and S. enterica, together with the most common intestinal bacteria E. coli, were used in our study to identify new antibiotic molecules from endophytes.
Endophytes are ubiquitous organisms that are valued for their ability to synthesize various functional natural products. In particular, endophytic fungi isolated from medicinal plants may produce metabolites that are similar to their hosts and have been shown to be a rich source of precious bioactive compounds (Egamberdieva et al. 2017; Gouda et al. 2016; Golinska et al. 2015). Astragalus, known by the Chinese name of huáng qí, is one of the most important Chinese traditional herbs and has been used for more than 2000 years. It has been proven to possess various pharmacological effects including antitumor, antioxidant, immunomodulatory, antiaging and antibacterial activities (Liu et al. 2017; Fan et al. 2012; Jiang et al. 2010). Gunatilaka’s group discovered several cytotoxic compounds from fungal endophytes of Astragalus lentiginosus. For example, secoemestrin D displayed significant cytotoxicity against six tumor cell lines with IC50 values ranging from 0.06 to 0.24 μM (Bashyal et al. 2017; Xu et al. 2013). Some other studies have also reported the antimicrobial potential of Astragalus endophytes (Mazinani et al. 2017; Liu et al. 2015).
In our course of finding antibacterial endophytes of Astragalus chinensis (widely distributed in northern China), three fungal strains were isolated and determined to be Chaetomium sp. HQ-1, Fusarium sp. HQ-7 and Fusarium sp. HQ-9. Chaetomium is a common endophytic fungus, and numerous novel bioactive molecules have recently been isolated from this genus. For example, chaetoconvosin B, which significantly inhibits the root elongation of wheat, was isolated from C. convolutum cib-100 (Xu et al. 2012). Chaetoglines B and F, which exhibited significant antibacterial activities against clinically pathogenic anaerobes, were obtained from C. globosum 1C51 residing in Epinephelus drummondhayi (Yan et al. 2014). Several chaetosemins with antifungal and antioxidant activities were discovered from C. seminudum (Li et al. 2014). Aureochaeglobosins A–C, three novel compounds with significant cytotoxicity against human cancer cells, were provided by the endophytic fungus C. globosum (Yang et al. 2018). In our study, Chaetomium sp. HQ-1 displayed great potential for producing antibiotics and was thus selected for further study.
Bioassay-guided fractionation of the ME fermentation broth of Chaetomium sp. HQ-1 led to the isolation of compounds 1 ~ 3, which were identified as differanisole A (1), 2,6-dichloro-4-propylphenol (2), and 4,5-dimethylresorcinol (3). Differanisole A, an inducer of the differentiation of Friend leukemic cells, has been proven to be an antitumor antibiotic (Kubohara et al. 1993; Kanatani et al. 1997). However, this paper is the first report of the antibacterial activity of differanisole A. Compared to ampicillin, differanisole A exhibited better antibacterial activity against the resistant pathogen MRSA. 2,6-Dichloro-4-propylphenol (2) was once obtained as an intermediate in chemical synthesis (Christie et al. 1977), and this was the first report of its isolation as a natural product and its antibacterial activity. 4,5-Dimethylresorcinol (3) has been proven to inhibit intestinal Cl− secretion through Cl− channels (Ogata and Shibata 2004), while this was the first discovery of its antimicrobial activity. The ME fermentation broth of strain HQ-1 showed antibacterial activity against 5 pathogenic bacteria, but the compounds could only inhibit 3 gram-positive bacteria at 128 μg/mL. This can be explained by the fact that some other active metabolites must be present in the fermentation broth of strain HQ-1 that can inhibit gram-negative bacteria and should be explored further.
Since the metabolites from the genus Chaetomium have shown antifungal activity and can be used for biological control (Zhao et al. 2017; Li et al. 2015), the antifungal activity of these three compounds was also determined in vitro. Interestingly, differanisole A and 4,5-dimethylresorcinol showed pronounced inhibition activity against S. rolfsii, a destructive soil-borne fungal pathogen that causes stem rot (Jogi et al. 2016), with IC50 values less than 16 and 32 µg/mL, respectively. Thus, these two compounds were the major contributors to the selective antifungal activity of the strain HQ-1. This is also the first report of the antifungal activity of differanisole A and 4,5-dimethylresorcinol.
Conclusion
This study reported the isolation and taxonomic study of three antibacterial endophytic fungi from Astragalus chinensis. Among them, strain Chaetomium sp. HQ-1 showed the best bioactivity and was further fermented to research its metabolites. Three bioactive molecules, differanisole A (1), 2,6-dichloro-4-propylphenol (2) and 4,5-dimethylresorcinol (3) were obtained and elucidated. Moreover, compounds 1 and 3 also exhibited promising antifungal activity against Selerotium rolfsii. Thus, based on this study, it was concluded that fungal endophytes from A. chinensis have antibacterial and antifungal potential. Future investigations are needed for a better understanding of the other antimicrobial agents produced by this and other endophytic fungi of A. chinensis.
References
Alvin A, Miller KI, Neilan BA (2014) Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res 169:483–495
Asgari B, Zare R (2011) The genus Chaetomium in Iran, a phylogenetic study including six new species. Mycologia 103(4):863–882
Basak S, Singh P, Rajurkar M (2016) Multidrug resistant and extensively drug resistant bacteria: a study. J Pathog. https://doi.org/10.1155/2016/4065603
Bashyal BP, Wijeratne EMK, Tillotson J, Arnold AE, Chapman E, Gunatilaka AAL (2017) Chlorinated dehydrocurvularins and alterperylenepoxide A from Alternaria sp. AST0039, a fungal endophyte of Astragalus lentiginosus. J Nat Prod 80:427–433
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis 48:1–12
Christie RM, Rickards RW, Schmalzl KJ, Taylor D (1977) Ring contraction of 4-substituted 2, 6-dichlorophenols. The crystal structure of 2, 2, 4α, 5α-tetrachloro-1α, 3α-dihydroxycyclopentane-1, 4-carbolactone. Aust J Chem 30(10):2195–2204
Egamberdieva E, Wirth S, Behrendt U, Ahmad P, Berg G (2017) Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199
Fan YP, Hu YL, Wang DY, Liu JG, Zhang J, Zhao XJ, Liu X, Liu C, Yuan J, Ruan S (2012) Effects of Astragalus polysaccharide liposome on lymphocyte proliferation in vitro and adjuvanticity in vivo. Carbohydr Polym 88:68–74
Golinska P, Wypij M, Agarkar G, Rathod D, Dahm H, Rai M (2015) Endophytic actinobacteria of medicinal plants: diversity and bioactivity. Anton Leeuw 108:267–289
Gouda S, Das G, Sen SK, Shin HS, Patra JK (2016) Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol 7:1538
Hamon M, Bierne H, Cossart P (2006) Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434
Hoffmann S, Batz MB, Morris JG Jr (2012) Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302
Ibrahim LF, Marzouk MM, Hussein SR, Kawashty SA, Mahmoud K, Saleh NAM (2013) Flavonoid constituents and biological screening of Astragalus bombycinus. Nat Prod Res 27:386–393
Jiang JB, Wu CH, Gao H, Song JD, Li HQ (2010) Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine 28:5614–5616
Jogi A, Kerry JW, Brenneman TB, Leebens-Mack JH, Gold SE (2016) Identification of genes differentially expressed during early interactions between the stem rot fungus (Sclerotium rolfsii) and peanut (Arachis hypogaea) cultivars with increasing disease resistance levels. Microbiol Res 184:1–12
Kanatani Y, Makishima M, Ken-i Asahi, Sakurai A, Takahashi N, Motoyoshi K, Nagata N (1997) Differanisole A, a novel antitumor antibiotic, enhances growth inhibition and differentiation of human myeloid leukemia cells induced by 9-cis retinoic acid. BBA Mol Cell Res 1359:71–79
Kubohara Y, Okamoto K, Tanaka Y, Ken-i Asahi, Sakurai A, Takahashi N (1993) Differanisole A, an inducer of the differentiation of Friend leukemic cells, induces stalk cell differentiation in Dictyostelium discoideum. FEBS Lett 322(1):73–75
Lewis K (2012) Antibiotics: recover the lost art of drug discovery. Nature 485:439–440
Li R, Chen WC, Wang WP, Tian WY, Zhang XG (2010) Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr Polym 82(2):240–244
Li X, Qu L, Dong Y, Han L, Liu E, Fang S, Zhang Y, Wang T (2014) A review of recent research progress on the Astragalus genus. Molecules 19(11):18850–18880
Li H, Tian JM, Tang HY, Pan SY, Zhang AL, Gao JM (2015) Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv 5:29185–29192
Liu X, Li H, Zhou F, Wang R (2015) Secondary metabolites of Fusarium sp., an endophytic fungus in Astragalus membranaceus. Chem Nat Compd 51(6):1199–1201
Liu P, Zhao H, Luo Y (2017) Anti-aging implications of Astragalus membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis 8(6):868–886
Liu D, Chen L, Zhao J, Cui K (2018) Cardioprotection activity and mechanism of Astragalus polysaccharide in vivo and in vitro. Int J Biol Macromol 111:947–952
Martinez-Klimova E, Rodríguez-Peña K, Sánchez S (2017) Endophytes as sources of antibiotics. Biochem Pharmacol 134:1–17
Mazinani Z, Zamani M, Sardari S (2017) Isolation and identification of phyllospheric bacteria possessing antimicrobial activity from Astragalus obtusifolius, Prosopis juliflora, Xanthium strumarium and Hippocrepis unisiliqousa. Avicen J Med Biotechnol 9(1):31–37
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Ogata N, Shibata T (2004) Inhibition of rat intestinal Cl− secretion by 4,5-dimethylresorcinol. Pharmacology 72(4):247–253
Oka H, Asahi KI, Morishima H, Sanada M, Shiratori K, Iimura Y, Sakurai T, Uzawa J, Iwadare S, Takahashi N (1985) Differanisole A, a new differentiation inducing substance. J Antibiot 38(8):1100–1102
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15
Schulz B, Wanke U, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Sharma D, Pramanik A, Agrawal PK (2016) Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech 6(210):1–14
Spellberg B (2012) New antibiotic development: barriers and opportunities in 2012. APUA Clin Newsl 30:8–10
Strobel GA (2003) Endophytic as sources of bioactive products. Microbes Infect 5:535–544
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tian Y, Jiang N, Zhang AH, Chen CJ, Deng XZ, Zhang WJ, Tan RX (2015) Muta-mycosynthesis of naphthalene analogs. Org Lett 17:1457–1460
Visser AA, Nobre T, Currie CR, Aanen DK, Poulsen M (2012) Exploring the potential for Actinobacteria as defensive symbionts in fungus-growing termites. Microb Ecol 63:975–985
Vuong C, Yeh AJ, Cheung GYC, Otto M (2016) Investigational drugs to treat methicillin-resistant Staphylococcus aureus. Expert Opin Investig Drug 25(1):73–93
Willyard C (2017) The drug-resistant bacteria that pose the greatest health threats. Nat New. https://doi.org/10.1038/nature.2017.21550
Wu CY, Ke Y, Zeng YF, Zhang YW, Yu HJ (2017) Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int 17:115
Xu GB, Li LM, Yang T, Zhang GL, Li GY (2012) Chaetoconvosins A and B, alkaloids with new skeleton from fungus Chaetomium convolutum. Org Lett 14:6052–6055
Xu YM, Artiles PE, Liu MX, Arnold AE, Gunatilaka AAL (2013) Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A–E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J Nat Prod 76:2330–2336
Yan W, Ge HM, Wang G, Jiang N, Mei YN, Jiang R, Li SJ, Chen CJ, Jiao RH, Xu Q, Ng SW, Tan RX (2014) Pictet-Spengler reaction-based biosynthetic machinery in fungi. Proc Natl Acad Sci 111:18138–18143
Yang MH, Gu ML, Han C, Guo XJ, Yin GP, Yu P, Kong LY (2018) Aureochaeglobosins A-C, three [4 + 2] adducts of chaetoglobosin and aureonitol dserivatives from Chaetomium globosum. Org Lett 20(11):3345–3348
Zhang YL, Li S, Jiang DH, Kong LC, Zhang PH, Xu JD (2013) Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J Agric Food Chem 61:1521–1524
Zhao SS, Zhang YY, Yan Y, Cao LL, Xiao Y, Ye YH (2017) Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol Lett 364(3):1–6
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21602152), Shandong Provincial Natural Science Foundation (ZR2016BB01), Shandong Provincial Key Laboratory of Agricultural Microbiology Open Fund (SDKL2017015).
Author information
Authors and Affiliations
Contributions
PL, DZ and RS performed the experiments and analyzed data. ZY and FZ edited the manuscript. YT designed the experiments. All authors revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, P., Zhang, D., Shi, R. et al. Antimicrobial potential of endophytic fungi from Astragalus chinensis. 3 Biotech 9, 405 (2019). https://doi.org/10.1007/s13205-019-1948-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1948-5