Abstract
The aim of this study is to determine the key laccase-encoding gene in the life cycle of Morchella importuna SCYDJ1-A1, and to characterize the biochemical properties of the laccase. Two laccase-like multicopper oxidase (LMCO) genes were identified in the genome of M. importuna SCYDJ1-A1 as putative laccase-encoding genes. The two genes, belonging to Auxiliary Activity family 1 subfamily 3, were named as MiLacA and MiLacB. Phylogenetic analysis of deduced amino acid sequences showed that MiLacA is closest to a LMCO of M. importuna 22J1, while MiLacB had low similarity with known Morchella LMCOs. Real-time quantitative PCR results showed that MiLacA was expressed at much higher levels than MiLacB throughout the entire course of artificial cultivation. MiLacA was overexpressed in Pichia pastoris as a recombinant protein. Biochemical characterization of the purified enzyme showed that MiLacA simultaneously possessed laccase and polyphenol-oxidase activities. MiLacA could be strongly inhibited by Fe2+, which is unusual. The optimum pH was four and optimum temperature was 60 °C. The enzyme retained over 74% of the laccase activity after 16-h incubation at 60 °C, which means that its thermostability is at the forefront among the currently known laccases. Our findings may help to elucidate how the laccase of M. importuna is involved in decaying lignin in plant litter, and could also provide a candidate thermostable laccase for potential industrial application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Morchella importuna, the ladder-ridged morel, is a delicious ascomycete mushroom. A strain of the morel, M. importuna SCYDJ1-A1, was domesticated successfully recently, making the mushroom artificially cultivable (Liu et al. 2018; Peng et al. 2016). As a soil-inhabiting ascomycete, M. importuna can utilize decayed plant litter in soil as a carbon (C) source (Liu et al. 2018). For instance, isotopic evidence has shown that saprotrophic morel tends to assimilate old organic matter in soils, which is unlikely to be photosynthesized in the same year (Hobbie et al. 2016). Among recalcitrant organic matter, lignin in plant litter is a major source of aromatic polymers in soils (Theuerl and Buscot 2010; Thevenot et al. 2010), and is highly resistant to chemical and biological degradation (Martínez et al. 2005).
Laccase is a phenol-oxidizing enzyme with four chelated copper ions in the catalytic center (Lyashenko et al. 2006). It is effective in oxidative degradation of the phenol units in lignin, and is also used for industrial purposes such as removing polyphenols in fruit juice and wine, de-coloring dyes in waste water, and biodegrading xenobiotic chemicals (Hildén et al. 2009). Previous studies have shown that laccase is actively involved in degradation and recycling of lignin in soils, as well as derivatives of lignin catabolites (Christopher et al. 2014) such as humus (Zavarzina et al. 2004). In addition, breakdown of lignin in lignocellulosic complexes could further improve the accessibility of cellulose and hemicellulose to hydrolytic enzymes in plant litter (Pinto et al. 2012; Shirkavand et al. 2016). Thus, laccase plays an important role in the morel to utilize plant litter in the soil (Baldrian 2006; Eichlerová et al. 2012; Theuerl and Buscot 2010). While Kanwal and Reddy (2014) reported that laccase activity was significant during sclerotia formation of M. crassipes, Kellner et al. (2007) inferred that LMCO genes were responsible for soil extracellular activities related to plant-litter decay by Morchellaceae species. These findings could be strengthened by estimating the contribution of different LMCO genes to the total laccase expression of the morel, as well as by obtaining biochemical evidence from isolated enzymatic protein.
In the present study, the importance of two LMCO genes of M. importuna SCYDJ1-A1 was compared by monitoring transcript levels throughout its life cycle. To verify the actual activity of the key LMCO, the recombinant enzyme was overexpressed in Pichia pastoris, and was then purified and characterized for its biochemical properties.
Materials and methods
Identification of LMCO genes
LMCO genes were searched in the M. importuna SCYDJ1-A1 genome (NCBI BioSample accession number SAMN05442956) with tblastx, using the LMCO gene fragments of M. importuna 22J1 (EMBL accession numbers AM269495 and AJ704782), reported by Kellner et al. (2007), as templates. The signal peptide was predicted with SignalP 4.1 (Petersen et al. 2011).
Phylogenetic analysis
Deduced amino acid (AA) sequences of MiLacA and MiLacB were compared with the 31 LMCOs in the Morchellaceae family reported by Kellner et al. (2007). Nucleotide sequences of the 31 LMCO fragments were converted into AA sequences by removing predicted introns with the same procedures described by Kellner et al. (2007). The AA sequences were aligned with MAFFT (Katoh and Toh 2008) to construct a neighbor-joining phylogenetic tree, using the procedures described previously (Tan et al. 2016c).
Structural modeling
Structural models of MiLacA and MiLacB were predicted by homolog modeling using Phyre2 (Kelley et al. 2015), and visualized with UCSF Chimera (Pettersen et al. 2004).
Sample collection
The morel strain used in the present study was M. importuna strain SCYDJ1-A1. The origin of the strain was described previously by Tan et al. (2017). Artificial cultivation of the morel was carried out, as instructed by Peng et al. (2015) and reviewed by Liu et al. (2018). The growth period of the morel was divided into the following six stages: (I) sclerotium in mushroom spawn; (II) surface-soil mycelium before contact with exogenous nutrient bag; (III) surface-soil mycelium after contact with exogenous nutrient bag; (IV) surface-soil mycelium before primordium formation; (V) primordium; (VI) fruiting body. Samples were collected from three biological replicates. Mycelium in surface soil (0–2-cm depth) of the mushroom bed, primordium and fruiting body were collected, snap-frozen by liquid nitrogen and kept at − 80 °C.
RNA extraction
RNA in surface-soil mycelium was extracted with the RNA PowerSoil Total RNA Isolation Kit (MO BIO Laboratories, Carlsbad, California, USA). RNA in the primordium and fruiting body was extracted with the Fungal Total RNA Isolation Kit (Sangon Biotech, Shanghai, China).
Real-time quantitative PCR
A constantly expressing actin gene (Act) of M. importuna SCYDJ1-A1 was used as a reference for transcript level. Both cDNA synthesis and real-time fluorescent quantitative PCR (qPCR) were carried out as described previously (Jia et al. 2017). Briefly, cDNA was synthesized with the HiScript First Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd, Nanjing, Jiangsu Province, China). MiLacA, MiLacB and ActB genes were amplified using the primers listed in Table 1. The amplification was carried out on a qTOWER 2.2 real-time fluorescent qPCR cycler (Analytik Jena AG, Jena, Thuringia State, Germany), with a program of 95 °C for 3 min, then (95 °C 10 s, 58 °C 30 s, plate read) × 45 cycles. Melting-curve analysis was carried out from 58 °C to 95 °C, with a ramp of + 1 °C and a hold of 4 s per cycle. The linear amount of target-gene transcription was compared to the calibrator and was calculated by the 2−△△Ct method.
Cloning and overexpression
The coding sequence of MiLacA was codon-optimized for yeast expression and was then chemically synthesized without the region of predicted signal peptide. An octamer-histidine tag was attached to the C-terminal end to facilitate Ni-affinity chromatography. The synthesized coding-fragment was inserted into a pPIC9 K vector between EcoRI and NotI sites, down-stream of an α-factor signal peptide provided by the plasmid, to facilitate secreting expression. The recombinant plasmid was transformed into P. pastoris GS115 (Invitrogen-Thermo Fisher Scientific, Waltham, Massachusetts, US) as described previously (Tan et al. 2017). The P. pastoris cells were grown at 28 °C in 2500 ml of liquid medium in a 7000-ml glass automatic fermenter and induced by continuous methanol feeding to overexpress the protein. The stirring speed was automatically controlled to be between 250 and 1000 rpm to maintain a dissolved oxygen content that was above 20% of the saturation point of oxygen in water. The pH was automatically controlled to be between 4.95 and 5.05. All the procedures and medium recipes were followed according to the instructions in the user manual “Pichia Fermentation Process Guidelines” issued by the manufacturer (Invitrogen-Thermo Fisher Scientific, Waltham, Massachusetts, US).
Protein purification
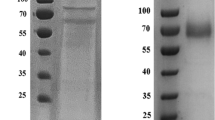
After 30 h of fermentation, the culture supernatant was collected, concentrated by ultra-filtration, de-glycosylated and purified with an AKTA pure automatic chromatography system, using the procedures described previously (Tan et al. 2018). The buffer was replaced with 20-mM PBS buffer (pH 5.0) using an ultra-filtration centrifugal tube with a 3-kDa molecular weight (Mw) cut-off (Merck-Millipore, Shanghai, China), and the total volume was then adjusted to 9.5 ml. The obtained recombinant protein was examined with HPLC to guarantee a purity ≥ 90%. The protein was quantified with a Bradford Assay, using bovine serum albumin as a standard (Bradford 1976). The Mw of the de-glycosylated MiLacA was estimated with the Matrix Assisted Laser Desorption Ionization–Time of Flight (MALDI–TOF) Mass Spectrometry.
Laccase activity assay
Laccase activity was measured with a colorimetric method as previously described by Liu et al. (2017). 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) was used as a substrate, with a final concentration of 1 mM in the reaction. A 200-μl reaction volume was loaded in a 96-well microplate. An optical absorbance at 420 nm was read by a microplate spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, US). One unit of laccase activity was defined as oxidizing 1 μmol of the substrate per minute.
Determination of pH and temperature profiles
To determine the pH profile, laccase activity was measured at pH 2–12 using buffer recipes described previously (Tan et al. 2016c). To determine the temperature profile, laccase activity was measured between 0 and 80 °C at 10 °C intervals.
Determination of pH and temperature stabilities
The pH stability and temperature stability were characterized by decline curves of laccase activity under different incubating conditions, using a method described previously (Tan et al. 2016b). The pH stability was characterized at pH 2, 4 and 6, during incubation at optimal temperature. The temperature stability was characterized at 10, 60, 70 and 80 °C, during incubation at optimal pH.
Determination of kinetic parameters
Laccase activity was measured at substrate concentrations of 0.05, 0.1, 0.5, 1, 2.5 and 5 mM. Kinetic parameters were obtained from Eadie–Hofstee plots as described previously (Tan et al. 2016a).
Determination of substrate specificity
Substrate specificity was tested using the procedures described by Zhang et al. (2013). Guaiacol, syringaldazine, N,N-dimethyl-p-phenylenediamine (DMPPDA), catechol and tyrosine were tested as potential substrates, at a final concentration of 1 mM. Activity levels of catalyzing these substrates were compared with catalyzing ABTS, which was defined as 100%.
Estimating the influences of cations
Laccase activity was measured with cations supplemented at a final concentration of 5 mM. The tested cations were Na+, K+, Ca2+, Mg2+, Al3+ Fe2+, Fe3+, Zn2+, Mn2+, Cu2+, Hg2+ and Pb2+, while the anion of the chemicals was Cl− for all cases. Laccase activity without supplementing any of these cations was defined as 100%.
Estimating the influences of inhibitory chemical agents
Laccase activity was measured with EDTA supplemented at a final concentration of 5 mM and 50 mM, sodium dodecyl sulfate (SDS) at 0.1% and 1%, and urea at 5 mM and 50 mM. Laccase activity without supplementing any of these chemicals was defined as 100%.
Replicate and statistical analysis
RNA for assessing transcript levels of MiLacA and MiLacB was extracted from three biological replicates. qPCR was carried out on the cDNA derived from the three biological replicates, respectively. Laccase activity assay for determining the biochemical properties was carried out with three technical replicates.
Significant differences were assessed with one-way analysis of variance (ANOVA) from three replicates, with a criterion of p < 0.05, using PASW Statistics version 18 (IBM Corporation, Armonk, New York, US).
Accession number
Nucleotide and amino acid sequences of MiLacA and MiLacB are accessible at the MycoCosm genome portal of M. importuna SCYDJ1-A1 at DOE Joint Genome Institute (https://genome.jgi.doe.gov/Morimp1/Morimp1.home.html) with transcript IDs 572050 and 546337, protein IDs 571670 and 546357, respectively.
Results
LMCO genes of M. importuna SCYDJ1-A1
Two LMCO gene hits were identified in the M. importuna SCYDJ1-A1 genome and were named as MiLacA and MiLacB, both of which belong to Auxiliary Activity family 1 subfamily 3 (AA1_3). The MiLacA gene is 2522 base pairs (bp) in length, with five exons and four introns (Fig. 1a), encoding for a 606 AA protein with a 19 AA N-terminal signal peptide. The MiLacB gene is 2382 bp with three exons and two introns (Fig. 1a), encoding for a 561 AA protein with a 22 AA signal peptide. The presence of signal peptides suggests that MiLacA and MiLacB are both secreted as extracellular enzymes.
a Schematic diagram of the two laccase-like multicopper oxidase (LMCO) genes of M. importuna SCYDJ1-A1, MiLacA and MiLacB. b Phylogenetic analysis of amino acid (AA) sequences of MiLacA and MiLacB with 31 reference LMCOs of the Morchellaceae family. The accession numbers are in brackets. The length of the bar scale indicates 0.1 unit of phylogenetic distance in the neighbor-joining tree, equal to 0.1 substitution rate per site
The AA sequences of MiLacA and MiLacB have a similarity of 53%, as compared by blastp. Compared with the AA fragments of other Morchellaceae LMCOs known to date (Fig. 1b), MiLacA is closest to the LMCO of M. importuna 22J1 (EMBL accession number AM269495), with 98% similarity; whereas, MiLacB is in a unique branch in the phylogenetic tree.
Structural model
The Botrytis aclada laccase (PDB accession number: 3SQR) and the H253D mutant of Aspergillus niger laccase McoG (PDB accession number: 5LWX) were the best structural templates for MiLacA and MiLacB, respectively, as screened and estimated by Phyre2. MiLacA had 89% alignment coverage and 53% sequence identity with the B. aclada laccase, while MiLacB had 93% alignment coverage and 53% sequence identity with the H253D mutant of A. niger laccase. The predicted structural models of MiLacA and MiLacB both had a homolog confidence of 100% with their respective templates. The structural models of MiLacA and MiLacB were both near a trimerous radial symmetric shape (Fig. 2a, b), containing three pair of β-sheets, linked with short α-helices and free coils. It is a typical structure of laccase protein. MiLacA is closer to a standard radial symmetric trigonal prism, while MiLacB is closer to an elliptical cylinder.
Gene expression during artificial cultivation
Expression of MiLacA and MiLacB was monitored by transcript levels throughout six stages of the course of artificial cultivation. The qPCR results showed that the MiLacA gene had a much higher transcript level than that of MiLacB, particularly at the primordium stage (Fig. 3). This result suggests that MiLacA may play a more effective role than MiLacB in the life cycle of the morel, and is, therefore, inferred as the key LMCO of the two.
Transcript levels of MiLacA (blue) and MiLacB (yellow) as monitored by qPCR of cDNA, during six stages in the course of artificial cultivation: (I) sclerotium in mushroom spawn; (II) surface-soil mycelium before contact with exogenous nutrient bag; (III) surface-soil mycelium after contact with exogenous nutrient bag; (IV) surface-soil mycelium before primordium formation; (V) primordium; (VI) fruiting body
Overexpression of recombinant protein
To verify the actual enzymatic activity of the key LMCO, MiLacA was overexpressed in P. pastoris GS115. A total of 5.64 mg of purified protein was obtained. Mass spectrometry of MALDI–TOF (Fig. S1) showed an estimated Mw of 66 kDa, similar to its theoretical monomeric Mw of 66.3 kDa. This result confirms that MiLacA was overexpressed as a monomer.
pH and temperature profiles
MiLacA showed the highest laccase activity level at a pH of 4 (Fig. 4a). The enzyme possessed over half of the maximal activity level between a pH of 2 and 5, low activity at a pH of 6, and the activity almost disappeared at pH > 7. The temperature profile showed that the laccase activity reached a maximum around 50–60 °C but decreased greatly over 70 °C (Fig. 4b), indicating that the enzyme is between mesophilic and thermophilic. At optimal pH and temperature (pH 4, 60 °C), MiLacA showed the maximal activity level at 28.59 ± 0.77 U/mg.
pH and temperature stabilities
When incubated at a pH of 4 or 6 (Fig. 5a), MiLacA retained over 80% residual activity after 8 h, and about 75% after 16 h. However, the laccase activity quickly declined when incubated at a pH of 2. The residual activity dropped to 58% after only 10 min and to 37% after 2 h. This result demonstrates that the enzyme is long-lasting between near-neutral to weak-acidic pH, but has poor resistance to strong-acidic pH. When incubated at the optimal temperature of 60 °C, the enzyme was almost as stable as when kept at 10 °C, retaining about a 75% residual-activity level after 16 h (Fig. 5b). In comparison, the enzyme retained only 53% residual activity after 4-h incubation at 70 °C. When incubated at 80 °C, the laccase activity declined even more quickly, losing over half of the laccase activity after only 3 h.
Kinetic features
At the optimal pH and temperature, MiLacA showed a Michaelis constant (Km) of 0.193 ± 0.004 mM, a maximal-reaction rate (Vmax) of 34.71 ± 0.23 U/mg, and a catalytic efficiency (kcat/Km) of (4.79 ± 0.07) × 104/M/s.
Substrate specificity
MiLacA showed the highest activity on guaiacol, while the activities on syringaldazine and DMPPDA were much lower than on ABTS and guaiacol (Fig. 6a). Oxidase activity was observed on catechol, indicating that the enzyme also possesses a polyphenol-oxidase activity. No activity could be detected on tyrosine.
a Relative activity of MiLacA on different substrates. The activity level on the ABTS substrate was defined as 100%. b Influence of metallic cation on laccase activity. The activity level without adding any of the metallic cations was defined as 100%. c Influence of activity inhibitors on laccase activity. The activity level without adding any of the chemicals was defined as 100%
Effects of cations
Among the usual mineral cations, Na+, K+, Ca2+, Mg2+, Al3+, and Cu2+ showed slight or small impacts on laccase activity, while Zn2+ significantly stimulated laccase activity (Fig. 6b). The laccase activity was decreased greatly by Mn2+ and Fe3+. The heavy metallic cation, Pb2+, showed less inhibition than Hg2+. Fe2+ showed a surprisingly high inhibition on laccase activity, which was almost as severe as that by Hg2+.
Effects of inhibitory chemical agents
The chelating agent, EDTA, inhibited the laccase activity to 29% and 23% at concentrations of 5 mM and 50 mM, respectively (Fig. 6c). This result demonstrated that cation-depriving, caused by chelating agents, has a markedly negative influence on laccase activity. Additionally, 0.1% SDS caused 56% loss of laccase activity, while 1% SDS caused a 70% loss. Similarly, residual activity in the presence of 5 mM and 50 mM urea was 26% and 27%, respectively. These results indicated that MiLacA is sensitive to protein denaturants SDS and urea, and that the inhibitory effect is obvious even at low denaturant concentrations.
Discussion
Morchella LMCO genes have previously been surveyed (Kellner et al. 2007) by amplification of partial fragments of LMCO genes, from various species in the Morchellaceae family. Kellner et al. (2007) revealed that some Morchella strains likely possess only one LMCO gene, while some possess two and some even possess 4–6 LMCO genes. This suggests that the number of LMCO genes possessed by morels is not unified among different species and strains. In our present study, two different LMCO genes were identified in a special morel strain that can be artificially cultivated. Only one of the two LMCO genes was actively expressed throughout the growth stages of the morel life cycle, while the other LMCO gene always showed slight expression. It is not surprising that fungi can possess multiple alternative genes for the same function, among which some alternatives play more important roles than other alternatives (Alfaro et al. 2016; Bánfi et al. 2015; Doré et al. 2015; Xie et al. 2016).
Kellner et al. (2007) verified laccase activity of morel strains with crude protein secreted from hypha. Our present study further verified laccase activity by obtaining purified recombinant protein of MiLacA via heterologous overexpression. In addition, biochemical properties of purified MiLacA enzyme were characterized. Besides laccase activity, our present study revealed that MiLacA also possesses a polyphenol-oxidase function. This feature is similar to the laccase of Lepiota ventriosospora (Zhang et al. 2013).
The laccase activity of M. importuna could be strongly induced by guaiacol and o-dianisidine, as reported by Kellner et al. (2007). Coincidently, our present study showed that MiLacA of M. importuna SCYDJ1-A1 had a high activity level on guaiacol, and was even higher than that on ABTS. The result demonstrates that guaiacol is a proper inducer and substrate for M. importuna laccase. This suggests that guaiacol may have an important role in lignin metabolism of M. importuna. Indeed, M. importuna usually distributes in soils under coniferous forest (Loizides 2017). Since lignin in coniferous wood is mainly composed of guaiacol units, plant-litter decomposition might input guaiacol units into the soil. This may support the growth and metabolism of M. importuna.
The severe inhibition by Fe2+ is surprising and unusual, since there is only one such example reported previously (Ling et al. 2015). Laccase is known to participate actively in oxidative breakdown of aromatic substrates in soils (Eichlerová et al. 2012; Theuerl and Buscot 2010; Zavarzina et al. 2004). Therefore, highly reductive soil may convert a higher proportion of ferric iron into ferrous iron, and could thereby hamper the function of MiLacA.
MiLacA is not as acidophilic as most of the other fungal laccases reported previously (Baldrian 2006), although its optimal pH is lower than those of bacterial laccases (Christopher et al. 2014; Mate and Alcalde 2017). MiLacA has slight laccase activity at a pH above 7. This indicates that MiLacA may not be optimal in neutral or slight-alkalic soils, since MiLacA is a predicted extracellular enzyme. However, mycelium of the morel may secrete organic-acid exudates into the soil and create a local low-pH condition for this enzyme. MiLacA has limited thermostability at 70 °C and 80 °C. However, the much slower decline of laccase activity at 60 °C compared with most of the previously reported laccases (Baldrian 2006; Bo et al. 2018; Chefetz et al. 1998; Endo et al. 2003; Hildén et al. 2007, 2009; Kiiskinen et al. 2004; Liu et al. 2017; Michniewicz et al. 2006; Zhang et al. 2013) indicates that MiLacA still possesses good thermostability around 60 °C.
Conclusion
The black morel M. importuna SCYDJ1-A1 possesses two LMCO genes, namely MiLacA and MiLacB. MiLacA is the dominant LMCO throughout the morel’s course of artificial cultivation. MiLacA showed laccase and polyphenol-oxidase activity, which demonstrates its physiological role in the mechanism of organic-matter decomposition in M. importuna SCYDJ1-A1. MiLacA is thermostable, with optimal laccase activity at weak-acidic pH and medium–high temperatures. The biochemical properties of MiLacA make it promising for industrial application as a long-lasting, mesophilic–thermophilic biocatalyst.
References
Alfaro M, Castanera R, Lavín JL, Grigoriev IV, Oguiza JA, Ramírez L, Pisabarro AG (2016) Comparative and transcriptional analysis of the predicted secretome in the lignocellulose-degrading basidiomycete fungus Pleurotus ostreatus. Environ Microbiol 18:4710–4726
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Bánfi R, Pohner Z, Kovács J, Luzics S, Nagy A, Dudás M, Tanos P, Márialigeti K, Vajna B (2015) Characterisation of the large-scale production process of oyster mushroom (Pleurotus ostreatus) with the analysis of succession and spatial heterogeneity of lignocellulolytic enzyme activities. Fung Biol 119:1354–1363
Bo W, Yan Y, Xu J, Fu X, Han H, Gao J, Li Z, Wang L, Tian Y, Peng R, Yao Q (2018) Heterologous expression and characterization of a laccase from Laccaria bicolor in Pichia pastoris and Arabidopsis thaliana. J Microbiol Biotechnol 28:2057–2063
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chefetz B, Chen Y, Hadar Y (1998) Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl Environ Microbiol 64:3175–3179
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energ Res 2:12
Doré J, Perraud M, Dieryckx C, Kohler A, Morin E, Henrissat B, Lindquist E, Zimmermann SD, Girard V, Kuo A, Grigoriev IV, Martin F, Marmeisse R, Gay G (2015) Comparative genomics, proteomics and transcriptomics give new insight into the exoproteome of the basidiomycete Hebeloma cylindrosporum and its involvement in ectomycorrhizal symbiosis. New Phytol 208:1169–1187
Eichlerová I, Šnajdr J, Baldrian P (2012) Laccase activity in soils: considerations for the measurement of enzyme activity. Chemosphere 88:1154–1160
Endo K, Hayashi Y, Hibi T, Hosono K, Beppu T, Ueda K (2003) Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J Biochem 133:671–677
Hildén K, Hakala TK, Maijala P, Lundell TK, Hatakka A (2007) Novel thermotolerant laccases produced by the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol 77:301–309
Hildén K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31:1117
Hobbie EA, Rice SF, Weber NS, Smith JE (2016) Isotopic evidence indicates saprotrophy in post-fire Morchella in Oregon and Alaska. Mycologia 108:638–645
Jia D, Wang B, Li X, Peng W, Zhou J, Tan H, Tang J, Huang Z, Tan W, Gan B, Yang Z, Zhao J (2017) Proteomic analysis revealed the fruiting-body protein profile of Auricularia polytricha. Curr Microbiol 74:943–951
Kanwal HK, Reddy MS (2014) Influence of sclerotia formation on ligninolytic enzyme production in Morchella crassipes. J Basic Microbiol 54:S63–S69
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858
Kellner H, Luis P, Buscot F (2007) Diversity of laccase-like multicopper oxidase genes in Morchellaceae: identification of genes potentially involved in extracellular activities related to plant litter decay. FEMS Microbiol Ecol 61:153–163
Kiiskinen L-L, Kruus K, Bailey M, Ylösmäki E, Siika-aho M, Saloheimo M (2004) Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiology 150:3065–3074
Ling Z-R, Wang S-S, Zhu M-J, Ning Y-J, Wang S-N, Li B, Yang A-Z, Zhang G-Q, Zhao X-M (2015) An extracellular laccase with potent dye decolorizing ability from white rot fungus Trametes sp. LAC-01. Int J Biol Macromol 81:785–793
Liu Y, Huang L, Guo W, Jia L, Fu Y, Gui S, Lu F (2017) Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochem 53:125–134
Liu Q, Ma H, Zhang Y, Dong C (2018) Artificial cultivation of true morels: current state, issues and perspectives. Crit Rev Biotechnol 38:259–271
Loizides M (2017) Morels: the story so far. Field Mycol 18:42–53
Lyashenko AV, Bento I, Zaitsev VN, Zhukhlistova NE, Zhukova YN, Gabdoulkhakov AG, Morgunova EY, Voelter W, Kachalova GS, Stepanova EV, OgV Koroleva, Lamzin VS, Tishkov VI, Betzel C, Lindley PF, AbM Mikhailov (2006) X-ray structural studies of the fungal laccase from Cerrena maxima. J Biol Inorgan Chem 11:963–973
Martínez ÁT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S, Guillén F, Martínez MJ, Gutiérrez Suárez A, Río Andrade JCd (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
Mate DM, Alcalde M (2017) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467
Michniewicz A, Ullrich R, Ledakowicz S, Hofrichter M (2006) The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl Microbiol Biotechnol 69:682–688
Peng W, Chen Y, Tan H, Tang J, Gan B (2015) Artificial cultivation of morels is blooming in Sichuan. In: bulletin for 8th international conference on mushroom biology and mushroom products 13
Peng W, Tang J, He X, Chen Y, Tan H (2016) Status analysis of morel artificial cultivation in Sichuan. Edible Med Mushrooms 24:145–150
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth 8:785–786
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Pinto PA, Dias AA, Fraga I, Marques G, Rodrigues MAM, Colaço J, Sampaio A, Bezerra RMF (2012) Influence of ligninolytic enzymes on straw saccharification during fungal pretreatment. Bioresour Technol 111:261–267
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—a review. Renew Sust Energ Rev 54:217–234
Tan H, Miao R, Liu T, Cao X, Wu X, Xie L, Huang Z, Peng W, Gan B (2016a) Enhancing the thermal resistance of a novel Acidobacteria-derived phytase by engineering of disulfide bridges. J Microbiol Biotechnol 26:1717–1722
Tan H, Wu X, Xie L, Huang Z, Peng W, Gan B (2016b) Identification and characterization of a mesophilic phytase highly resilient to high-temperatures from a fungus-garden associated metagenome. Appl Microbiol Biotechnol 100:2225–2241
Tan H, Wu X, Xie L, Huang Z, Peng W, Gan B (2016c) A novel phytase derived from an acidic peat-soil microbiome showing high stability under acidic plus pepsin conditions. J Mol Microbiol Biotechnol 26:291–301
Tan H, Tang J, Li X, Liu T, Miao R, Huang Z, Wang Y, Gan B, Peng W (2017) Biochemical characterization of a psychrophilic phytase from an artificially cultivable morel Morchella importuna. J Microbiol Biotechnol 27:2180–2189
Tan H, Miao R, Liu T, Yang L, Yang Y, Chen C, Lei J, Li Y, He J, Sun Q, Peng W, Gan B, Huang Z (2018) A bifunctional cellulase–xylanase of a new Chryseobacterium strain isolated from the dung of a straw-fed cattle. Microb Biotechnol 11:381–398
Theuerl S, Buscot F (2010) Laccases: toward disentangling their diversity and functions in relation to soil organic matter cycling. Biol Fertil Soils 46:215–225
Thevenot M, Dignac MF, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–1211
Xie C, Luo W, Li Z, Yan L, Zhu Z, Wang J, Hu Z, Peng Y (2016) Secretome analysis of Pleurotus eryngii reveals enzymatic composition for ramie stalk degradation. Electrophoresis 37:310–320
Zavarzina AG, Leontievsky AA, Golovleva LA, Trofimov SY (2004) Biotransformation of soil humic acids by blue laccase of Panus tigrinus 8/18: an in vitro study. Soil Biol Biochem 36:359–369
Zhang G-Q, Chen Q-J, Wang H-X, Ng TB (2013) A laccase with inhibitory activity against HIV-1 reverse transcriptase from the mycorrhizal fungus Lepiota ventriosospora. J Mol Cat B: Enz 85–86:31–36
Acknowledgements
This research was supported by the Sichuan Science and Technology Program (Applied Fundamental Research Project, 2018JY0637), Innovative Improvement Projects of Sichuan Province (2016ZYPZ-028, 2016LWJJ-007), and the Special Fund for Agro-scientific Research in the Public Interest (201503137).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Q., Miao, R., Liu, T. et al. Biochemical characterization of a key laccase-like multicopper oxidase of artificially cultivable Morchella importuna provides insights into plant-litter decomposition. 3 Biotech 9, 171 (2019). https://doi.org/10.1007/s13205-019-1688-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1688-6