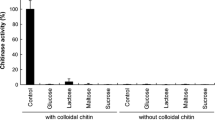
Abstract
The filamentous fungus Aspergillus niveus produced extracellular antifungal chitinase when cultured under submerged fermentation (SbmF) using crab shells as the carbon source. Maximal chitinase production was achieved at 192 h of cultivation using minimal medium containing 1% chitin. The enzyme was purified 1.97-fold with 40% recovery by ammonium sulfate precipitation and Sephadex G-100 gel filtration. The molecular mass was estimated to be 44 kDa by both 12% SDS-PAGE and Sepharose CL-6B gel filtration. Maximal A. niveus chitinase activity was obtained at 65 °C and pH 5.0. The enzyme was fully stable at 60 °C for up to 120 min and the enzymatic activity was increased by Mn2+. In the presence of reducing and denaturing compounds, the enzyme activity was not drastically affected. The chitinase was able to hydrolyze colloidal chitin, azure chitin, and 4-nitrophenyl N-acetyl-β-D glucosaminide; for the latter, the K0.5 and maximal velocity (Vmax) were 3.51 mM and 9.68 U/mg of protein, respectively. The A. niveus chitinase presented antifungal activity against Aspergillus niger (MIC = 84 µg/mL), A. fumigatus (MIC = 21 µg/mL), A. flavus (MIC = 24 µg/mL), A. phoenicis (MIC = 24 µg/mL), and Paecilomyces variotii (MIC = 21 µg/mL). The fungus A. niveus was able to produce a thermostable and denaturation-resistant chitinase able to inhibit fungal development, signaling its biotechnological potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitin is a homopolymer of β-1,4-linked N-acetyl-d-glucosamine that is widely distributed in nature, being the second most abundant polymer after cellulose. It is the major structural component of fungal cell walls and the exoskeleton of arthropods (Seidl 2008). The enzymatic hydrolysis of chitin occurs through the action of chitinolytic enzymes that cleave the β-1,4-glycosidic bonds of this polymer. This group of enzymes can be classified as endochitinases, exochitinases, or N-acetylglucosaminidases according to the cleavage mechanism of the chitin polymer (Rathore and Gupta 2015). Endochitinases randomly cleave β-1,4-glycosidic bonds of chitin, whereas exochitinases cleave the chitin from the non-reducing end to form diacetyl-chitobiose [(GlcNAc)2]. N-acetylglucosaminidases hydrolyze (GlcNAc)2 producing GlcNAc. They also can produce GlcNAc from the non-reducing end of chitin and N-acetyl-chitooligosaccharides (Li 2006). These enzymes can be produced by different organisms, from bacteria to human. Filamentous fungi can contain between 10 and 25 different chitinase genes and the role of the chitinases in these microorganisms includes degradation and the use of exogenous chitin for nutrition, cell wall remodeling during the fungal life cycle, and competition or defense against other fungi or arthropods. Entomopathogenic and nematode-trapping fungi use chitinases as “weapons” to attack insects and nematodes, respectively (Seidl 2008).
Fungal chitinases show a biotechnological potential for different applications such as the biocontrol of pests that attack different plantations causing economic loss worldwide, reducing or eliminating the use of chemical pesticides, and minimizing the negative impact on the environment. In addition, these enzymes can be used for the isolation of protoplasts from fungi and yeasts, for the preparation of single-cell protein, and for the treatment of chitinous waste from the fishery industry (Rathore and Gupta 2015). N-acetyl-d-glucosamine (an important therapeutic agent in the treatment of osteoarthritis) and bioactive chitooligosaccharides (which are important as antitumor agents) can also be obtained using chitinases (Lodhi et al. 2014). Antifungal chitinases have also been reported (Berini et al. 2017; Swiontek Brzezinska and Jankiweicz 2012). Considering the biotechnological potential of chitinases associated with the importance of finding new fungal strains as sources of enzymes with distinctive characteristics, we describe the production and characterization of an extracellular chitinase from Aspergillus niveus under submerged fermentation using crab shells as an inducer. Moreover, the antifungal potential of the chitinase is also evaluated.
Materials and methods
Fungal strain and production of chitinase
The filamentous fungus Aspergillus niveus, deposited in the culture collection of the Laboratory of Microbiology of the Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, University of São Paulo (USP), was maintained on PDA (potato dextrose agar) slants previously autoclaved at 121 °C, 1 atm, for 15 min. The cultures were kept at 30 °C for 5 days and then stored at 4 °C.
The chitinase production by A. niveus was carried out using Adams (Adams 1990), Czapek (Wiseman 1975), Khanna (Khanna et al. 1995), minimal (Hill and Kafer 2001), SR (Rizatti et al. 2001), M5 (Peralta et al. 1990), and YPD (Gun Lee et al. 1999) culture media containing 0.5% of chitin from crab shells (Sigma-Aldrich). Flasks with 50 mL of sterile media were inoculated with 1 mL of a spore suspension (5 × 104 spore/mL) and incubated at 30 °C for 96 h at 100 rpm.
To find the chitin concentration and time of cultivation that produced the highest enzyme production by A. niveus, chitinase production was carried out using minimal medium (50 mL) containing different chitin concentrations (w/v, 0.5, 1, 1.5, and 2%) for 48, 96, 144, 192, and 240 h of cultivation at 30 °C at 100 rpm.
Retrieval of the filtrate containing chitinase
The crude filtrate was separated from the mycelial mass by vacuum filtration using Whatman filter paper No. 1 in a Büchner funnel. The resulting crude filtrate was dialyzed against distilled water at 4 °C for 24 h, and used to determine enzyme activity and protein concentration.
Determination of chitinase activity and protein quantitation
The exochitinase activity was evaluated using 1 mM of the synthetic substrate 4-nitrophenyl N-acetyl-β-d glucosaminide (Sigma-Aldrich) in 100 mM sodium acetate buffer, pH 5.0. The reaction was performed using 200 µL of the substrate solution and 200 µL of the enzyme sample. After incubation at 40 °C for 5 min., the reaction was stopped by adding 1 mL of 1 M NaOH. The p-nitrophenolate released was quantified at λ = 405 nm in a spectrophotometer. One unit of enzyme activity (U) was defined as the amount of enzyme required to hydrolyze 1 µmol of substrate per minute under the assay conditions.
The chitinase activity was also evaluated using 10% (m/v) colloidal chitin as a substrate, using 50 mmol/L sodium acetate buffer pH 5.0 according to Rojas-Avelizapa et al. (1999). The reaction was performed at 50 °C for 1 h and stopped by adding 1 mL of 1% NaOH solution, followed by boiling for 5 min. The tubes containing the reaction medium were centrifuged at 7000×g for 5 min and the supernatants were used to determine the reducing sugars using DNS (3,5-dinitrosalicylic acid) (Miller 1959) at 490 nm. One unit of chitinase activity was defined as the amount of enzyme required to produce 1 µmol of NAG (N-acetyl-d-glucosamine) per hour under the assay conditions.
The enzymatic assay was also performed using chitin azure (Sigma®) in 50 mmol/L sodium acetate buffer, pH 5.0 as the substrate as described by Gómez Ramírez et al. (2004). The reaction was conducted at 65 °C for 3 h. Thereafter, the tubes containing the reaction medium were centrifuged at 7000×g for 10 min and the supernatants obtained measured at 560 nm. One unit of chitinase activity was defined as the amount of enzyme that produced an increase of 0.01 in absorbance. All experiments were performed in triplicate.
Protein quantitation was performed according to that described by Bradford (1976) using bovine serum albumin (BSA) as a standard. The protein concentration was expressed as mg of protein per mL of sample.
Chitinase purification
The proteins in crude filtrate (200 mL) were precipitated using ammonium sulfate to 80% saturation, at 4 °C. After overnight incubation, the solution was centrifuged at 10,000×g for 20 min at 4 °C. The sediment was dissolved in 20 mM sodium acetate buffer (pH 5.0) and dialyzed against distilled water overnight at 4 °C.
Two milliliters of the concentrated proteins was loaded into Sephadex G-100 (Sigma-Aldrich) gel filtration chromatographic column (1 × 60 cm) equilibrated with sodium acetate buffer 20 mM, pH 5. The enzyme was eluted using the same buffer at a flow rate of 0.4 mL/min and fractions of 1 mL were collected. Both the enzyme activity and the absorbance at 280 nm for each fraction were analyzed. Fractions with chitinase activity were pooled, dialyzed against distilled water for 24 h at 4 °C, and used for enzyme characterization.
Electrophoresis
12% SDS-PAGE was performed according to Laemmli (1970). After running at 120 V and 40 mA for 45 min, the gel was stained using Coomassie blue-silver. The Precision Plus Protein™ (Bio-Rad®) (10–250 kDa) was used as molecular mass marker.
The native molecular mass of the A. niveus chitinase was estimated using a Sepharose CL-6B (1 × 70 cm) chromatographic column equilibrated with Tris/HCl buffer 50 mM pH 7.0 containing KCl 100 mM. Fractions of 1 mL were collected at a flow rate of 0.4 mL/min. The β-amylase proteins (200 kDa), alcohol dehydrogenase (150 kDa), and bovine serum albumin (66 kDa) were used as standards. The void volume (Vo) was determined to be 56 mL using Blue Dextran.
Influence of temperature and pH on enzyme activity
The influence of temperature on chitinase activity was analyzed by testing enzyme activity from 30 to 85 °C. Thermal stability was assessed throughout the pre-incubation of the enzymatic samples in aqueous solution at 40, 50, and 60 °C for different periods of time (5–120 min) followed by the determination of chitinase activity.
The influence of the pH on the enzyme activity was determined by performing the enzyme assay using 50 mM citric acid buffer at different pH values (2–6). To determine the pH stability, enzyme samples were incubated in 50 mM citric acid buffer at different pH values (2–8) at 4 °C for 2 and 24 h. Thereafter, the enzymatic reactions were performed as described above.
Effect of chemical compounds on chitinase activity
The effect of several chemical compounds [1 mM: MnCl2, MgSO4, KI, CuSO4, FeSO4, CoCl2, CaCl2, ZnSO4, EDTA, SDS, urea, β-mercaptoethanol, and dithiothreitol (DTT); 1% (v/v): HCl and acetone] on chitinase activity was analyzed under optimal temperature and pH conditions.
Kinetic parameter determination (K 0.5 and V max)
The kinetic parameters K0.5 and Vmax for the purified enzyme were determined using the synthetic substrate 4-nitrophenyl-N-Acetyl-β-d-glucosaminide (0.05–1.7 mM) in 100 mM sodium acetate buffer pH 5. The K0.5 and maximal velocity (Vmax) were calculated using the SigrafW software.
Antifungal activity
The antifungal activity from the purified A. niveus chitinase was evaluated against A. fumigatus CAS21, A. flavus, A. niger, A. phoenicis, and Paecilomyces variotii according to Mania et al. (2010) with modification. Briefly, 100 µL of minimal medium was added to each well in a 96-well microtiter plate. Thereafter, purified chitinase and Amphotericin B (positive control) were added to the wells at different concentrations (0.65–84 µg/mL) and 104 spores/mL from each fungal strain were inoculated. The plates were maintained at 37 °C for 72 h. After this period, the indicator resazurin (7-hydroxy-3H-phenoxazin-3-one-10-oxide; Aldrich®) was added to the wells at a final concentration of 100 µmol/L and monitored at 490 nm using a microplate reader (SpectraMax M2, Molecular Devices, CA, USA). All fungal strains used are deposited in the culture collection of the Laboratory of Microbiology of the Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, University of São Paulo (USP), and maintained on potato dextrose agar (PDA) slants.
Results and discussion
Production of chitinase by A. niveus
The potential of the fungus A. niveus to produce chitinase under different medium compositions was analyzed. Among all culture media tested, the best chitinase production (6.5 U/mg of protein) was achieved using minimal medium with added chitin from crab shells, 3.6-fold higher than that obtained using the Czapeck medium (1.8 U/mg of protein) in the same condition (96 h, 30 °C, 100 rpm) (Fig. 1). The enzyme production in M5 and YPD media was greatly reduced. According to Khan et al. (2010), the presence of MgSO4 and KH2PO4 in the medium (as observed for minimal medium, Adams, Czapek, and Khanna media) can positively affect chitinase production in the presence of chitin. The chitinase production by A. niveus was induced by chitin when any another carbon source was present. All media used, except for minimal medium, contain yeast extract, providing carbon and nitrogen sources that can be used promptly, reducing the need for enzyme production. In minimal medium (in the absence of the yeast extract), fungal metabolism is directed towards the degradation of chitin, which is the only available carbon/nitrogen source in this medium.
Several fungal species of Aspergillus, Penicillium, Metarhizium, and Trichoderma have been described as chitinase producers (Farag et al. 2016; Swiontek Brzezinska and Jankiweicz 2012; Lee et al. 2009; Binod et al. 2005; Rustiguel 2014). The induction of the enzyme production by these fungi has been performed using chitin from different sources, such as shrimp-shell powder, used for A. terreus (Farag et al. 2016) and A. niger LOCK 62 (Swiontek Brzezinska and Jankiweicz 2012), and shrimp shellfish waste used for Aspergillus sp. S1-13 (Rattanakit et al. 2007).
Considering the importance of chitin for inducing chitinase production by A. niveus, the influence of different concentrations of this inducer on enzyme production as a function of the cultivation time course was evaluated. As evident, the highest level of enzyme was obtained when the microorganism was cultured in minimal medium with 1% added chitin from 144 to 240 h (36 U/mg of protein) (Fig. 2a). The addition of high chitin content (2%) in the culture medium for long periods of cultivation reduced chitinase production significantly. A high concentration of chitin in the medium leads to increased viscosity, reducing the oxygen supply and leading to reduced fungal growth and enzymatic production. The influence of chitin concentration on chitinase production was also evaluated by Farag and Al-Nusarie (2014), who observed increments in chitinase content when A. terreus was cultured using shrimp-shell powder chitin from 5 to 20 g/L, whereas, at a concentration above 25 g/L, production decreased.
Influence of different concentrations of chitin and culture time on the production of chitinases by A. niveus in minimal medium (a). Chitin concentrations in the medium: 1% (circle), 0.5% (square), 1.5% (triangle), and 2% (inverted triangle) at the culture times of 48, 96, 144, 192, and 240 h. SDS-PAGE profile for the proteins secreted by A. niveus cultured in minimal medium, containing 1% chitin, for different time periods (b). Lane 1 48 h; Lane 2 96 h; Lane 3 144 h; Lane 4 196 h, Lane 5 240 h, and Lane M molecular weight markers. 80 µg of each sample was applied to the gel
Fungal cultivation for 48 h resulted in low chitinase production for all chitin concentrations used, as well as reduced fungal growth. Incubation for short periods may not lead to maximal enzyme production, as the microorganism is in the vegetative growth phase. In addition, the growth of the microorganism over a long period can lead to nutrient exhaustion and, consequently, to a decline in growth and enzyme production. Below 1% chitin in the culture medium, the chitinase production by A. niveus at 192 h of culture (36 U/mg of protein) was 2.8-fold higher than that observed at 96 h (12.5 U/mg of protein). Lee et al. (2009) observed maximal chitinase production by Penicillium sp. LYG 0704 with 3-day-old cultures, while, for A. terreus, maximum production was obtained with 5-day-old cultures (Farag and Al-Nusarie 2014). According to Swiontek Brzezinska and Jankiweicz (2012), the maximal chitinase production by A. niger LOCK 62 was observed with 6-day-old cultures.
The protein profile for the fungal growth in optimal conditions (minimal medium containing 1% chitin) for different periods at 30 °C (100 rpm) for the production of high chitinase levels was analyzed using 12% SDS-PAGE (Fig. 2b). Protein bands with different molecular masses (from 28 to 138 kDa) can be observed, especially the band corresponding to a 44 kDa protein. Increased 44 kDa protein expression is noted as a function of the increase in culture duration; this protein being the most prevalent in the cultures.
Chitinase purification
The A. niveus chitinase was initially precipitated with ammonium sulfate, followed by Sephadex G-100 gel filtration chromatography. A single peak corresponding with enzymatic activity was obtained in this last step (Fig. 3a), recording a 1.97-fold purification with 40% recovery and a specific activity of 13.03 U/mg of protein (Table 1). In general, the purification of most chitinases is inefficient, which can be explained by the presence of different isoforms with synergistic actions present in the crude supernatant and, consequently, loss during the purification procedure (Li 2006).
Chitinase purifications steps. After precipitation with 80% ammonium sulfate, the proteins were applied on Sephadex G-100 and the fractions with enzymatic activity were joined in a single pool as indicated by arrows in the chromatographic profile (a). Analysis of chitinase purification steps by 12% SDS-PAGE (b). Lane 1 chitinase after 80% ammonium sulfate precipitation; Lane 2 purified chitinase; Lane M molecular weight marker. 50 µg of proteins was applied to the gel
Each step of the purification process was monitored by 12% SDS-PAGE (Fig. 3b), and at the end of the purification, a single protein band was observed. Fungal chitinases have previously been purified by different methods, including ammonium sulfate precipitation and different chromatographic procedures. For example, the chitinases produced by A. terreus (Farag et al. 2016), A. niger LOCK 62 (Swiontek Brzezinska and Jankiweicz 2012), and A. fumigatus YJ-407 (Xia et al. 2001), were purified using ammonium sulfate. Ammonium sulfate is the most commonly used salt in protein precipitation owing to some of its characteristics such as high solubility, low cost, and low toxicity (Duong-Ly and Gabelli 2014).
Determination of molecular mass
The native molecular mass of A. niveus chitinase was found to be 44.57 and 44.03 kDa estimated by Sepharose CL-6B and 12% SDS-PAGE (Fig. 3b), respectively, indicating a monomeric enzyme. These values were near to the value estimated for the chitinases from produced A. fumigatus YJ-407, with a molecular mass of 46 kDa (Xia et al. 2001) and from A. niger LOCK 62, with a molecular mass of 43 kDa (Swiontek Brzezinska and Jankiweicz 2012). According to Li (2006), the molecular mass of fungal chitinases can vary from 27 to 190 kDa. The different chitinases produced by Penicillium aculeatum NRRL 2129 presented molecular masses of 82.7, 44.6, 28.2, and 26 kDa (Binod et al. 2005). Rattanakit et al. (2007) purified three chitinases from Aspergillus sp. S1-13 with molecular masses of 45, 51, and 73 kDa. For the chitinase from A. terreus, the molecular mass was estimated as 60 kDa (Farag et al. 2016).
Effect of the temperature and pH on the chitinase activity
The highest A. niveus chitinase activity was obtained at 65 °C (Fig. 4a). This value was higher than that observed for the enzymes produced by Penicillium aculeatum NRRL 2129 and A. terreus, with an optimum temperature of 50 °C (Farag et al. 2016; Binod et al. 2005), and by A. fumigatus YJ-407, with an optimum temperature of 60 °C (Xia et al. 2001). In addition, the A. niveus enzyme was remarkably thermostable (Fig. 4c); at 40 °C, the enzyme was fully stable. Interestingly, when maintained at 50 °C, chitinase activity was increased for all periods analyzed because of structural changes in the enzyme, which favor a better interaction with the substrate. At 60 °C, the half-life (t50) of the enzyme was estimated to be 120 min. The A. fumigatus chitinase retained 40% of its initial activity after heating at 60 °C for 30 min (Xia et al. 2001), while the A. niger LOCK 62 chitinase completely lost its activity after incubation at the same temperature for 90 min (Swiontek Brzezinska and Jankiweicz 2012). The A. terreus chitinase retained 66% and 42% of its initial activity when maintained at 60 and 70 °C for 60 min, respectively, and lost 90% of its activity at 90 °C for the same period of incubation (Farag et al. 2016). According to Guo et al. (2008), thermostable chitinase had major advantages over industrial catalysis due its high activity at higher temperatures. For example, the production of chito-oligosaccarides from chitin waste conducted at high temperature can minimize the contamination and the viscosity of the medium. Another possible biotechnological application is the biocontrol agents containing chitinase to combat plant pathogens (fungi) and insects that should be sprayed on the plant, exposing them to environmental conditions, such as high temperatures, for long periods. The preservation of the enzymatic activity under adverse conditions is important to achieve positive results in plant protection.
Influence of temperature (a) and pH (b) on enzyme activity, and stability over time at different temperatures (c) at 40 °C (squares), 50 °C (circles), and 60 °C (triangles), and to pH (d) using 50 mM citric acid buffer (pH 2.0–8.0) for 2 h (black bars) and 24 h (white bars) for the purified A. niveus chitinase
The optimal pH for A. niveus chitinase was 5.0, retaining 65–80% of its activity at pH 5.5 and 6.0 (Fig. 4b). At pH 2.0–3.5, the enzyme activity was strongly reduced. Most fungal chitinases have optimum pH of activity from pH 4.0 to 7.0, such as the enzymes produced by A. niger LOCK 62 (Swiontek Brzezinska and Jankiweicz 2012), A. fumigatus YJ-407 (Xia et al. 2001), A. terreus (Farag et al. 2016), and Penicillium aculeatum NRRL 2129 (Binod et al. 2005). Considering the enzyme stability to pH for 2 h, the activity of the A. niveus chitinase was slightly increased when maintained at a pH range from 2 to 5 (Fig. 4d). Progressive reduction in chitinase activity was observed when the enzyme was incubated at pH 6.0, 7.0, and 8.0. At pH 2.0 and 3.0 for 24 h, a slight increase in the chitinase activity was observed. However, at basic pH (8.0), 46% of activity loss was observed. Rustiguel (2014) reported that the chitinase from Metarhizium anisopliae IBCB 384 retained 100% of its activity when incubated at pH 7.0, while the chitinase from M. anisopliae IBCB 425 maintained 70% of activity in pH values from 2.5 to 7.0. The chitinase from M. anisopliae IBCB 167 maintained 60% of its activity at pH 5.5.
Effect of chemical compounds on chitinase activity
The effect of several chemical compounds on chitinase activity is presented in Table 2. The salt MnCl2 improved the chitinase activity by around 122% when compared with the absence of chemical compounds. Chitinase activity from M. anisopliae (Rustiguel 2014), A. terreus (Farag et al. 2016), and Penicillium sp. (Lee et al. 2009) were also improved in the presence of Mn2+. On the other hand, the enzyme activity from A. niveus was inhibited by 20–26% in the presence of KI, CuSO4, and ZnSO4. A slight inhibition (maximum 17%) was observed for the use of the other salts.
Interestingly, the presence of denaturing agents, HCl and acetone, did not significantly influence A. niveus chitinase activity. However, in the presence of urea, the enzyme maintained 85% of its initial activity. According to other authors, urea may cause conformational changes in the structure of the enzymes, promoting denaturation (Pace et al. 2010). In the presence of β-Mercaptoethanol and DTT, denaturing agents able to attack disulfide bonds between cysteine residues, the enzyme retained 95 and 90% of its initial activity, respectively, demonstrating that cysteine residues are not involved in the formation of the catalytic center. In the presence of SDS and acetone, the enzyme maintained 100% of its activity, while, in the presence of HCl, the enzyme maintained 90% of its activity. The A. fumigatus YJ-407 chitinase maintained its activity when incubated in the presence of urea and presented a reduction of only 20% of its activity in the presence of SDS (Xia et al. 2001). In contrast to the results found in this work, Farag et al. (2016) observed that a chitinase from A. terreus was strongly inhibited in the presence of acetone. Resistance to denaturation in organic solvents, acid, and reducing and denaturing compounds is favorable characteristics for the industrial application of A. niveus chitinase.
Kinetic parameter determination (K 0.5 and V max)
The A. niveus chitinase was able to hydrolyze colloidal chitin (0.05 U/mL), azure chitin (0.02 U), and 4-nitrophenyl N-acetyl-β-d glucosaminide (2.9 U/mL) as substrates. However, the hydrolysis was more pronounced for the last substrate, indicating the predominance of the exochitinase activity. Using 4-nitrophenyl N-acetyl-β-d glucosaminide as the substrate, the chitinase presented a K0.5 of 3.51 mM and a Vmax of 9.68 U/mg of protein. The Hill coefficient (n) was estimated as 2.98, indicating a positive cooperativity, which causes the enzyme to be much more sensitive to substrate concentration. Likewise, the activity can vary strongly even at very narrow substrate concentration intervals (Porter and Miller 2012). Thimoteo et al. (2017) observed that the chitinase MetaChi18A presented an n of 4.12 using colloidal chitin as a substrate. This phenomenon can occur because of hysteresis, a delay in the enzyme reaching its fully active form involving conformational changes. As chitinase is a multidomain enzyme, the substrate must interact with binding domains that can organize the conformational change, facilitating the access of the catalytic domain to the substrate (Thimoteo et al. 2017).
Rustiguel (2014) evaluated the kinetic parameters of the chitinase produced by M. anisopliae IBCB 360 using the substrate 4-nitrophenyl-N-acetyl-β-d-glucosaminide, and found a Km of 0.57 mM and a Vmax of 3.38 U/mg of protein. The A. fumigatus YJ-407 chitinase presented Km of 1.12 and 1.84 mg/mL when the chitin and chitosan were used as substrates, respectively (Xia et al. 2001).
Antifungal activity
The A. niveus chitinase was able to inhibit the development of A. fumigatus CAS21, A. flavus, A. niger, A. phoenicis, and P. variotii (Table 3). The minimum inhibitory concentration (MIC) was 21 µg/mL for P. variotii and A. fumigatus, while it was 24 µg/mL for A. phoenicis and A. flavus, and 84 µg/mL for A. niger. According to our results, the antifungal chitinase activity was similar to Amphotericin B for A. phoenicis and A. flavus, but not for that observed for P. variotii and A. niger. On the other hand, when the assay was conducted with A. fumigatus, the A. niveus chitinase was more effective compared with the result obtained with Amphotericin B. A. flavus is an important aflatoxin-producing fungus responsible for the contamination of food supplies of humans and animals, resulting in health hazards and sometimes mortality (Kumar et al. 2017). Other fungal species, including A. niger and P. variotii, are also able to produce mycotoxins (Hameed et al. 2012). Therefore, the use of chitinase from A. niveus is an attractive alternative method to combat mycotoxigenic fungi, minimizing food contamination problems. In addition, the fungus A. fumigatus is an important agent that causes allergic bronchopulmonary aspergillosis in immunocompromised patients (Singh et al. 2014).
Other antifungal chitinases produced by filamentous fungi have also been reported. For example, the chitinase produced by A. niger LOCK 62 inhibited the growth of Fusarium culmorum, Fusarium solani, and Rhizoctonia solani, but not the growth of Botrytis cinerea, Alternaria alternata, and Fusarium oxysporum (Swiontek Brzezinska and Jankiweicz 2012). The chitinase isolated from A. griseoaurantiacus KX010988 inhibited the growth of F. solani (Shehataa et al. 2018).
Conclusion
The chitinase secreted by A. niveus presented very interesting characteristics for possible industrial applications, such as high temperature of activity and thermostability, besides the ability to maintain its activity in the presence of different compounds, including denaturing and reducing agents. The chitinase secreted by A. niveus may also have other biotechnological applications such as in the production of chitin derivatives by enzymatic hydrolysis and in the control of mycotoxigenic fungi.
References
Adams PR (1990) Mycelial amylase activities of thermophilic species of Rhizomucor, Humicola and Papulaspora. Mycopathologia 112:35–37. https://doi.org/10.1007/BF01795178
Berini F, Presti I, Beltrametti F, Pedroli M, Varum KM, Pellegioni L, Sjöling S, Marinelli F (2017) Production and characterization of a novel antifungal chitinase identified by functional screening of a suppressive-soil metagenome. Microb Cell Fact 16:16. https://doi.org/10.1186/s12934-017-0634-8
Binod P, Pusztaheyi T, Nagy N, Sandhya C, Szakács G, Pócsi I, Pandey A (2005) Production and purification of extracellular chitinases from Penicillium aculeatum NRRL 2129 under solid-state fermentation. Enzyme Microbial Technol 36:880–887. https://doi.org/10.1016/j.enzmictec.2004.12.031
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilization the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Duong-Ly KC, Gabelli SB (2014) Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol 541:85–94. https://doi.org/10.1016/B978-0-12-420119-4.00007-0
Farag MA, Al-Nusarie ST (2014) Production, optimization, characterization and antifungal activity of chitinase produced by Aspergillus terreus. Afr J Biotechnol 13:1567–1578. https://doi.org/10.5897/AJB2014.13628
Farag AM, Abd-Elnabey HM, Ibrahim HAH, El-Shenawy M (2016) Purification, characterization and antimicrobial activity of chitinase from marine derived Aspergillus terreus. Egypt J Aquat Res 42:185–192. https://doi.org/10.1016/j.ejar.2016.04.004
Gun Lee D, Shin SY, Maeng CY, Jin ZZ, Kim KL, Hahm K-S (1999) Isolation and characterization of a novel antifungal peptide from Aspergillus niger. Biochem Biophys Res Commun 263:646–651. https://doi.org/10.1006/bbrc.1999.1428
Guo R-F, Shi B-S, Li D-C, Ma W, Wei Q (2008) Purification and characterization of a novel thermostable chitinase from Thermomyces lanuginosus SY2 and cloning of its encoding gene. Agric Sci China 7(12):1458–1465
Hameed AAA, Ayesh AM, Mohamed MAR, Mawla HFA (2012) Fungi and some mycotoxins producing species in the air of soybean and cotton mills: a case study. Atmos Pollut Res 3:126–131
Hill TW, Kafer E (2001) Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet Newsl 48:20–21. https://doi.org/10.4148/1941-4765.1173
Khan MA, Hamid R, Ahmad M, Abdin MZ, Javed S (2010) Optimization of culture media for enhanced chitinase production from a novel strain of Stenotrophomonas maltophilia using response surface methodology. J Microbiol Biotechnol 20(11):1597–1602. https://doi.org/10.4014/jmb.0909.09040
Khanna P, Sundari SS, Kumar NJ (1995) Production, isolation and partial purification of xylanase from Aspergillus sp. World J Microbiol Biotechnol 11:242–243. https://doi.org/10.1007/BF00704661
Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG (2017) Aflatoxins: a global concern for food safety, human health and their management. Front Microbiol 7:2170. https://doi.org/10.3389/fmicb.2016.02170
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Lee YG, Chung KC, Wi SG, Lee JC, Bae HJ (2009) Purification and properties of a chitinase from Penicillium sp. LYG 0704. Protein Expr Purif 65:244–250. https://doi.org/10.1016/j.pep.2008.12.004
Li D-C (2006) Review of fungal chitinases. Mycopathologia 161:345–360. https://doi.org/10.1007/s11046-006-0024-y
Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YD, Je JY, Ahn BB, Moon SH, Jeon BT, Park PJ (2014) Chitooligosaccharide and its derivatives: preparation and biological applications. Biomed Res Int 2014:1–13. https://doi.org/10.1155/2014/654913 (Article ID 654913).
Mania D, Hilpert K, Ruden S, Fischer R, Takeshita N (2010) Screening for antifungal peptides and their modes of action in Aspergillus nidulans. Appl Environ Microbiol 76(21):7102–7108
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Pace CN, Huyghues-Despointes BMP, Fu H, Takano K, Scholtz JM, Grimsley GR (2010) Urea denatured state ensembles contain extensive secondary structure that is increased in hydrophobic proteins. Protein Sci 19:929–943. https://doi.org/10.1002/pro.370
Peralta RM, Terenzi HF, Jorge JA (1990) β-d-glycosidase activities of Humicola grisea var. thermoidea: biochemical and kinetic characterization of a multifunctional enzyme. Biochim Biophys Acta 1033:243–249. https://doi.org/10.1016/0304-4165(90)90127-l
Porter CM, Miller BG (2012) Cooperativity in monomeric enzymes with single ligand-binding sites. Bioorg Chem 43:44–50. https://doi.org/10.1016/j.bioorg.2011.11.001
Gómez Ramírez M, Rojas Avelizapaa LI, Rojas Avelizapab NG, Cruz Camarillo R (2004) Colloidal chitin stained with Remazol Brilliant Blue RR, a useful substrate to select chitinolytic microorganisms and to evaluate chitinases. J Microbiol Methods 56:213–219
Rathore AS, Gupta RD (2015) Chitinases from bacteria to human: properties applications, and perspectives. Enzyme Res 2015:1–8. https://doi.org/10.1155/2015/791907 (Article ID 791907)
Rattanakit N, Yano S, Plikomol A, Wakayama M, Tachiki T (2007) Purification of Aspergillus sp. S1-13 chitinases and their role in saccharification of chitin in mash of solid-state culture with shellfish waste. J Biosci Bioeng 103:535–541. https://doi.org/10.1263/jbb.103.535
Rizatti AC, Jorge JA, Terenzi HF, Rechia CG, Polizeli ML (2001) Purification and properties of a thermostable extracellular β-d-xylosidase produced by a thermotolerant Aspergillus phoenicis. J Ind Microbiol Biotechnol 3:156–160. https://doi.org/10.1038/sj/jim/7000107
Rojas-Avelizapa LI, Cruz-Camarillo R, Guerrero MI, Rodríguez-Vázquez R, Ibarra JE (1999) Selection and characterization of a proteo-chitinolytic strain of Bacillus thuringiensis, able to grow in shrimp waste media. World J Microbiol Biotechnol 15(2):299–308
Rustiguel CB (2014) Comparison of the biochemical properties of the chitinases produced by different isolates of Metarhizium anisopliae. Thesis, University of São Paulo, Brazil
Seidl V (2008) Chitinases of filamentous fungi: a large group of diverse proteins with multiple physiological functions. Fungal Biol Rev 22:36–42. https://doi.org/10.1016/j.fbr.2008.03.002
Shehataa AN, Abd El Aty AA, Darwishc DA, Wahabb WAA, Mostafa FA (2018) Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated Aspergillus griseoaurantiacus KX010988. Int J Biol Macromol 107:990–999
Singh B, Singh S, Asif AR, Oellerich M, Sharma GL (2014) Allergic aspergillosis and the antigens of Aspergillus fumigatus. Curr Protein Pept Sci 15(5):403–423. https://doi.org/10.2174/1389203715666140512120605
Swiontek Brzezinska M, Jankiweicz U (2012) Production of chitinase by Aspergillus niger LOCK 62 and Its potential role in the biological control. Curr Microbiol 65:666–672. https://doi.org/10.1007/s00284-012-0208-2
Thimoteo SS, Glogauer A, Faoro H, Souza EM, Huergo LF, Moerschbacher BM, Pedrosa FO (2017) A broad pH range and processive chitinase from metagenome library. Braz J Med Biol Res 50(1):e5658. https://doi.org/10.0.6.54/1414-431x20165658
Wiseman A (1975) Industrial practice with enzymes. In: Wiseman A (ed) Handbook of enzyme biotechnology, 1rd edn. Horwood, Chichester, pp 243–272
Xia G, Jin CZ, Hou JY, Ang SZ, Hang S, Jin C (2001) A novel chitinase having a unique model of action from Aspergillus fumigatus YJ-407. Eur J Biochem 208:4079–4085. https://doi.org/10.1046/j.1432-1327.2001.02323.x
Acknowledgements
We thank Mauricio de Oliveira for technical assistance. The authors also kindly acknowledge the financial support from FAPESP (Process no. 2011/50880-1) and the research scholarships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This manuscript is part of the doctoral thesis by T.B.A.
Author information
Authors and Affiliations
Contributions
LHSG and TBA designed the study; TBA performed the experiments on chitinase production, purification, and characterization; PHOO performed the experiments on antifungal activity; LHSG, TBA, AHCO, and JAJ analyzed the results; LHSG and TBA wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Alves, T.B., de Oliveira Ornela, P.H., de Oliveira, A.H.C. et al. Production and characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation. 3 Biotech 8, 369 (2018). https://doi.org/10.1007/s13205-018-1397-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1397-6