Abstract
Lignin is a source for obtaining natural phenols with high commercial value that can act as redox mediators enhancing effects in dye decolorization. In this study Trametes hirsuta Bm-2 was grown on wheat bran to produce laccases and phenol extracts (PE). Ultrafiltered phenols obtained at different times were evaluated in their potential as redox mediators of laccase activity and indigo carmin decolorization. Laccase activity (L) on ABTS increased up to 12.4 times with L/PE72 compared with laccase alone and L/PE48 showed the highest level of dye decolorization (97%) compared with laccase (12%). The chromatographic analysis by HPLC showed variation in the profile and concentration of phenols at different times of culture. Stability of the laccase mediator system (LMs) in dye decolorization was maintained over 3 months. Our results suggest the use of natural mediators as a strategy for improving efficiency in dye biodegradation by laccase-producing fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes present in effluents are environmentally hazardous xenobiotic compounds. During processing, 10–15% of the total world textile dye production (800,000 tons/year) is released in industrial effluent (Zollinger 2004). Many dyes are known to have mutagenic and carcinogenic effects and their removal is a priority for most environmental agencies (Viswanath et al. 2014). Indigo carmine plays a key role in various sectors such as the food, textile and cosmetic industries and in medicine because of its color, but it is highly toxic and contact with skin or eyes may cause irritation (Legerská et al. 2016). Today, it is important to search for an environmentally friendly alternative for textile effluent treatment, and therefore, microbial and enzymatic treatments are attracting interest as substitutes for chemicals.
White rot fungi (WRF) are the most efficient organisms at degrading lignin. Enzymes from WRF, especially laccases, are able to degrade azo- and heterocyclic, reactive and polymeric dyes (Zeng et al. 2011). Laccase (benzenediol oxygen oxidoreductase, EC 1.10.3.2) belongs to a family of multicopper oxidases which can oxidize several phenolic compounds by one electron transfer with the concomitant reduction of oxygen to water. Laccase application provides some advantages related to processing conditions, such as low temperature, low pressure and the possibility of using the oxidant O2 directly (Baldrian 2006). A diversity of industrial applications have been proposed for laccase including organic synthesis, the food industry, dye decolorization and nanobiotechnological applications (Kunamnenni et al. 2008; Upadhyay et al. 2016). However, many substrates cannot be oxidized directly by the enzyme due to laccases having low redox potential (0.4–0.84). This limitation can be addressed through the addition of low molecular weight compounds that are continuously oxidized by laccase to stable mediators with high redox potential.
Redox mediators acted as redox shuttles between the enzyme active site and the substrate to allow the oxidization of high redox-potential substrates that would not be substrates of laccases (Zeng et al. 2017). The ideal mediator should be non-toxic, economic and efficient with a stable oxidized and reduced form that does not inhibit the enzymatic reaction. Moreover, mediators should be able to continuously maintain cyclic redox conversion (Morozova et al. 2007). Synthetic mediators such as 2-2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), -NOH-type (violuric acid, hydroxybenzotriazole) and TEMPO have improved dye decolorization, degradation of polycyclic aromatic hydrocarbons, pesticides and lignin (Strong and Claus 2011; Tran et al. 2013). Industrial scale application of synthetic mediators is not convenient due to the high cost of these chemicals and the generation of toxic species that can cause biocatalytic inactivation of laccase (Bibi et al. 2011; Wells et al. 2006).
There are phenols related with lignin structure that are capable of acting in a similar way to synthetic mediators. Lignin from lignocellulosic substrates represents a potential source of phenols and aromatic compounds. These natural mediators can be released from the degradation of lignin present in lignocellulosic substrates through laccase activity produced by white rot fungi (Andreu and Vidal 2011). The biological breakdown of the lignin polymer avoids the formation of undesirable by-products, and the biocatalytic process takes place under mild conditions and reduces environmental impact. To date, there are few studies on obtaining natural mediators produced by fungi, despite the huge availability of lignocellulosic substrates that can be used as a source for these compounds. The obtention of phenolic extracts by fungal sources has been reported in Trametes versicolor and Pleurotus ostreatus cultured on bran and straw (Collins et al. 1996; Li et al. 2014). In these works crude laccase and the aqueous ultrafiltrate improved the benzopyrene and anthracene biotransformation. However, fungal natural mediators were not identified.
Trametes hirsuta Bm-2 is a white rot fungi isolated from decaying wood in Yucatan, Mexico, which was selected due to its high laccase activity and effectiveness in removing colorants in vinasse (Tapia-Tussell et al. 2015). Laccases produced by this strain on wheat bran showed a loss of decolorization activity during the purification process, but this was recovered with the addition of phenol monomers, suggesting the presence of mediators in the crude extract (Zapata-Castillo et al. 2015). The objective of the present study was to obtain phenolic extracts (PE) from the culture of T. hirsuta Bm-2 on wheat bran and to determine their potential as redox mediators in a dye decolorization reaction catalyzed by laccase. Also, identification of phenols by HPLC and the stability of extracts in dye decolorization process were reported.
Materials and methods
Microorganism and propagation
A fungal strain, T. hirsuta Bm-2 collected from decayed wood in Yucatan, Mexico, was used in this work (Tapia-Tussell et al. 2011). The growth medium for propagation consisted of (g/L): malt extract 20, bacteriological agar 20 (pH 6.0). Plates were incubated for 4 days at 35 °C.
Laccase and phenol production
The liquid medium to obtain inoculum was (g/L): glucose, 10; malt extract, 10; peptone, 2; yeast extract, 2; KH2PO4; MgSO4·7H2O, 2; thiamine-HCl, 1 mg/mL. 250 mL Erlenmeyer flasks containing 30 mL of medium were inoculated with two 1 cm2 agar pieces from an actively growing fungus on a malt extract plate (Bm-2). The flasks were incubated at 35 °C and shaken at 150 rpm. After 4 days, the culture was homogenized using a sterilized blender. The liquid media for laccase and phenol production was wheat bran 3% (w/v) in 60 mM phosphate buffer (pH 6.0). Four milliliters of mycelia suspension was inoculated to 200 mL of medium and the culture was grown at 35 °C for 10 days. Aliquots of the growth medium were withdrawn each 24 h, filtered and centrifuged for 15 min (5000 rpm, 4 °C). To obtain phenolic extracts from the crude culture fluid the method described by Collins et al. (1996) was used. The crude supernatant was filtrated by ultrafiltration tubes (10 kDa, Millipore, USA) to separate phenolic extracts (PE) and laccases.
Assay for laccase activity
Laccase activity in cell-free filtrates was measured at 40 °C using 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS). The assay mixture contained 1 M sodium acetate buffer (pH 4.5) and 0.5 mM ABTS in a total volume of 1 mL. The oxidation of ABTS was measured by the increase in absorbance at 420 nm as described by Johannes and Majcherczyk (2000). One enzyme unit (U) is defined as the amount of enzyme required to oxidize 1 µmol of ABTS per min under the assay conditions. The amount of the enzyme production was expressed as U/mL.
Effect of phenol extracts on laccase activity
The effect of PE on laccase activity was studied by using laccase obtained at 264 h (L) and with the addition of PE (0, 24, 48, 72 h). The reaction system contained: 720 µL distilled water, 100 µL of 1M acetate buffer (pH 4.5), 30 µL of laccase extract, 50 µL of PE, and 100 µL ABTS. Laccase activity was also determined with inactivated enzyme addition (boiled 30 min) that was used as a negative control.
Determination of total phenol content
The concentration of total phenols in cell-free filtrates was determined by reduction of Folin–Ciocalteau reagent (Singleton and Rosi 1965). The estimation of total phenols in the extracts was carried out in triplicate. Gallic acid was used as a standard and the results obtained were expressed as mg of gallic acid/mL.
Decolorization of indigo carmine dye
Decolorization of indigo carmine was investigated using laccase with and without PE. The reaction mixture contained 4 mL indigo carmine solution (20 mg/L) in an acetate buffer (1 M, pH 4.5) and 0.5 mL laccase produced at 264 h. To evaluate the effect of PE on laccase-catalyzed indigo carmine oxidation, 0.5 mL of each extract was added. The treatment with inactivated enzyme addition (boiled 30 min) was used as negative control. The reaction was initiated with enzyme addition and incubated at 45 °C. Decolorization was determined as a percentage of the absorbance reduction at 600 nm. Rate of color removal was calculated using the following formula:
where D is the degree of decolorization (in percent), A1 is the absorbency of the control without enzyme and A2 represents the absorbency of samples.
Determination of phenols by high performance liquid chromatography
Phenol extracts (0–264 h) were filtered using a 0.45 µM Teknokroma filter. A sample (20 µL) was injected to nucleosil C18 column (250 × 4.6 mm, particle size 2.2 µM, 280 nm), with a mobile phase with 1% formic acid in 98% water (A phase) and 2% acetonitrile (B phase) at a constant flow rate (0.5 mL/min, 25 °C) were used to separate phenols (Yahia et al. 2011). Phenol standards were gallic acid, ferulic acid, p-coumaric acid, guaiacol, syringaldehyde, vanillic acid and 4-hydroxybenzoic acid (Sigma-Aldrich). Identification of phenols was made considering retention times of phenol standards separated using a constant gradient 2–100% of B phase (0–70 min). The concentration of phenol standard solutions was determined at 280 nm. Phenolic compounds in the samples were identified according to their elution order and by comparing their retention times with standards. Phenol compound concentration (mg/L) was interpolated from the corresponding peak areas.
Statistical analysis
The data are the average of the results of three replicates and are presented as the mean ± S.D. One-way ANOVA was used to determine the difference between treatments at a significant level of 0.01 or 0.05.
Results and discussion
Obtention of laccases and phenol extracts
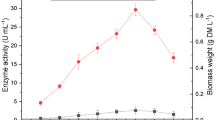
The efficiency of the laccase mediator system in different processes has led to the search for efficient alternative, low-cost natural mediators from renewable lignocellulose sources (Johannes and Majcherczyk 2000; Arias et al. 2003). Phenol mediators can be obtained from plant residue, effluent streams and fungal culture broth, from which they can be easily extracted (Gutiérrez et al. 2007). With a view to obtaining an efficient, natural laccase mediator in decolorization of dyes, a culture of T. hirsuta Bm-2 fungus was produced on wheat bran. Laccase activity began after 48 h, and showed a progressive increase until reaching a maximum activity of 4081.2 U/mL at 264 h (Fig. 1a). The production of laccase by WRF has been carried out widely in lignocellulosic substrates such as banana peel, wheat bran, coffee grounds and sugar cane bagasse among others, where high levels of activity have been reported (Gonzalez et al. 2013). However, it is difficult to make a comparison of activity levels since different methods are used to measure and define the units of enzymatic activity.
Total phenols showed irregular behavior, with increase and decrease in concentration (2.18–3.46 mg/L) during the culture (Fig. 1b). This situation may be attributed to the fact that fungal culture is a dynamic event, in which the fungi simultaneously liberate phenols from the lignin and use them as inductors and/or carbon sources. Also, the speed with which these events occur can vary depending on the condition of the culture and the physiological stage of the organism. The induction of laccases by phenolic substances may provide an answer to the oxidative stress caused by the phenols, which affect the growth of the fungi. Herpoël et al. (2000) reported that phenolic monomers from lignin, such as synapilic acid, guaiacol and vanillic acid, stimulated the synthesis of laccases in the Pycnoporius cinnabarius fungus. It has also been demonstrated that laccase induction by phenols in WRF occurs at a transcriptional level (Piscitelli et al. 2011). The phenols present in the culture can also act as redox mediators of laccases, contributing to an improved degradation of the lignin. The laccase-rich extract produced by T. hirsuta after 264 h, and the ultrafiltered phenolic extracts after 24, 48 and 72 h were selected to continue with the study.
Effect of PE addition on laccase activity
The effect of adding phenolic extracts on laccase activity was evaluated using ABTS as a substrate. Adding PE0 and PE24 reduced laccase activity by 12.4 and 20%, respectively (Table 1). On the other hand, the addition of PE after 48 and 72 h increased laccase activity by 1.3 and 12.4 times, respectively, which suggests that the phenols present in the extracts act as redox mediators. Laccase inactivation in a laccase-mediator system depends in great measure on the mediator. The laccase inactivation mechanism by different mediators is still not well understood, although it is known that laccases respond to stimuli based on their allosteric properties, and are subject to inhibition phenomena. Extracellular laccases from Trametes versicolor and Cerrena unicolor were inhibited by the addition of synthetic mediators such as ABTS and 1-hydroxybenzotriazole (1-HBT), but there was a significant activation of enzymes with the addition of highly diluted mediators. In these conditions the demethylation properties of veratric acid by the laccases increased (Malarczyk et al. 2009). The increase in laccase activity observed with PE48 and PE72 could be attributed to the redox action of the phenols presents in extract, which act as co-oxidizing agents on the laccase to oxidize a larger number of molecules of the ABTS substrate. Also, differences in laccase activity could be related to the isoenzymes produced by T. hirsuta Bm2 on wheat bran (Zapata-Castillo et al. 2015). Several laccase isoenzymes have been detected in fungal species and their affinity toward substrates or mediators can vary, which may increase or decrease ABTS oxidation.
Effect of PE addition on dye decolorization
The study was carried out to determine the efficiency of the laccase/PE system in the decolorization of indigo carmine (Fig. 2). Laccase alone was practically unable to decolorize the dye (12%), a fact which could be connected to the aromatic nature of the dye or to its high redox potential. Figure 2a shows the decolorization efficiency of the laccase/PE. Compared to the L, both L/PE24 and L/P48 show a great increase in decolorization (five- and sixfold, respectively). This behavior could be related to phenols in extracts, 4-hydroxybenzaldehyde, guaiacol, vanillin and gallic acid that have been reported as substrates for laccase activity and also could be useful as redox mediators (Pardo and Camarero 2015). It was also observed that the efficiency of L/PE72 is significantly lower than that of L/PE48, although the content of phenols is not significantly different between PE48 and PE72. This result could be related to the high content of total phenol detected in PE72 (Fig. 1b). Phenols not identified could play a role in inhibiting the capacity for decolorization. On the other hand, treatments with L/PE24, L/PE48 and L/PE72 increased the decolorization of the dye five-, six- and twofold in comparison with the enzyme alone which indicates that these extracts contain effective mediators for the decolorization of indigo carmine.
Effect of phenol extracts ultrafiltrates on decolorization of indigo carmine by laccase at 40 °C, 50 h. The reaction system included laccase and indigo carmine (L) and Laccase with PE (24, 48, 72 h). Values are the means of triplicate and error bars are standard deviations. Treatments were monitored 50 h
The results of this study showed a variation between the effect of the PEs on the efficiency of ABTS oxidation (laccase activity) and the decolorization of the dye. The L/PE24 system inhibited the oxidation of the ABTS and improved decolorization of the dye, L/PE48 increased oxidation of the ABTS (1.3-fold) and attained the greatest decolorization (sixfold). L/PE72 stimulated oxidation of ABTS (12-fold) with increase (twofold) in decolorization. Laccases from Trametes villosa, Miceliophthora thermophile and P. cinnabarius were inactivated in the presence of the synthetic mediators 1-hydroxybenzotriazole (1-HBT) and violuric acid, but with the addition of red phenol, the inactivation decreased and the dye was rapidly oxidized (Li et al. 1999). The difference in behavior of the extracts in enzyme activity on ABTS and dye decolorization may depend on different factors such as the mix of phenols, the type of substrate, reversibility of the substrate reaction, the stability of the phenoxy radical and the redox potential of the mediator (Fernández-Sanchez et al. 2002; Rosado et al. 2012).
The effectiveness of reactive grade natural mediators in the decolorization of dyes has been widely reported. Purified laccases of T. hirsuta and Sclerotium rolfsii increased the decolorization of indigo carmine when mediators such as acetosyringone and 1-hydroxybenzotriazole were added (Campos et al. 2001). Phenols such as phloroglucinol and thymol allowed complete decolorization of indigo carmine with laccases from Fomes lignosus (Hu et al. 2009). The natural mediators, syringaldehyde, acetosyringone, p-coumaric acid, vanilline, and acetovainilline proved effective in decolorizing different dyes when they were used with laccases from P. cinnabarinus and T. villosa (Camarero et al. 2005).
In order to identify the phenols in the extracts, an HPLC chromatography was performed. The chromatograms of the phenolic extracts at 0 and 48 h of T. hirsuta Bm-2 culture on wheat bran showed peaks corresponding to lignin phenols: gallic acid (GA), 4-hydroxybenzoic acid (4HB), vanillic acid (VA), ρ-coumaric acid (CA), syringaldehyde (SYR), ferulic acid (FA) and guaiacol (G) with retention times of (RT) 10.8, 18.2, 19.9, 23.5, 24.4, 24.6, and 28.5 min, respectively, which served as references to identify and quantify phenols in extracts (Fig. 3a). The PE showed differences in phenolic profile; at time zero, GA, VA, SYR, and FA were present (Fig. 3b), whereas at 48 h, GA, 4HB, VA, FA, and G were detected (Fig. 3c), as well as other peaks of higher magnitude which were not identified. Table 2 clearly shows the variations in profile and concentration of phenols during the culture. The phenolic content in PE24 is lower than that in PE48. However, Fig. 1b shows that the total phenol in PE24 is higher than that in PE48. Differences could be explained because PE24 contains more phenols quantified but not identified by the Folin method; however, it is possible that the proportion of individual phenols (HPLC) is lower than that of PE48. Ultrafiltered phenolic extracts demonstrated their redox action during the degradation of aromatic polycyclic hydrocarbons by laccases (Collins et al. 1996; Li et al. 2014); however, the composition of phenols in the extracts was not identified. Gutierrez et al. (2004) reported the chemical composition of non-woody plant lignin which showed high syringil/guaiacil ratio, also sinapyl and coniferyl acetate were identified. Del Rio et al. (2001) identified the presence of guaiacol, eugenol, syringol and acetosyringone during the degradation of eucalyptus wood by WRF, but these extracts were not evaluated as laccase redox mediators. The phenolic extracts obtained in our study contain a mixture of phenols which demonstrate their efficiency as redox mediators in the decolorization of indigo carmine.
Stability of the laccase mediator system
In order to determine stability of phenol extract in dye decolorization, PE48 was selected to study the stability of the laccase mediator system because this extract increased the laccase activity on ABTS and also showed the best level of dye decolorization. The LMs showed a high rate of color removal (80–97%) over three months, compared to laccase (30–40%) (Fig. 4). These results show the stability of laccase and the phenolic extract during frozen storage (− 20 °C). The effect of the mediators could be linked to the concentration of the phenolic compound, or the combination of two or more compounds that might have a synergic effect on the oxidizing activity of the laccase on the indigo carmine (Jeon et al. 2008).
Conclusion
In this study it was shown that wheat bran is a lignocellulosic source for the production by T. hirsuta Bm-2 of an extract rich in laccases and natural mediators. The LMs decolorized indigo carmine up to 97%. The efficiency varied depending on the phenolic extract and as well as the substrate. The phenols generated during the biodegradation of lignin from lignocellulosic residue are raw extracts that provide a low-cost, eco-friendly system of low toxicity for the treatment of effluent from the textile industry, and its application could be extended to the degradation of other xenobiotic compounds. Furthermore, the obtention of phenols through microbial activity represents a biotechnological opportunity for assessing the value of agro-industrial by-products with lignin, since the deconstruction of the polymer into phenols represents a first-stage source for obtaining various valuable products.
References
Andreu G, Vidal T (2011) Effects of laccase-natural mediator systems on kenaf pulp. Bioresour Technol 102:5932–5937
Arias ME, Arenas M, Rodriguez J, Soliveri J, Ball AS, Hernandez M (2003) Kraft pulp biobleaching and mediated oxidation of a nonphenolic substrate by laccase from Streptomyces cyaneus CECT 3335. Appl Environ Microbiol 69:1053–1958
Baldrian P (2006) Fungal laccase-occurrence properties. FEMS Microbiol Rev 30:215–242
Bibi I, Bhatti HN, Ashger M (2011) Comparative study of natural and synthetic phenolic compounds as efficient laccase mediators for the transformation of cationic dyes. Biochem Eng J 56:225–231
Camarero S, Ibarra D, Martínez MJ, Martinez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Campos R, Kandelbauer A, Robra KH, Cavaco-Paulo A, Gübits GM (2001) Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J Biotechnol 89:131–139
Collins PJ, Kotterman MJJ, Field JA, Dobson ADW (1996) Oxidation of anthracene and bezo [a] pyrene by laccases from Trametes versicolor. Appl Environ Microbiol 62:4563–4567
Del Rio JC, Gutierrez A, Martinez MJ, Martinez AT (2001) Py-GC/MC study of Eucalyptus globulus wood treated with different fungi. J Anal Appl Pyrol 58–59:441–452
Fernández-Sánchez CT, Tzanov CT, Gübitz GM, Cavaco-Paulo A (2002) Voltametric monitoring of laccase-catalyzed mediated reactions. Bioelectrochemistry 58:149–156
Gonzalez JC, Medina SC, Rodriguez A, Osma JF, Alméciga-Díaz CJ, Sánchez OF (2013) Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro-industrial wastes. PLos one 8(9):e73721. https://doi.org/10.1371/journal.pone.0073721
Gutierrez A, Rodriguez IM, Del Rio JC (2004) Characterization of lignin and lipid fractions in kenaft bast fibers used for manufacturing high quality papers. J Agric Food Chem 52(1):4764–4773
Gutiérrez A, Rencoret J, Ibarra D, Molina S, Camarero S, Romero J, Del Rio JC, Martinez AT (2007) Removal of lipophilic extractives from paper pulp by laccase and lignin phenols as natural mediators. Environ Sci Technol 41:41254–44129
Herpoël I, Moukha S, Lesage-Meessen L, Sigoillot JC, Asther M (2000) Selection of Pycnoporus cinnabarinus strains for laccase production. FEMS Microbiol Lett 183:301–306
Hu MR, Chao YP, Zhang GQ, Xue ZQ, Qian S (2009) Laccase-mediator system in the decolorization of different types of recalcitrant dyes. J Ind Microbiol Biotechnol 36:45–51
Jeon J, Murugesan K, Kim J, Kim E, Chang Y (2008) Synergistic effect of laccase mediators on pentachlorophenol removal by Ganoderma lucidum laccase. Appl Microbiol Biotechnol 81:783–790
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Kunamnenni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microbiol Cell Fact 7:1–32
Legerská B, Chmelová D, Ondrejovič M (2016) Degradation of synthetic dyes by laccases—a mini-review. Nova Biotechnol Chim 15(1):90–106
Li K, Xu F, Eriksson KEL (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Li X, La G, Cheng Q, Wang F, Feng F, Zhang B, Zhang Z (2014) Profile of natural mediators production of laccase producing fungus Pleurotus ostreatus. Bull Environ Contam Toxicol 93:478–482
Malarczyk E, Kochmanska-Rdest J, Joroz-Wilkolazka A (2009) Influence of very low doses of mediators on fungal laccase activity—nonlinearity beyond imagination. Nonlinear Biomed Physic 3:10. https://doi.org/10.1186/1753-4631-3-10
Morozova OV, Shumakovich GP, Gorvacheva MA, Shleev SV, Yaropolov YI (2007) Laccase mediator systems and their applications: a review. Appl Biochem Microbiol 43(5):523–535
Pardo I, Camarero S (2015) Exploring the oxidation of lignin-derived phenols by a library of laccase mutants. Molecules 20:15929–15943
Piscitelli A, Giardina P, Lettera V, Pezzella C, Sannia G, Faraco V (2011) Induction and transcriptional regulation of laccases in fungi. Curr Genomics 12(2):104–112
Rosado T, Bernardo P, Koci K, Coello A, Robalo MP, Martinez L (2012) Methyl syringate: an efficient phenolic mediator for bacterial and fungal laccases. Bioresour Technol 124:371–378
Singleton V, Rossi J (1965) Colorimetry of total phenolics with phosphomolybdic-phoshotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Strong PJ, Claus H (2011) Laccase: A review of its past and its future in bioremediation. Crit Rev Environ Sci Technol 41:373–434
Tapia-Tussell R, Pérez-Brito D, Rojas-Herrera R, Cortes-Velazquez A, Rivera-Muñoz G, Solis-Pereira S (2011) New laccase-producing fungi isolates with biotechnological potential in dye decolorization. Afr J Biotechnol 10:10134–10142
Tapia-Tussell R, Perez-Brito D, Torres-Calzada C, Cortes-Velazquez A, Alzate-Gaviria L, Chable-Villacis R, Solis-Pereira S (2015) Laccase gene expression and vinasse biodegradation by Trametes hirsuta strain Bm-2. Molecules 20:15147–15157
Tran NH, Hu J, Urase T (2013) Removal of the insect repellent N,N diethyl-m-toluamide (DEET) by laccase-mediated systems. Bioresour Technol 147:667–671
Upadhyay P, Shrivastava R, Agrawal PK (2016) Bioprospecting and biotechnological application of laccases. 3 Biotech 6(1):15
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res https://doi.org/10.1155/2014/163242
Wells A, Teria M, Eve T (2006) Green oxidations with laccase-mediator systems. Biochem Soc Trans 34(2):304–308
Yahia EM, Gutierres-Orosco F, Arvizu-De Leon C (2011) Phytochemical and antioxidant characterization of the fruit black sapote (Diospyros digyna Jacq.). Food Res Int 44:2210–2216
Zapata-Castillo P, Villalonga-Santana L, Islas-Flores I, Rivera-Muñoz G, Ancona-Escalante W, Solís-Pereira S (2015) Synergistic action of laccases from Trametes hirsuta Bm-2 improves decolourization of indigo carmine. Lett appl microbiol 61(3):252–258
Zeng X, Cai Y, Liao X, Zeng X, Li W, Zhang D (2011) Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agroindustrial residue. J Hazard Mater 187:517–525
Zeng S, Quin X, Xia L (2017) Degradation of the herbicide isoproturon by laccase-mediator system. Biochem Engin J 119:92–100
Zollinger H (2004) Color Chemistry. synthesis, properties and applications of organic dyes and pigments. 3rd revised edn. Angewandte Chemie 43(40):5291–5292
Acknowledgements
The authors wish to express their gratitude to National Science and Technology Council, Mexico, for providing the financial support for this research (Project No. 248295).
Author information
Authors and Affiliations
Contributions
All the authors contributed to this work. Sara Solis-Pereira conceived, designed and wrote the paper; Ancona-Escalante performed the experiments and analyzed the data of laccase activity and phenol extract; Lizama-Uc, Pool-Yam and Can-Cahuich performed the experiments and analyzed the decolorization data and HPLC; and Raul Tapia-Tussell participated in the data analysis and writing of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this paper.
Rights and permissions
About this article
Cite this article
Ancona-Escalante, W., Tapia-Tussell, R., Pool-Yam, L. et al. Laccase-mediator system produced by Trametes hirsuta Bm-2 on lignocellulosic substrate improves dye decolorization. 3 Biotech 8, 298 (2018). https://doi.org/10.1007/s13205-018-1323-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1323-y