Abstract
Among the 194 Fusarium verticillioides isolates screened from 127 cereal samples, 176 were fumonisin producers and others were non-producers. Representative nine Fusarium verticillioides strains along with one reference standard strain MTCC156 were selected to study their morphological, pathological and mycotoxicological variations by conventional and molecular approaches. Fusarium verticillioides strains FVM86, FVM146, FV200 and FVS3 showed significant pathogenicity and also in pigmentation production but varied in fumonisin production. Fusarium verticillioides strain FVP19 recorded variations in all the assays. Fusarium verticillioides strain FVM42 showed drastic phenotypic variation and it also produced fumonisin. Genetic variation among the strains was independent of geographic area of origin but depended on their ability to produce fumonisin. The strains were independent in their cultural characteristics, pigmentation production, pathogenicity assays, fumonisin production and in their genetic variability without having any correlation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium verticillioides is the well-known pathogen of agriculturally important crops due to their great variability with respect to morphological, physiological and genetic properties (Leslie 1991, 1995). They are more frequent in certain climatic regions and on some host plants mainly associated with root, stalk and ear rot on maize affecting crop yield between 10 and 30% in Europe (Logrieco et al. 2003). It mainly causes Fusarium stalk rot in maize which is characterized by discoloration of pith tissue causing premature plant death (Afolabi et al. 2008; Yamamura and Shim 2008). Natural F. verticillioides populations in Iran are genetically highly divergent and are a potential risk for disease development (Gohari et al. 2008). High disease incidence was observed in maize fields during 2004 and 2005, and used multilocus markers to detect populations (Milgroom 1996). Seed infection was high in Costa Rica maize crops with potential risk for disease development and fumonisin accumulation (Danielsen and Jensen 1998) in association with symptomless infection along with stalk and ear rot (Bacon and Hinton 1996).
Fusarium verticillioides is a ubiquitous and versatile pathogen producing fumonisins, a group of polyketide-derived secondary metabolites toxic to when consumed along with food and feed by humans and animals and FB1 has classified it as a group 2B carcinogen by International Agency for Research on Cancer (1993) (Desjardins et al. 1998; Lazzaro et al. 2012). Kernel infection by Gibberella fujikuroi is of concern because of the loss of grain and seed quality, and the potential occurrence of fumonisins and other mycotoxins (Munkvold and Desjardins 1997). Fumonisin B1 is most abundantly produced and it causes leucoencephalomalacia in horses, pulmonary oedema syndrome in pigs, hepatocarcinogencity and hepatotoxicity in rats and also associated with humans causing oesophageal cancer.
The potentiality of fumonisin biosynthesis by fungal strains from different cereals helps to predict the risk of contamination by mycotoxins. Different toxicological profiles of F. verticillioides strains should show important differences in the risk for mycotoxin contaminations with implication for human and animal health, biological control of mycotoxin contamination and mycotoxin synthesis (Proctor et al. 2006). Mycotoxicological and pathogenic characterization of fungal plant pathogens can be complemented by fingerprinting. Wide range of pathogenicity in terms of effect on seed germination, seedling development and of symptoms produced on detached leaves, which were not correlated with the different in vitro fumonisin production. Amplified Fragment Polymorphisms Analysis (AFLP) analysis indicated the presence of genetic diversity not only between Italian strains and the American reference but also among the Italian isolates (Covarelli et al. 2012). Restriction fragment length polymorphism (RFLP) and Random amplified polymorphic DNA (RAPD) can also reveal genetic variability between different species and within the same species, giving us information about genetic differences which could correspond to different mycotoxicological or pathogenic profiles (Moretti et al. 2004; Reynso et al. 2009).
The present work was to carry out a comprehensive study to determine (i) the morphological differences of F. verticillioides strains isolated from the cereal samples from different districts of Karnataka, (ii) their pathological variations on stalk, cob and kernels, (iii) the ability of F. verticillioides strains to biosynthesize fumonisins, and (iv) the genetic variability among them with respect to MTCC reference strains. The study also demonstrated the individual strains of F. verticillioides for its substantial differences in their ability to produce fumonisins and differences among the geographical and host origins.
Materials and methods
Fungal strains/culture
A total of 194 F. verticillioides isolates were screened from 127 cereal samples (61 maize, 42 paddy, 24 sorghum) covering all districts of Karnataka State. Among which 18 isolates were non-fumonisin producers (Deepa et al. 2016a). All the 194 isolates were grown on potato dextrose agar (PDA) medium. Among them representative nine isolates [FVM42 (Kodagu), FVM86 (Mysore), FVM146 (Bellary) from maize, FVP200 (Mandya), FVP19 (Mysore), FVP31 (Hassan) from paddy, FVS3 (Haveri), FVS22 (Mysore), FVS36 (Mandya) from sorghum)] of F. verticillioides with cultural variability of pigmentation, sporulation, colony diameter from maize, paddy and sorghum were selected and used in the assays along with a positive control MTCC 156. Mycological evaluation for the collected cereal samples were carried out as per Sreenivasa et al. (2011).
Preliminary variations
Cultural and microscopic characterization of F. verticillioides strains
Different media of potato dextrose agar (PDA), Czapek–Dox agar (CZA), potato sucrose agar (PSA), Spezieller Nahrstoffarmer agar (SNA), malt extract agar (MEA) and yeast extract malt agar (YEMA) were prepared and autoclaved. About 15 mL of each medium were poured onto Petri plates and after solidification all the ten F. verticillioides strains (including MTCC156) were center-inoculated onto every medium plate and incubated for 10 days with alternating periods of 12 h darkness and 12 h light at 25 ± 2 °C. The colony diameter of each strain grown on each medium was measured on fourth and seventh day of incubation. At the end of incubation period, each plate was observed for its cultural and microscopic characteristics/variations and recorded.
Pigmentation variations among isolated F. verticillioides strains
Potato dextrose broth (PDB) of 50 mL was suspended in each conical flask and autoclaved. Fresh 106 spore suspensions of F. verticillioides strains were inoculated to all ten flasks and incubated in alternating periods of 12 h darkness and 12 h light at 25 ± 2 °C for 10 days. After incubation, pigmentation formed was observed and OD was measured at 560 nm.
Phenotypic variations
Pathogenicity assays
Inoculation of cobs
Fresh cobs from the fields were brought to the laboratory. A single-line kernel of each cob was inoculated with fresh 106 spore suspension of each F. verticillioides strains. Inoculated cobs were incubated in a controlled moisture conditions for 10 days. After incubation, kernels showing Fusarium infestation were harvested to analyze the percent infection in the respective cobs. Liquid chromatography–mass spectrometry (LC–MS) was performed for the infected kernels to study the pathogenicity assay of the isolates. The ability to colonize in the germ of maize kernels was also tested by maintaining cobs in triplicates with control lane inoculated with sterile distilled water.
Inoculation of stalk
Two-month-old maize plants were selected for this experiment. Wounds measuring 5 mm depth were made near the center of the second internode above the soil line of each maize stalk and fresh 106 spore suspensions of F. verticillioides strains were injected into wound. Sterile distilled water (10 µl) was injected into 5-mm deep wounded maize stalk and maintained as negative control. Wounded sites were covered and were incubated for 13 days. Stalk rot evaluation was done by vertically cut opening the maize stalk on the fourteenth day of incubation and area of pith discoloration was quantified using the formula A = πr2. Plants were maintained in triplicates (Ridenour and Bluhm 2014).
Inoculation of kernels
Maize kernels from fields were brought to the laboratory. Kernels were autoclaved followed by overnight suspension with 50 mL of each F. verticillioides spore suspension (106 spores/mL). The kernels were then incubated by blotter method at alternation periods of 12 h darkness and 12 h light at 25 ± 2 °C for 10 days. After incubation, kernel rot was estimated by determining the percent of kernel infection among different strains of F. verticillioides.
Fumonisin variations and LC–MS analysis
All the ten isolates of F. verticillioides were tested for their ability to synthesize fumonisin B1 and its variation among the isolates. The concentration of 106 spores/mL of 1 mL of each isolate was artificially inoculated into 5 g autoclaved cereals in culture tubes. The tubes were incubated up to 27 days. After incubation, samples were finely ground using liquid nitrogen, in a sterile pestle and mortar and further used for fumonisin extraction. The ground sample weighing 0.4 g was taken in a sterile glass vial and suspended with 2 mL acetonitrile/water (1:1) extraction solvent and allowed for equilibration overnight in a gel rocker at 28 ± 2 °C. The extracts were syringe filtered using 0.45-µm nylon membrane filters and liquid chromatography/mass spectrometry (LC/MS Waters Acquity Synapt G2, United States) was performed for the sample extracts. Column C18 was used at 50 °C and sample temperature being 24 °C for a run time of 8 min with mobile phase being water/acetonitrile. MassLynx SCN781 software was used to validate the LC/MS results. All sample extraction was carried out in triplicates.
Genotypic variations
Restriction fragment length polymorphism
DNA samples of all nine strains with reference to F. verticillioides MTCC 156 were subjected to PCR using VERTF-1/VERTR primers (Deepa et al. 2016a). Further, restriction fragment length polymorphism (RFLP) fingerprinting of the PCR-amplified products was performed for all ten isolates. RFLP analysis was performed on 6 µl of PCR product in a mixture containing the appropriate restriction enzyme buffer and restriction endonuclease as suggested by the manufacturer (Fermentas) and incubated at 37 °C for 3 h. The restriction endonucleases used to generate the RFLP patterns were HaeIII, HinfI, KpnI, HindIII, EcoRI and XhoI (Conventional Fermentas) for the ten strains of F. verticillioides. F. verticillioides (MTCC 156) was the reference control. The resultant restricted PCR products were analyzed by electrophoresis in 2% (w/v) agarose gel (Sigma). PCR product without enzyme was served as negative control (Kim et al. 2001).
Random amplified polymorphic DNA
Isolated nine DNA samples and reference strain were subjected to PCR with three RAPD primers (R1-5′–AGGHCTCGATAHCMGVY-3′, R14-5′–MTGTAMGCTCCTGGGGATTCHC–3′, R16-5′–AMGTAHGTGACTGGVGTGHGYG–3′) from Chromous Biotech. Pvt. Ltd., India. PCR conditions were: 94 °C for 5 min of initial denaturation, 40 cycles of 94 °C for 1 min of denaturation, 40 °C for 1 min of annealing, primer extension at 72 °C for 2 min and final extension at 72 °C for 2 min. The PCR reaction included 1 μL of dNTPs, 2.5 μL of Taq buffer, 2.5 μL of MgCl2, 0.5 μL of Taq polymerase, 1.5 μL DNA, 2 μL of each primer (5 pg/μL) and 15 μL of water in a total volume of 25 μL reaction. Later, the PCR products were analyzed in 2% agarose gel with 100-bp DNA ladder and gel, which was documented using a gel documentation system after staining with ethidium bromide. The assay was repeated thrice to authorize the repeatability (Vijayan et al. 2012).
Results
The selected nine strains [FVM42, FVM86, FVM186—isolated from Maize, FVP200, FVP19, FVP31—isolated from paddy, FVS3, FVS22, FVS36—isolated from sorghum (Fig. S1)] were confirmed as F. verticillioides by PCR using species-specific primers VERTF-1 and VERTR and its fumonisin producing variability by fumonisin-specific primers VERTF-1 and VERTF-2 (Deepa et al. 2016a, b, c). Based on its PCR results, six strains of fumonisin-producing strains (FVM42, FVM86, FVP200, FVP31, FVS3, FVS36) and three non-fumonisin producing strains (FVM186, FVP19, FVS22) were used for further assays (Fig. 1).
Gel image of Fusarium verticillioides strains distinguishing fumonisin production. a Lane B to J—F. verticillioides strains; b Lane B, C, E, G, H, J—fumonisin-producing strains; Lane D, F, I—Non-fumonisin-producing strains. M-100bpMarker; A FVMTCC 156; B FVM42; C: FVM86; D: FVM146; E: FVP200; F: FVP19; G: FVP31; H: FVS3; I: FVS22; J: FVS36
Preliminary variations
Cultural and microscopic characteristics of F. verticillioides strains
The ten isolates colony diameters were found to be more on SNA media and less on CZA medium. F. verticillioides FVMTCC156 strain grew moderately when compared to F. verticillioides strains FVM42, FVM86, FVM186, FVP200, FVP19, FVP31, FVS3, FVS22 and FVS36. PSA and YEMA media exhibited moderate growth of colony when compared to PDA and MEA medium (Fig. S2). Sporulation was more on SNA and PSA media when compared to other media. Colony growth variations among the isolates based on diameter was found drastically more on PDA, PSA and YEMA media when compared to SNA, MEA and CZA media (Fig. 2).
Pigmentation variations
Pigmentation production was found (Fig. S3) more varied among F. verticillioides strain FVM86, FVMTCC156 and FVM146 and F. verticillioides strain FVS22 was highly pigmented but moderately pigmented were F. verticillioides strains FVP19, FVP200 and FVS3. Less pigmented were F. verticillioides strains FVP31 and FVS36 and least pigmentation was in F. verticillioides strain FVM42 (Fig. 3).
Phenotypic variations
Pathogenicity assays
Inoculation of cobs
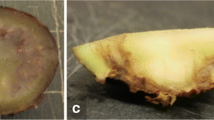
Fumonisin producing and non-producing isolates could not be easily distinguished for pathogenicity based on colonization, since F. verticillioides strains FVP200 and FVP19 were very highly colonized compared to F. verticillioides strains FVMTCC156 and FVM42 which are least colonizing among other strains. F. verticillioides strains FVM86 and FVM146 recorded high colonization and F. verticillioides strains FVS3 and FVS22 were found to be moderately colonized but F. verticillioides strains FVP31 and FVS36 were less colonized. The kernels from each cob lane were subjected to LCMS for their pathogenicity analysis (Fig. 4).
Inoculation of stalk
Area of necrosis for F. verticillioides strain FVM86 was 2.54 cm2 which was very high among all the isolates. F. verticillioides strains FVM146 and FVP200 recorded more than 1.8 cm2 necrosis and the area of necrosis shown by F. verticillioides strains FVP31, FVS3 and FVMTCC156 was moderate with 1.5 cm2 less, respectively. F. verticillioides strains FVM42 and FVS36 recorded necrotic area of more than 0.5 cm2 and F. verticillioides strains FVP19 and FVS22 recorded less than 0.5 cm2. Since F. verticillioides strain FVM146 which was non-fumonisin producer area of necrosis was about 2 cm2 (Figs. 5, 6).
Inoculation of kernel
Kernel rotness was measured to study the phenotypic variations (Fig. S4). Rotness was 100% in F. verticillioides strains FVP31 and FVS22 and F. verticillioides strains FVM146, FVM86, FVP19, FVP200 and FVS3 recorded above 85%, whereas in F. verticillioides strains FVS36, and FVM42 more than 75% kernel rot was recorded. Reference strain FVMTCC156 showed 54% rotness (Fig. 7a).
a Incidence of Kernel rot by F. verticillioides on maize kernels. A FVMTCC 156; B FVM42; C FVM86; D FVM146; E FVP200; F FVP19; G FVP31; H FVS3; I FVS22; J FVS36. b LCMS analysis to know the fumonisin producing variations among F. verticillioides strains. A FVMTCC 156; B FVM42; C FVM86; D FVM146; E FVP200; F FVP19; G FVP31; H FVS3; I FVS22; J FVS36
Fumonisin variations and LC–MS analysis
Among the F. verticillioides strains FVMTCC156, FVM42, FVP200 and FVS3 are fumonisin producers and F. verticillioides strains FVM86, FVP31 and FVS36 are negligible producers but F. verticillioides strains FVM146, FVP19 and FVS22 are non-fumonisin producers when compared to standard toxin Fumonisin B1 with molecular weight of 722.535 g/mol by LCMS (Fig. 7b).
Genotypic variations
Restriction fragment length polymorphism
RFLP analysis of the ten F. verticillioides strains isolated from three types of cereals showed their genetic diversity. PCR–RFLP analysis revealed their identical pattern in case of Hae III and Hinf I endonucleases. In case of Kpn I, Hind III, EcoR I and Xho I endonucleases, there was strain level differentiation. In Kpn I endonuclease, the positive control strain A (F. verticillioides strain MTCC 156) was differentiable from other nine isolates. Of the nine isolates, F. verticillioides strains FVM42, FVM86, FVM146 and FVP19 had identical RFLP pattern. F. verticillioides strains FVP200 and FVP31 had RFLP profile different from each other and rest of the strains, whereas F. verticillioides strains FVS3, FVS22 and FVS36 had similar pattern. In the RFLP pattern obtained using Hind III endonuclease, the strains could be formed into four differentiable groups. F. verticillioides strains FVMTCC156, FVM86 and FVP19 showed identical RFLP pattern and thus are different from the other isolates. F. verticillioides strains FVM42, FVM86 and FVP200 formed one group and these are different from other strains. F. verticillioides strain FVP31 showed unique RFLP pattern making it discreetly distinguishable from all the other strains. F. verticillioides strains FVS3, FVS22 and FVS36 were having similar RFLP patterns. The RFLP pattern derived using Xho I endonuclease showed that F. verticillioides strains FVMTCC156, FVM42, FVS3, FVS22 and FVS36 belonged to one group, whereas F. verticillioides strains FVM86, FVM146 and FVP200 formed a different group and F. verticillioides strains FVP19 and FVP31 formed yet another group. In case of EcoR I endonuclease, F. verticillioides strains FVMTCC156, FVS3, FVS22 and FVS36 constituted a different group from other six isolates, whereas F. verticillioides strains FVM42, FVM86, FVM146, FVP200, FVP19 and FVP31 demonstrated identical restriction profile comprising in one group (Fig. 8).
Random amplified polymorphic DNA
PCR amplification of total genomic DNA with random oligonucleotide primers generated unique banding patterns depending on primers and isolates. Three nucleotide primers selected for the RAPD analysis resulted in 162 bands for ten isolates of F. verticillioides (Fig. 9). Genetic variability was observed in isolates from F. verticillioides strains FVM86, FVP200, FVP19 and FVP31, FVS3, and FVS36 representing same banding pattern for R1 primer, F. verticillioides strains FVP19, FVP31 and FVP31, and FVS3 resembled banding pattern similar to R14, and F. verticillioides strains FVM86, FVM146, and FVP19 recorded similar banding pattern as R16 and remaining isolates differed from each other (Table 1).
Discussion
Fusarium verticillioides strains were characterized in terms of preliminary, pathogenic and genetic variability with respect to fumonisin production. Earlier it was shown that 18 out of 194 strains did not produce fumonisin, indicating phenotypic variability among F. verticillioides (Deepa et al. 2016a, b, c). The results showed that the fumonisin-producing ability among isolates is neither linked to geographical origin nor to host origin but it is uniformly distributed. FUM 1 is the gene responsible for fumonisin biosynthesis but it did not produce fumonisin (Moretti et al. 2004). Sanchèz-Rangel et al. (2005) also showed that the FUM 1 gene containing maize in Mexico did not produce fumonisin.
Present results support earlier reports that fumonisin production is not essential for pathogenicity (Logrieco et al. 2003) and it also does not act as virulence/aggressiveness factor (Jardine and Leslie 1999; Desjardins and Plattner 2000). There is no correlation between cultural characteristics, genetic variability, pathogenicity assay and fumonisin biosynthesis in different maize tissues such as cob, kernel and stalk were found. F. verticillioides strains FVM86, FVM146, FVP200 and FVS3 were having significance in all pathogenicity and pigmentation. F. verticillioides strains FVS36 and FVM42 were prominent in kernel rotness but FVM42 was having very less pigmentation and less colonized in cob and stalk. Similarly, F. verticillioides strain FVS36 recorded less pigmentation and less colonization in cob and stalk. F. verticillioides strains FVP31 and FVS22 recorded 100% kernel rotness, whereas F. verticillioides strain FVS22 was highly pigmented moderately colonized in cob and exhibited less necrosis in stalk. Similarly, F. verticillioides strain FVP31 was less colonized in cob and moderately necrotised in stalk and produced less pigmentation. F. verticillioides strain FVP19 was varied in all assays recording moderate pigmentation, high colonization in cob minimum rotness in kernel and less necrosis region in stalk. Development of pigmentation in strains was independent of growth of cultures on different media and pathogenicity assays.
Fumonisin non-producing strains/negligible producing strains possessed low level of reduced virulence which could possibly be used in biological control to reduce the level of toxin-producing strains in the field thereby reducing mycotoxin contamination in feed stuff (Desjardins and Plattner 2000, Sneh 1998). F. verticillioides strain FVM86 was greatly associated with all phenotypic variations and produced negligible amount of fumonisin B1 and F. verticillioides strain FVM146 recorded no fumonisin production. Fumonisin-producing strain F. verticillioides strain FVM42 had drastic phenotypic variations. Phenotypic and genotypic variations may vary in each isolate independent of its fumonisin production and cannot be concluded based on its growth or pigmentation variations.
Thirty-three F. verticillioides strains from different origin and hosts were analyzed for fumonisin production and were characterized by PCR–RFLP to detect variations and to discriminate the isolates (Patino et al. 2006). In present study, identical pattern was recorded for two restriction endonuclease enzymes HaeIII and HinfI. Strain level differentiation was observed for KpnI, HindIII, EcoRI and XhoI endonucleases. RFLP patterns generated were used to detect the occurrence of genetic-related groups of strains within the species. Group of non-fumonisin-producing strains showed less variability than fumonisin-producing group of strains according to (Kerengy et al. 1999). RAPD applied to fungal studies would be useful in providing marker for identification purpose. Several studies have reported about the molecular differentiation while examining variations of Fusarium species (Abd-Elsalam et al. 2004; Singh et al. 2006). F. oxysporoum infecting vanilla in South India consisted of a single clonal lineage with moderate level of genetic diversification as observed using RAPD markers (Vijayan et al. 2012). In the present study, genetic diversity was observed in respect of strains with same banding pattern for the primers used and some strains found to be unique (Table 1). Among 99 isolates of F. oxysporum sixty isolates were characterized by RAPD markers using DNA bulks with 40 primers and cluster analysis of RAPD data showed three clusters of isolates within Foc for the study of Foc races distribution (Jimenez-Gasco et al. 2001). Cramer et al. (2003) characterized genetic diversity and pathogenicity of 166 isolates of F. oxysporum from bean and sugar beet plants by 12 RAPD primers generating 105 polymorphic bands. Cluster analysis from RAPD analysis clearly separated isolates into two clusters A and B confirming genetic diversity among the isolates of F. oxysporum from onion (Malathi and Mohan 2012). Genetic diversity among isolates from maize pathogen was found to be very high but specific relation was not observed between vegetative compatibility groups and their geographical origin (Mohammadi and Mofrad 2011). In the study, RFLP and RAPD analysis showed genetic diversity among isolates which was not related to their geographic area of origin or host specific but depend on ability of strains to produce fumonisins.
Conclusion
The isolates collected from different geographic regions throughout Karnataka from maize, paddy, and sorghum were tested for their ability to produce fumonisin. All the ten isolates tested showed no correlation in their cultural characteristics, pigmentation production, pathogenicity assays, genetic variability and ability to produce fumonisin. The F. verticillioides strains FVM86 and FVM146 isolated from maize were dominant in their pigmentation production and pathogenicity but showed negligible amount or no fumonisin production. F. verticillioides strain FVS3 isolated from sorghum is a high fumonisin producer but least varied phenotypically with kernel rot and stalk rot variations but varied in pigmentation production. F. verticillioides strain FVP19 isolated from paddy varied drastically in all assays where it was moderately pigmented and highly colonized in cob and had minimum kernel rotness but very less necrosis region in stalk and it is a non-fumonisin producing strain with moderate cultural characteristics and sporulation. Individual strains demonstrated different fumonisin production and showed differences in pathogenicity, preliminary and genetic variations in which no correlations could be observed between these parameters. Overall, the findings increase the knowledge on mycotoxin production which is independent of pigmentation and pathogenic variations along with genetic variations and its cultural characteristics.
References
Abd-Elsalam KA, Omar MR, Migheli Q, Nirenberg HI (2004) Genetic characterization of F. oxysporum f. sp. vasinfectum isolates by random amplification of polymorphic DNA (RAPD) and amplified fragment length polymorphism (RFLP). J Plant Dis Prot 111:534–544
Afolabi CG, Ojiambo PS, Ekpo EJA, Menkir A, Bandyopadhyay R (2008) Novel sources of resistance to Fusarium stalk rot of maize in tropical Africa. Plant Dis 92:772–780
Bacon CW, Hinton DM (1996) Symptomless endophytic colonization of maize by Fusarium moniliforme. Can J Bot 74:1195–1202
Covarelli L, Stifano S, Beccari G, Raggi L, Lattanzio VMT, Albertini E (2012) Characterization of Fusarium verticillioides strains isolated from maize in Italy: fumonisin production, pathogenicity and genetic variability. Food Microbiol 31:17–24
Cramer RA, Byrne PF, Brick MA, Panella L, Wickliffe E, Schwartz HF (2003) Characterzation of Fusarium oxysporum isolates from common bean and sugar beet using pathogenecity assays and random amplified polymorphic DNA markers. J Phytopathol 151:352–360
Danielsen S, Jensen DF (1998) Relationships between seed germination, fumonisin content and Fusarium verticillioides infection in selected maize samples from different regions of Costa Rica. Plant Pathol 47:609–614
Deepa N, Adkar-Purushothama Charith Raj, Sreenivasa MY (2016a) Nested PCR method for early detection of fumonisin producing Fusarium verticillioides in pure cultures, cereal samples and plant parts. Food Biotechnol 30(1):18–29
Deepa N, Nagaraja H, Sreenivasa MY (2016b) Prevalence of fumonisin producing Fusarium verticillioides associated with cereals grown in Karnataka, India. FSHW 5(3):156–158
Deepa N, Adkar-Purushothama Charith Raj, Sreenivasa MY (2016c) Multiplex PCR for the early detection of fumonisin producing Fusarium verticillioides. Food Biosci 13:84–88
Desjardins AE, Plattner RD (2000) Fumonisin B1—nonproducing strains of Fusarium verticillioides cause maize (Zea mays) ear infection and ear rot. J Agric Food Chem 48:5773–5780
Desjardins AE, Plattner RD, Lu M, Claflin LE (1998) Distribution of fumonisins in maize ears infected with strains of Fusarium moniliforme that differ in fumonisin production. Plant Dis 82:953–958
Gohari AM, Nikkhah MJ, Hedjaroude GA, Abbasi M, Rahjoo V, Sedaghat N (2008) Genetic diversity of Fusarium verticillioides isolates from maize in Iran based on vegetative compatability grouping. J Plant Pathol 90(1):113–116
International Agency for Research on Cancer (IARC) (1993) Ochratoxin A. Monographs on the evaluation of carcinogenic risks to humans. In: Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins, vol 56. International Agency for Research on Cancer, Lyon, France, pp 489–521
Jardine DJ, Leslie JF (1999) Aggressiveness to mature maize plants of Fusarium strains differing in ability to produce fumonisins. Plant Dis 83:690–693
Jimenez-Gasco MM, Perez-Artes E, Jimenez Diaz RM (2001) Identification of pathogenic races 0,1 B/C, 5 and 6 of F. oxysporum f. sp. ciceris with random amplified polymorphic DNA. Eur J Plant Pathol 107:237–248
Kerengy Z, Zeller K, Hornok L, Leslie JF (1999) Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl Envi Microbiol 65:4071–4076
Kim BJ, Lee KH, Park BN, Kim SJ, Bai GH, Kim SJ, Kook YH (2001) Differentitaion of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB). J clin microbiol 39(6):2012–2019
Lazzaro I, Falavigna C, Dall’Asta C, Proctor RH, Galaverna G, Battilani P (2012) Fumonisins B, A and C profile and masking in Fusarium verticillioides strains on fumonisin-inducing and maize-based media. Int J Food Microbiol 159:93–100
Leslie JF (1991) Mating populations in Gibberella fujikuroi (Fusarium section Liseola). Phytopathol 81(9):1058–1060
Leslie JF (1995) Gibberella fujikuroi: available populations and variable traits. Can J Bot 73:S282–S291
Logrieco A, Bottalico A, Mule G, Moretti A, Perrone G (2003) Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur J Plant Pathol 109:645–667
Malathi S, Mohan S (2012) Analysis of genetic variability of F. oxysporum f.sp. cepae the causal agent of basal rot on onion using RAPD markers. Arch Phytopathol Plant Prot 45(13):1519–1526
Milgroom MG (1996) Recombination and the multilocus structure of fungal populations. Ann rev phytopathol 34:457–477
Mohammadi A, Mofrad NN (2011) Investigation on genetic diversity of Fusarium verticillioides isolated from corn using vegetative compatibility groups and relation of VCGs to the pathogenecity. J Agric Technol 7(1):143–148
Moretti A, Mule G, Susca A, Gonzalez-Jaen MT, Logrieco A (2004) Toxin profile, fertility and AFLP analysis of F. verticillioides from banana fruits. Eur J Plant Pathol 110:601–609
Munkvold GP, Desjardins AE (1997) Fumonisins in maize, can we reduce their occurrence? Plant Dis 81:556–565
Patino B, Mirete S, Vazquez C, Jimenez M, Rodriguez MT, Gonzalez-Jaen MT (2006) Characterization of F. verticillioides strains by PCR-RFLP analysis of the intergenic spacer region of the rDNA. J Sci Food Agric 86:429–435
Proctor RH, Plattner RD, Desjardins AE, Busman M, Butchko RAE (2006) Fumonisin production in the maize pathogen F. verticillioides: genetic basis of naturally occurring chemical variation. J Agric Food Chem 54:2424–2430
Reynso NN, Chulze SN, Zeller KA, Torres AM, Leslie JF (2009) Genetic structure of F. verticillioides populations isolated from maize in Argentina. Eur J Plant Pathol 123:207–215
Ridenour JB, Bluhm BH (2014) The HAP complex in Fusarium verticillioides is a key regulator of growth, morphogenesis, secondary metabolites and pathogenesis. Fung Gen Biol 69:52–64
Sanchèz-Rangel D, Sanjuan-Badillo A, Plasencia J (2005) Fumonisin production by Fusarium verticillioides strains isolated from maize in Mexico and development of a polymerase chain reaction to detect potential toxigenic strains in grains. J Agri Food Chem 53:8565–8571
Singh BP, Saikia R, Yadav M, Singh R, Chauhan VS, Arora DK (2006) Molecular characterization of F. oxysporum f. sp. ciceris casing wilt of chickpea. Afr J Biotechnol 5(6):497–502
Sneh B (1998) Use of non-pathogenic or hypovirulent fungal strains to protect plants against closely related fungal pathogens. Biotechnol Adv 16:1–32
Sreenivasa MY, Dass RS, Charith Raj AP, Janardhana GR (2011) Mycological evaluation of Maize grains produced in Karnataka (India) for the post harvest fungal contamination. World Appli Sci J 13(4):688–692
Vijayan AK, Sithara L, Sreelakshmi KP, Thomas J, Misra RS, Saju KA (2012) Molecular diversity of F. oxysporum causing rot disease of vanilla in south India. Arch phytopathol plant protect 45(11):1319–1326
Yamamura Y, Shim WB (2008) The coiled-coil protein-binding motif in Fusarium verticillioides Fsr1 is essential for maize stalk rot virulence. Microbiol 154:1637–1645
Acknowledgements
We thank the Indian Council of Medical Research (ICMR) for providing grants through ICMR-SRF. The authors acknowledge Bhavani P.V and Sowmya rao N for their kind help and support in conducting genotypic variation assay, Deepthi B.V., and Poorna Chandra Rao K for their kind co-operation for conducting stalk rot assay in the field and Institute of Excellence, Vijnana bhavan, Mysore, for their valuable support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deepa, N., Rakesh, S. & Sreenivasa, M.Y. Morphological, pathological and mycotoxicological variations among Fusarium verticillioides isolated from cereals. 3 Biotech 8, 105 (2018). https://doi.org/10.1007/s13205-018-1136-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1136-z