Abstract
The present study was conducted to investigate the antileishmanial activity of biogenic silver nanoparticles (AgNPs) compared to chemically synthesized AgNPs. A nano dimension size (10–15 nm) biogenic AgNPs was produced and characterized by UV–Vis spectroscopy and X-rays diffraction. The chemically synthesized AgNPs was recovering from our previous study with a nanoparticle (NP) size in the range of 10–40 nm. The antileishmanial activities were investigated through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay. The infectivity was determined by Giemsa staining of the infected macrophages cells. Nitric oxide (NO) accumulation was measured by Griess reagent using NaNO2 as a positive control. After 24 h of exposure with nanoparticles (NPs), a concentration-dependent growth inhibition was observed. The IC50 values were determined against promastigotes of L. infantum as 19.42 ± 2.76 µg/ml for leaves aqueous extract mediated AgNPs, 30.71 ± 1.91 µg/ml for stem mediated AgNPs and 51.23 ± 2.20 µg/ml for chemically synthesized AgNPs. It was also detected that all types of NPs produced NO at a significant level. However, the production of a high-level of NO in the biologically synthesized NPs activated macrophage cells, infected with L. infantum promastigotes indicates that NO radicals are mainly responsible for induced cell death and a decrease in the pathogenicity of the parasites. Since, biogenic nanoparticles are cost-effective, eco-friendly, simple to synthesize, and more effective than chemically synthesized silver nanoparticles, therefore, it could be used as a potential alternative for the development of antileishmanial drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Leishmaniasis is a crucial parasitic disease which is caused by the leishmania parasites. Most frequent clinical forms of the disease are cutaneous leishmaniasis (CL) characterized by skin lesions and visceral leishmaniasis (VL), characterized by sores on internal organs have a serious burden on mammals (De Freitas et al. 2016). The diagnosis and treatment of VL are difficult and 95% of VL cases are fatal if it is not treated properly (Gadisa et al. 2015). It has been estimated that nearly 350 million people are threatened and about 1.5 million cases appear in a year globally (Srivastava et al. 2016). Moreover, risk factors such as late wars, migration, hunger, climate changes and resistance to existing treatment modalities carry an important role in the progression of the disease (Dawit et al. 2013).
So far, chemotherapy-based treatments are considered to control and prevent leishmaniasis. However, many disadvantages of current antileishmanial drugs including toxic side effects for humans, high cost and drug-resistance of the parasites (Beaumier et al. 2013; Das and Ali 2012). To overcome these drawback, it is the prime interest of the scientist to focus on the development of new approaches to an ideal antileishmanial drug which prevent resistive immunity and present long-term T cell responses near safety and financial availability (Gillespie et al. 2016).
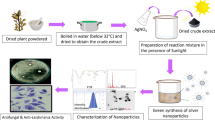
A possibility to treat or improve the therapeutic potency of drugs against leishmaniasis, nanobiotechnology-based strategies is very promising and cost effective. The development of nanoscale materials such as silver, gold, zinc and titanium has attracted significant attention in the field of medicine. Among these, silver nanoparticles have significant importance due to their broad spectrum antimicrobial properties. Both chemical and physical methods are used for the synthesis of AgNPs (Zhu et al. 2000; Hiramatsu and Osterloh 2004; Rodriguez-Sanchez et al. 2000). However, chemical methods are costly and use toxic chemicals that are a potential risk for the environment and biological stakes (Li et al. 2011). Biomimetic synthesis of silver nanoparticles involves oxidation and reduction reactions. Utilizing plants as a source for the reduction of silver into silver nanoparticles is easily accessible, safe and rich source of metabolites that help in the reduction of silver ions (Ahmad et al. 2003). Therefore, in the present study green chemistry approach was made to synthesize AgNPs using the aqueous extracts of Teucrium stocksianum Boiss.
Teucrium stocksianum is a well-known medicinal herb used in folklore medicine for the treatment of hypertension, diabetes, epilepsy, skin rashes, pain in the throat and as a blood purifier (Iqbal and Hamayun 2004). In scientific literature, various studied shows that its leaves crude extract is having antispasmodic properties, cytotoxic and anthelminthic effects (Ali et al. 2011), hepatotoxicity (Rasheed et al. 1995), anti-ulcerogenic and gastric cytoprotective (Wasfi et al. 1995; Islam et al. 2002). Teucrium stocksianum is a rich source of biomolecules that has excellent reducing/capping ability for the synthesis of silver nanoparticles and its associated kinetics would benefit to prepare desired size ranges of nanoparticles. In this study, for the first time, we report, the comparative antileishmanial activities of chemically synthesized and green synthesized AgNPs mediated by leaves and stem aqueous extract of T. stocksianum Boiss.
Materials and methods
Synthesis of silver nanoparticles (AgNPs)
The silver nanoparticles were prepared from leaves and stem aqueous extract of T. stocksianum Boiss as described previously (Ullah et al. 2017a). Briefly, 10 ml aqueous extracts (5 mg/ml) was added to a vigorously stirred 90 ml aqueous solution of AgNO3 (1 mM) at 90 °C in the dark. The reduction of the Ag ions, took place rapidly as indicated by the dark brown color of the solution. The UV–visible spectra were recorded on a Jasco V-550 UV–visible spectrophotometer with samples in a quartz cuvette. The colloidal solution of the nanoparticles (NPs) was rapidly centrifuged 13,000 rpm for 15 min and washed with distilled water. The pellet obtained was freeze-dried using lyophilizer machine. The size and crystallinity of AgNPs were determined using X-rays diffractometer (XRD). The chemically prepared silver nanopowder synthesized in our previous study, (Allahverdiyev et al. 2011) was used for comparative study.
Parasite culture
Parasites were cultured as described by Allahverdiyev and co workers (Allahverdiyev et al. 2013). Briefly, Leishmania infantum promastigotes were cultured at 26 °C in culture flasks containing RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), (Gibco), l-glutamine (Gibco), and gentamicin (80 µg/ml). Cultures were passaged every 4 days during the study period. The growth of promastigotes was monitored daily using an inverted microscope (Olympus CK 40). Samples of promastigote culture (2 × 106 promastigotes/ml of medium) were transferred to a culture flask with 7 ml of RPMI 1640 + 10% FCS. They were fixed in formalin 2% (v/v) in PBS solution and counted using a hemocytometer with a 20× objective under standard light microscopy.
Cytotoxicity assay on L. infantum promastigotes
Parasites were resuspended in RPMI 1640 medium supplemented with 10% FBS and gentamicin (80 µg/ml) and seeded (1 × 105 promastigotes/well) in 96-well flat bottom microplates with 100 µl of the medium (Allahverdiyev et al. 2011). The parasites were exposed to different concentrations of nanoparticles and incubated for 48 h at 27 °C. The parasites were washed with PBS, then incubated with MTT 100 µg/well for 4 h in the dark at 37 °C. MTT solution was removed and the cells were resuspended in 100 µl of DMSO and the absorbance measured in an ELISA reader at 540 nm (Bio-Rad).
Macrophage (J774) culture
The J774 cells were grown in plastic 25 cm2 cell culture flasks in RPMI 1640 medium (Gibco) containing l-glutamine, buffered with 10 mM HEPES, and supplemented with 10% heat-inactivated fetal bovine serum (FBS) and gentamicin (80 µg/ml), in a humidified incubator 5% CO2 at 37 °C, and sub passaged once a week (Allahverdiyev et al. 2013).
Cytotoxicity assay on macrophages
J774 macrophages were seeded (10,000 cells/well) in 96-well flat bottom microplates with 100 µl of the medium. The cells were allowed to attach to the bottom of the dish for 24 h at 37 °C and then exposed to the different concentrations of extracts and nanoparticles for 24 h. After, the cells were washed with PBS and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) 100 µg/well for 4 h in the dark at 37 °C. MTT solution was removed, then cells were resuspended in 100 µl of dimethylsulfoxide (DMSO) and the absorbance measured in an ELISA reader (Bio-Rad) at 540 nm (Ullah et al. 2017b).
Infectivity of L. infantum promastigotes in macrophages
Infectivity was investigated by the methods described by (Tanaka et al. 2007) with little modifications. Briefly, stationary phase L. infantum parasites were exposed to the IC50 concentration of the nanoparticles for 4 h. Nanoparticles were then removed by washing twice by centrifugation (1.500g for 5 min) in PBS, at pH 7.2. Macrophage cells were transferred into 24-well cell-culture plates that contain sterile cover-slips. Cells were then incubated at 37 °C with 5.0% CO2 for 2 h for adhesion. The cover-slips were washed with RPMI-1640 medium to remove unattached cells. An aliquot of 500 µl RPMI-1640 was added into the culture, and cells were incubated for an additional 24 h before the infection. The infection of macrophage cells was based on a ratio of ten parasites per macrophage, and this procedure was performed in triplicate. Promastigotes were added to macrophage monolayers and maintained for 4 h in RPMI 1640 at 37 °C. Non-adherent parasites were removed by washing the monolayers with culture medium and incubated for additional 24 h. İn order to determine the infection index, the coverslips were washed with PBS, fixed in absolute methyl alcohol for 5 min, and stained with Giemsa for 45 min. The number of amastigotes in infected macrophage cells was counted under oil emersion microscope.
Determination of nitrite accumulation
After 24 h of macrophage–Leishmania interaction in culture, the supernatants (100 µl) were collected to evaluate the levels of NO production. The concentration of nitrite (NO2) released by macrophages as determined by the Griess reaction (Mauel et al. 1991) was used as an indicator of NO production. Equal volumes of cell culture medium were mixed with Griess reagents (1% sulfanilamide, 0.1% naphthyl ethylenediamine, and 2.5% H3PO4) for 10 min at room temperature. This mixture was distributed in a 96-well plate and the absorbance measured in an ELISA reader at 540 nm (Bio-Rad). The results were expressed as µM of NO based on a standard curve formed by known concentrations of sodium nitrite (NaNO2 dilutions from 200 to 1 µM) dissolved in culture medium.
Data analysis
Each concentration was assayed in triplicate, and corresponding cell growth controls were used in each measurement. Each assay was also performed in triplicate. The percentage of infected cells and the geometric mean of the number of parasites per infected cell were evaluated. The infection index was calculated by multiplication of both parameters to account for the overall parasite load. The graphs were made on GraphPad prism and Origen Pro softwares. The cytotoxicity against leishmania parasites and macrophage cells was determined using the formula;
Results
Green synthesis and characterization of silver nanoparticles (AgNPs)
The silver nanoparticles were prepared by reducing AgNO3 with an aqueous extract obtained from T. stocksianum leaves and stem parts. The optimal concentration of AgNO3 was 1 mM combined with extract in the ratio of 1–10 (1 ml extract to 9 ml AgNO3). The color change was observed as the first indication of the reaction. The color of the colloidal solution is light and dark brown in leaves and stem mediated synthesis, respectively (Fig. 1 inset). The change in color indicates that the reduction and capping of silver are proceeding into the formation of the nano-metallic complex. Figure 1 shows the UV–visible spectra of the silver nanoparticles obtained after 2 h of the reaction. The surface plasmon resonance of the colloidal solution indicates peaks at 424 and 431 nm in the leaves and stem extract mediated synthesis, respectively. The specific surface plasmon resonance observed by the reduction of Ag+ into Ag0 by UV confirm the synthesis of silver nanoparticles in the nano range.
Characterization of silver nanoparticles (AgNPs) using UV–Vis spectrophotometer. The figure shows the UV–Vis spectrum of leaves and stem extract mediated AgNPs and color of the reaction mixture after 2 h of incubation (Fig. 1 inset)
The powder AgNPs was obtained by repeated washing and centrifugation at 13,000 rpm for 15 min. The pellet obtained was freeze dried using lyophilizer. The size was further confirmed by X-rays diffraction (XRD). The XRD pattern of powdered AgNPs is shown in Fig. 2a, b. The intensity of diffraction was counted in the range of 20°–80° at 2θ angles. The characteristic crystalline face-centered cubic nature of the silver nanoparticles diffraction peak was compared to JCPD card (04-0783) of silver nanoparticles. The characteristic peak was observed at a 2θ angel of 38.15° (111), 46.20° (200) and 64.57° (220) in leaves mediated silver nanoparticles. Whereas in stem mediated synthesis the brag reflection was observed at 2θ angle 27.78° (101), 32.22° (111), 46.19° (200) and 66.19° (220). The average size of leaves mediated silver nanoparticles was calculated as 13.12 nm, whereas for stem AgNPs it was 14.13 nm. The XRD pattern shows pure silver crystals. Our XRD pattern confirmed that the silver particles formed are nanocrystals with an average size in the nanometer scale as calculated by Scherrer equation.
Chemically synthesized AgNPs was used for the comparative study that we prepared in our previous study (Allahverdiyev et al. 2011). The transmission electron microscopy (TEM) revealed an average size in the range of 10–40 nm. The nanoparticle was in rounded shape with a smooth surface morphology. The dynamic light scattering (DLS) analysis reveals an average hydrodynamic zeta size of about 65 nm.
Anti-leishmanial activity of silver nanoparticles
The efficacy of the tested nanoparticles was determined as percent growth inhibition against different concentration (6, 12, 25, 50, 100, 200, 300, 400 µg/ml). MTT cell viability assay was performed to investigate the growth of L. infantum promastigotes. Twenty-four hours after incubation with the nanoparticles, concentration-dependent growth inhibition was observed (Fig. 3). A very fewer formazan crystal formed at a higher concentration of the nanoparticles. Each concentration has a suppressive effect on the growth and metabolic activity of L. infantum promastigotes. The highest percent growth inhibition was calculated for leaves mediated silver nanoparticles at highest concentration followed by chemically synthesized silver nanoparticle and stem mediated biogenic silver nanoparticles, respectively. Cell viability decreased with increasing AgNPs concentration. The IC50 was determined for promastigotes of L. infantum as 19.42 ± 2.76 µg/ml for leaves mediated AgNPs, 30.71 ± 1.91 µg/ml for stem mediated AgNPs and 51.23 ± 2.20 µg/ml of chemically synthesized silver nanoparticles. The IC50 values indicate that biogenically synthesized AgNPs are more effective to inhibit the growth of L. infantum promastigotes than the chemically synthesized AgNPs.
Cytotoxicity assay on J774 macrophage cells
The inhibition of cell growth was assessed by cultivating macrophage J774 cell lines with increasing concentration of the nanoparticles. MTT cell viability assay was performed after 24 h of incubation. Figure 4 demonstrates the percent growth inhibition and IC50 value of the respective green synthesized and chemically synthesized AgNPs. Figure 4 shows that the respective nanoparticles are not much toxic to macrophage cells as compared to Leishmania parasites (Fig. 3). IC50 values described that 50% of macrophages maintained their viability at 100.02 ± 5.67 µg/ml (Leaves-AgNPs), 116.81 ± 7.32 µg/ml (Stem-AgNPs) and 62.99 + 8.24 µg/ml (Chem-AgNPs) concentration of nanoparticles. The figure also describes that leaves and stem extract mediated silver nanoparticles are less toxic compared to chemically synthesized silver nanoparticles.
Infectivity of Leishmania parasites on macrophages cells
After 4 h, the L. infantum promastigotes treated with different types of silver nanoparticles was used to infected macrophage cells J774. The amazogenesis of promastigotes into amastigotes was observed by Giemsa staining in the infected macrophages.
Table 1 shows the infection percentage for macrophages cultured with L. infantum amastigotes after 24 h of incubation. Intracellular amastigotes were observed as 2.32, 2.81 and 2.4 in the nanoparticles synthesized from leaves/stem of T. stocksianum and chemically synthesized silver nanoparticles, respectively. In contrast, 80% of control macrophages were infected (average 3.9 amastigotes/macrophage). The viability of the macrophages was intact, as this concentration of nanoparticles as shown in the MTT viability assay. The highest infectivity index was calculated in the control group that was 312, leaves extract mediated silver nanoparticles (85.84), and stem extract mediated nanoparticles (112.4) and 100.8 in the chemically synthesized AgNPs. However, the MTT cell viability assay shows that chemically synthesized silver nanoparticles are toxic at a lower concentration to macrophage cells as compared to biogenically synthesized AgNPs.
Nitrogen oxide (NO) production
Nitric oxide production in cell supernatants of nanoparticle-treated macrophages was monitored after 24 h of treatment. Figure 5 describes the production of nitric oxide in the nanoparticles activated macrophage cells, infected with L. infantum promastigotes. The figure indicates that NO is produced at a significant level by macrophage cells activated with all types of silver nanoparticles as compared to control group. From the infectivity index it also clear that the infectivity of amastigotes is also less in nanoparticles activated macrophage cells than control groups (Table 1). The production of nitrogen oxide species is dependent on the size of the nanoparticles. The leaves extract mediated AgNPs with small size (13.12 nm) produce an elevated level of NO as compared to stem AgNPs (14.13 nm). Similarly, there is a significant difference in the NO production of chemically synthesized and biologically synthesized NPs. the production of NO is directly related to the antileishmanial activity of the nanoparticles as demonstrated in Fig. 3 with less IC50 value for biological nanoparticles as compared to chemical nanoparticles.
Discussion
Nanobiotechnology is gaining much interest in the development of nanomaterials that have leishmanicidal properties and other significant application in the field of medicines. Among the various nanomaterials, silver nanoparticles possess a broad spectrum of antimicrobial properties. Therefore, silver nanoparticles have been used as an alternative treatment agent against various emerging microbial resistance (Ahmad et al. 2016). We synthesized silver nanoparticles mediated by T. stocksianum aqueous extract. The UV–visible spectrum shows a characteristic surface plasmon resonance of silver nanoparticles. The XRD pattern shows a crystalline nature of the nanoparticles with an average size of nanometer scale. The slight difference in the surface plasmon resonance in leaves and stem nanoparticles may be due to the difference in the biomolecules found in the leaf tissues and stem tissues of the plant, that ultimately results in differences in capping and reduction of silver ions into silver nanoparticles. The corresponding biogenic silver nanoparticles were used to evaluate the antileishmanial activities of the silver nanoparticles compared to chemically synthesized nanoparticles. There are many reports about the antileishmanial effects of biogenic silver, gold and titanium oxide nanoparticles (Ahmad et al. 2016; Rossi-Bergmann et al. 2012; Zahir et al. 2015) and chemically synthesised metal nanoparticles (Allahverdiyev et al. 2013; Baiocco et al. 2010). However, there is no study to our knowledge that reports the comparative antileishmanial effects of biogenically and chemically synthesized silver nanoparticles. In this study, we for the first time report that biogenically synthesized silver nanoparticles of T. stocksianum aqueous extract are more effective in killing leishmania parasites and nontoxic to macrophage cells as compared to chemically synthesized silver nanoparticles. The antileishmanial potential of the nanoparticles after 24 h of evaluation indicates that a viable number of parasites are decreasing and the nanoparticles inhibit the growth of the parasites in a concentration-dependent manner. Biogenic AgNPs exhibit a maximum of inhibition (98% in leaves AgNPs), (81% in stem-AgNPs) and chemically synthesised silver nanoparticles (95% inhibition) at at higher concentration (400 µg/ml). Similar in vitro anti promastigote activity was obtained by Rossi-Bergmann in the silver nanoparticles chemically synthesized (AgCHEM), biogenic silver nanoparticles (AgBIO) and amphotericin B. They observed that the parasitic load decreased up to 13, 61, and 68%, in chemical, biogenic and amphotericin B treated cells, respectively. They found the IC50 of Chem-AgNPs and Biogenic AgNPs as 103.5 ± 11.5 and 31.6 ± 8.2 μM, respectively (Rossi-Bergmann et al. 2012). Similarly, we observed that biogenic AgNPs are effective at lower IC50 values (Fig. 3), than chemically synthesized silver nanoparticles. A number of studies are available that report the antileishmanial effect of different metal nanoparticles (Minodier and Parola 2007; Visbal et al. 2008; Zahir et al. 2015; Ghosh et al. 2015; Kalangi et al. 2016). The non-toxicological concentration of the biogenic nanoparticles and chemically synthesized nanoparticles was higher for macrophage cells than leishmania parasites. However, there was a significant difference in the toxicity of chemically synthesized nanoparticles to macrophage cells compared to biogenic nanoparticles (Fig. 4).
Living organism protect themselves from invading intracellular parasites by activating macrophages and or Kupffer cells in the liver (James 1995). Macrophages act as phagocytic agent for the invading pathogen and produce various reactive oxygen and nitric oxide species that act as leishmanicidal (Carneiro et al. 2016; Roma et al. 2016). The infectivity of Leishmania promastigotes and the formation of amastigotes in macrophage cell was assessed by prior incubation of the parasites with the IC50 concentration of the nanoparticles. The results show that the infectivity of the parasite decreased on exposure to NP as compared to control group. The possible mechanism for the increase in the antileishmanial activity of the biological nanoparticles is due to its small size compared to chemically synthesized nanoparticles. There is also a significant difference in the infection index of the parasites against all type of NPs as compared to control group (Table 1). However, a slight difference were observed in the infection percentage and parasite load in all the three groups of NP. Our observations shows that after exposure to the NPs, leishmania promastigotes lost their ability to infect the in vitro cultured macrophage cells. This could be the fact that NPs change the surface morphology, chemical composition or structure of the parasitic cell by producing a high level of nitric oxides (NO).
Previously, the in vitro cytotoxicity studies reports that intracellular form of the leishmania is killed by the production nitric oxide species (Green et al. 1991; Elcicek et al. 2013; Cunningham 2002). Nitric oxides (NO) is a free radical, produced in the biological system. It reacts with superoxides in a living organism to produce harmful nitrite (NO2−) and nitrate (NO3−) that have toxic effects on microbes and parasites. NO free radical stimulate multiple cellular processes that cause DNA damage (Jaiswal et al. 2000), damage to the cell membrane, alteration in protein structure, inhibition of protein synthesis and lipid peroxidation (Banuls et al. 2014; Ullah et al. 2017b; Ahamed et al. 2010). NO production can be measured by various methods. The simplest method is detecting the level of NO in activated macrophage cells by Griess reaction (Green et al. 1982; Shavandi et al. 2011). We detect a significant difference in the production of NO level in all types of NPs activated macrophage cells. However, sufficient amount of NO produced by all types of nanoparticles that lead to decrease in the parasite load in the macrophage cells. This indicates that biogenically synthesized AgNPs of T. stocksianum leaves and stem aqueous extracts and chemically synthesized silver nanoparticles kills the Leishmania parasite on the same mechanism by producing NO free radicals in Leishmania and macrophage cells. However, the elevated amount of NO produced by biological nanoparticles indicates its potential therapeutic efficacy in the development of antileishmanial drugs in the future.
Conclusion
The strong antileishmanial activity of biogenic silver nanoparticles as compared to chemically synthesized silver nanoparticles may be due to the inhibitory effect of some metabolically active enzymes responsible for the growth of Leishmania or due to the production of nitrite-free radicals that kill Leishmania parasites. Furthermore, it is suggested that biogenic silver nanoparticles are easy to synthesize, cost-effective and biologically more active in low concentration than chemically synthesized silver nanoparticles, and thus T. stocksianum extract-mediated silver nanoparticles can be a promising agent for the development of antileishmanial drug in the future.
References
Ahamed M, AlSalhi MS, Siddiqui M (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23):1841–1848
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28(4):313–318
Ahmad A, Wei Y, Syed F, Khan S, Khan GM, Tahir K, Khan AU, Raza M, Khan FU, Yuan Q (2016) Isatis tinctoria mediated synthesis of amphotericin B-bound silver nanoparticles with enhanced photoinduced antileishmanial activity: a novel green approach. J Photochem Photobiol B 161:17–24
Ali N, Shah SW, Shah I, Ahmed G, Ghias M, Khan I (2011) Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium Stocksianum boiss. BMC Complement Altern Med 11(1):106
Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, Rafailovich M (2011) Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomed 6:2705–2714
Allahverdiyev AM, Abamor ES, Bagirova M, Baydar SY, Ates SC, Kaya F, Kaya C, Rafailovich M (2013) Investigation of antileishmanial activities of TiO2@Ag nanoparticles on biological properties of L. tropica and L. infantum parasites, in vitro. Exp Parasitol 135(1):55–63
Baiocco P, Ilari A, Ceci P, Orsini S, Gramiccia M, Di Muccio T, Colotti G (2010) Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med Chem Lett 2(3):230–233
Banuls C, Rocha M, Rovira-Llopis S, Falcon R, Castello R, Herance RJ, Polo M, Blas-Garcia A, Hernandez-Mijares A, Victor MV (2014) The pivotal role of nitric oxide: effects on the nervous and immune systems. Curr Pharm Des 20(29):4679–4689
Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME (2013) New vaccines for neglected parasitic diseases and dengue. Transl Res J Lab Clin Med 162(3):144–155. https://doi.org/10.1016/j.trsl.2013.03.006
Carneiro PP, Conceição J, Macedo M, Magalhães V, Carvalho EM, Bacellar O (2016) The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with cutaneous leishmaniasis. PLoS One 11(2):e0148084
Cunningham AC (2002) Parasitic adaptive mechanisms in infection by Leishmania. Exp Mol Pathol 72(2):132–141
Das A, Ali N (2012) Vaccine development against Leishmania donovani. Front Immunol 3:99. https://doi.org/10.3389/fimmu.2012.00099
Dawit G, Girma Z, Simenew K (2013) A review on biology. Epidemiology and public health significance of leishmaniasis. J Bacteriol Parasitol 4(166):2. https://doi.org/10.4172/2155-9597.1000166
De Freitas EO, de Souza Leoratti FM, Freire De Lima CG, Morrot A, Ferreira Feijó D (2016) The contribution of immune evasive mechanisms to parasite persistence in Visceral Leishmaniasis. Front Immunol 7:153. https://doi.org/10.3389/fimmu.2016.00153
Elcicek S, Bagirova M, Allahverdiyev AM (2013) Generation of avirulent Leishmania parasites and induction of nitric oxide production in macrophages by using polyacrylic acid. Exp Parasitol 133(3):237–242
Gadisa E, Tsegaw T, Abera A, Elnaiem DE, den Boer M, Aseffa A, Jorge A (2015) Eco-epidemiology of visceral leishmaniasis in Ethiopia. Parasit Vectors 8:381. https://doi.org/10.1186/s13071-015-0987-y
Ghosh S, Jagtap S, More P, Shete UJ, Maheshwari NO, Rao SJ, Kitture R, Kale S, Bellare J, Patil S (2015) Dioscorea bulbifera mediated synthesis of novel Au core Ag shell nanoparticles with potent antibiofilm and antileishmanial activity. J Nanomater 2015:161
Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME (2016) Status of vaccine research and development of vaccines for leishmaniasis. Vaccine 34(26):2992–2995. https://doi.org/10.1016/j.vaccine.2015.12.071
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126(1):131–138
Green SJ, Nacy CA, Meltzer MS (1991) Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J Leukoc Biol 50(1):93–103
Hiramatsu H, Osterloh FE (2004) A simple large-scale synthesis of nearly monodisperse gold and silver nanoparticles with adjustable sizes and with exchangeable surfactants. Chem Mater 16(13):2509–2511
Iqbal I, Hamayun M (2004) Studies on the traditional uses of plants of Malam Jabba valley, District Swat, Pakistan. Ethnobot Leafl 1:15
Islam M, Zakaria M, Radhakrishnan R, Kamil M (2002) Effect of Teucrium stocksianum on gastric ulceration and secretion in rats. Pharm Biol 40(3):216–220
Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ (2000) Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 60(1):184–190
James SL (1995) Role of nitric oxide in parasitic infections. Microbiol Rev 59(4):533–547
Kalangi SK, Dayakar A, Gangappa D, Sathyavathi R, Maurya R, Rao DN (2016) Biocompatible silver nanoparticles reduced from Anethum graveolens leaf extract augments the antileishmanial efficacy of miltefosine. Exp Parasitol 170:184–192
Li X, Xu H, Chen Z-S, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011(2011):1–16
Mauel J, Ransijn A, Buchmüller-Rouiller Y (1991) Killing of Leishmania parasites in activated murine macrophages is based on an l-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol 49(1):73–82
Minodier P, Parola P (2007) Cutaneous leishmaniasis treatment. Travel Med Infect Dis 5(3):150–158
Rasheed R, Ali B, Bashir A (1995) Effect of Teucrium stocksianum on paracetamol-induced hepatotoxicity in mice. Gen Pharmacol Vasc Syst 26(2):297–301
Rodriguez-Sanchez L, Blanco M, Lopez-Quintela M (2000) Electrochemical synthesis of silver nanoparticles. J Phys Chem B 104(41):9683–9688
Roma EH, Macedo JP, Goes GR, Gonçalves JL, De Castro W, Cisalpino D, Vieira LQ (2016) Impact of reactive oxygen species (ROS) on the control of parasite loads and inflammation in Leishmania amazonensis infection. Parasit Vectors 9(1):193
Rossi-Bergmann B, Pacienza-Lima W, Marcato PD, De Conti R, Durán N (2012) Therapeutic potential of biogenic silver nanoparticles in murine cutaneous leishmaniasis. J Nano Res 20:89–97
Shavandi Z, Ghazanfari T, Moghaddam KN (2011) In vitro toxicity of silver nanoparticles on murine peritoneal macrophages. Immunopharmacol Immunotoxicol 33(1):135–140
Srivastava S, Shankar P, Mishra J, Singh S (2016) Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 9(1):1–15. https://doi.org/10.1186/s13071-016-1553-y
Tanaka AK, Valero VB, Takahashi HK, Straus AH (2007) Inhibition of Leishmania (Leishmania) amazonensis growth and infectivity by aureobasidin A. J Antimicrob Chemother 59(3):487–492
Ullah I, Abamor EŞ, Bağirova M, Shinwari ZK, Allahverdiyev AM (2017a) Biomimetic production, characterisation, in vitro cytotoxic and anticancer assessment of aqueous extract-mediated AgNPs of Teucrium stocksianum Boiss. IET Nanobiotechnol 1–7. https://doi.org/10.1049/iet-nbt.2017.0092
Ullah I, Shinwari ZK, Khalil AT (2017b) Investigation of the cytotoxic and antileishmanial effects of Fagonia indica L. extract and extract mediated silver nanoparticles (AgNPs). Pak J Bot 49(4):1561–1568
Visbal G, Marchán E, Maldonado A, Simoni Z, Navarro M (2008) Synthesis and characterization of platinum–sterol hydrazone complexes with biological activity against Leishmania (L.) mexicana. J Inorg Biochem 102(3):547–554
Wasfi I, Bashir A, Amiri M, Abdalla A, Banna N, Tanira M (1995) Gastric cytoprotective activity of Teucrium stocksianum extract in rats. Int J Pharmacogn 33(2):164–171
Zahir AA, Chauhan IS, Bagavan A, Kamaraj C, Elango G, Shankar J, Arjaria N, Roopan SM, Rahuman AA, Singh N (2015) Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob Agents Chemother 59(8):4782–4799
Zhu J, Liu S, Palchik O, Koltypin Y, Gedanken A (2000) Shape-controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods. Langmuir 16(16):6396–6399
Acknowledgements
This study was supported by the Scientific Researches and Project Coordinatorship of Yıldız Technical University Istanbul Turkey, with project support number 2013-07-04-KAP02. We also would like to thanks TUBITAK (the scientific and technological research council of Turkey) for providing research opportunity to the first author under 2216 Ph.D. fellowship for international researcher.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ullah, I., Cosar, G., Abamor, E.S. et al. Comparative study on the antileishmanial activities of chemically and biologically synthesized silver nanoparticles (AgNPs). 3 Biotech 8, 98 (2018). https://doi.org/10.1007/s13205-018-1121-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1121-6