Abstract
In this study, we surveyed the abundance and diversity of three sulfur oxidation genes (sqr, soxB, and dsrA) using quantitative assays and Miseq high-throughput sequencing. The quantitative assays revealed that soxB is more abundant than sqr and dsrA and is the main contributor to sulfur oxidation. In the diversity analysis, the SOB community mainly comprised the classes Nitrospira, Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. The genera Gallionella, Hydrogenophaga, Limnohabitans, Methylomonas, Nitrospira, Rhodoferax, and Sulfuritalea were abundant in the communities for sqr; Dechloromonas, Limnohabitans, Paracoccus, Sulfuritalea, Sulfitobacter, and Thiobacillus were abundant in communities for soxB; Sulfuritalea, Sulfurisoma, and Thiobacillus were abundant in communities for dsrA. This study presented a high diversity of SOB species and functional sulfur-oxidizing genes in Pearl River via high-throughput sequencing, suggesting that the aquatic ecosystem has great potential to scavenge the sulfur pollutants by itself.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the reform and opening, China’s economy has grown quickly and is maintaining a high growth rate, producing nearly 11.6% of the total global GDP and consuming about 46.3% of the world’s total steel in 2012 (Zhao et al. 2017). The Pearl River Delta Region is one of the most developed and industrialized zones in China. It contributed to 9.1% of the national GDP in 2014. However, rapid population growth, industrial development, and agricultural production have led to increasing environmental pollution in this region. The Pearl River is the 2nd largest river in China and the 13th largest river in the world. Anthropogenic activity and economic development have caused an unforeseen damage to the ecosystem of the Pearl River due to the presence of large amount of pollutants, such as the sulfur compounds that are being illegally discharged from the cloth printing and dyeing factories, tanneries, paper mills, petrochemical refineries, etc. Thus, a good understanding of sulfur biogeochemistry in the Pearl River ecosystem is helpful to know how the ecosystem itself scavenges sulfur pollutants.
Biological oxidation of inorganic sulfur compounds is an important process in the global sulfur cycle. In this process, the energy generated from sulfur oxidation is used by aerobic chemoautotrophs for carbon dioxide fixation, while the electrons derived from the reduced sulfur compounds are used by anoxygenic photoautotrophs for carbon dioxide reduction (Friedrich et al. 2005). Because sulfur occurs in a broad range of oxidation states (− 2 to + 6), a wide variety of redox enzymes produced by different microorganisms have been detected in artificial or natural ecosystems. These enzymes include the sulfur-oxidizing (Sox) enzyme system, which is encoded by the soxTRSVWXYZABCDEFGH gene cluster (Rother et al. 2001; Friedrich et al. 2005; Ghosh and Dam 2009); the sulfide:quinone oxidoreductase (SQR), which is encoded by sqr (Ghosh and Dam 2009; Chan et al. 2009); and the reverse dissimilatory sulfite reductase (Dsr) system, which is encoded by the dsrABEFHCMKLJOPNRS gene cluster (Dahl et al. 2005).
Many studies using one or more of these functional sulfur oxidation genes as molecular biomarkers have been conducted to examine communities of sulfur-oxidizing bacteria (SOB) and their roles in various environments. Thiobacillus-like Betaproteobacteria has been found to be the dominant SOB group in soil after sulfur application using soxB clone library sequencing (Tourna et al. 2014); Sulfurimonas, Thiobacillus, Thioclava, Thiohalomonas, and Dechloromonas have been detected as the most frequent SOB in water-flooded petroleum reservoirs (Tian et al. 2017); Thioalkalivibrio, Halothiobacillus, Marinobacter, and Halochromatium have been detected in hypersaline and soda lakes using soxB as molecular marker (Tourova et al. 2013); soxB clone library analyses suggested that Chromatiales and Thiotrichales were dominant SOB members in vegetated salt marsh sediments (Thomas et al. 2014). In a previous work, we had designed three primer pairs specific for soxB, sqr, and dsrA, which are known parts of sulfur oxidation genes, to reveal the main SOB that participated in the removal of sulfide from sulfide-rich wastewater in a treatment plant. The results as well as some other reports based on our designed primers validated the suitability of these primers for studying the SOB community (Luo et al. 2011; Kojima et al. 2014). Until now, the SOB communities distributed in various environments were studied using functional genes-based clone library, but no study has been reported via high-throughput sequencing on Illumina (Miseq and Hiseq) or Roche (454 pyrosequencing) platforms. In this study, we applied the Miseq high-throughput sequencing method to survey the SOB community in the Pearl River water using three kinds of sulfur oxidation genes as molecular biomarkers. Diverse SOB species were detected along the Pearl River and the result provides useful information regarding the potential bacterial groups for scavenging sulfur pollutants in the Pearl River.
Materials and methods
Water sampling
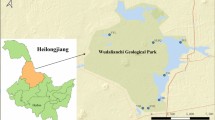
Four water samples were collected from the Guangzhou section of the Pearl River in September 2014; distribution of the sampling sites represents the upper, middle, and lower reaches of the Pearl River that flows through Guangzhou City; the geographical location and chemical characteristics of each site are shown in Fig. 1. For each site, five water samples were randomly collected from the top 0.5 m, mixed together, and considered as one water sample. The samples were pre-filtered through a filter paper to remove macroparticles and then filtered through a 0.22 μm membrane (Millipore, USA) to obtain a microbial pellet. The pellets were stored at − 80 °C until extraction of total genomic DNA. The filtered water was used to determine the concentrations of sulfide, sulfur, and sulfate.
Determination of sulfur compounds
The concentration of sulfate was measured by ion chromatography (ICS 900, Dionex, USA) using a precolumn Dionex IonPac® AS14, with 10 mM carbonate buffer solution as the mobile phase. The sulfide concentration was measured by the methylene blue method (Fogo and Popowsky 1949). Elemental sulfur was extracted with chloroform, and the air-dried extract was dissolved in ethanol and oxidized by alkaline sodium hypobromite solution, as described by Tabatabai and Bremner (Tabatabai and Bremner 1970); the resultant sulfate was measured by ion chromatography as described above.
Genomic DNA extraction
Genomic DNA from water samples was extracted using the BioFast Soil Genomic DNA extraction kit (Biofux, Hangzhou, China), according to the manufacturer’s instructions. The extracted DNA was dissolved in Tris–EDTA (TE) buffer (50 mmol L−1; pH 8.0) and its purity was determined using a UV spectrophotometer (NanoDrop 2000, Thermo Scientific, USA) and by agarose gel electrophoresis. The DNA samples were stored at – 80 °C for further analysis such as PCR amplification and quantitative PCR.
High-throughput sequencing of functional genes
The three functional genes, sqr, soxB, and dsrA, were PCR-amplified using previously designed primer pairs sqr-473F/982R, soxB-704F/1199R, and dsrA-625F/877R, respectively (Luo et al. 2011). A unique 8 bp barcode was added at the 5′-end of each forward primer. PCRs were performed in a 25 μl reaction mixture containing 1 μl of DNA template, 12.5 μl of PCR mix (TIANGEN, China), and 1 μl of forward and reverse primers (10 μM). The PCR products were purified using the TaKaRa Agarose Gel DNA Purification Kit ver. 4.0 (TaKaRa, Dalian, China) and quantified using a spectrophotometer (NanoDrop 2000, Thermo Scientific, USA). Sequencing was performed on a MiSeq 300 sequencer (Illumina, San Diego, USA) by IGE Biotechnology (Guangzhou, China).
Clean data were obtained after filtering reads containing ambiguities and mismatches with specific primers or those with average quality values less than 20. Data were processed using Mothur (Schloss et al. 2009) and QIIME (Caporaso et al. 2010) and OTUs were clustered at the 90 and 95% nucleotide sequence identity levels. The taxonomic classifications of sqr, soxB, and dsrA were determined using the BLAST× program in BLAST+ by searching against the NR database.
Alpha diversity indices including the Chaol, Shannon, and Simpson were calculated and the SOB communities from all the sites were compared using the Mothur program. A neighbor-joining tree was created using Mega 5.0 software (Tamura et al. 2011) after computing the evolutionary distances via the Poisson correction method. A heat map analysis was conducted to compare the communities in different samples using the vegan package in R ver. 3.2.3 (https://www.r-project.org/). Correlations between the communities and environmental variables were tested by canonical correlation analysis (CCA), followed by a manual deselection of collinear environmental variables, and significance tests of Monte Carlo permutations were performed to construct optimal models (ter Braak and Smilauer 2002).
Quantitative PCR
PCR products of sqr, soxB, and dsrA from the genomic DNA isolated from the river water were cloned using the Mighty TA-cloning kit (TaKaRa, Dalian, China). Plasmids containing sqr, soxB, or dsrA were extracted using the TaKaRa MiniBEST Plasmid Purification kit (TaKaRa, Dalian, China) and digested using EcoRV (TaKaRa, Dalian, China). After separation on an agarose gel, the sqr, soxB, and dsrA fragments were purified using the TaKaRa Agarose Gel DNA Purification kit (TaKaRa, Dalian, China) and used to construct the respective standard curves. Concentration of DNA was determined using a spectrophotometer (NanoDrop 2000, Thermo Scientific, USA). Standard curves were prepared using 6 serial tenfold dilutions ranging from 103 to 108 gene copies/mL. The DNA was quantified by determining the copy number as well as the concentration and base pair composition of related functional genes. Quantitative PCR was performed in triplicate on an ABI 7500 Fast real-time PCR system (Applied Biosystems) using SYBR Premix Ex Taq™ II (TaKaRa, Dalian, China). The cycling protocol was as follows: initial denaturation for 30 s at 95 °C, followed by 40 cycles of denaturation for 10 s at 95 °C, annealing for 30 s at 52 °C for sqr, 55 °C for soxB, and 60 °C for dsrA, and elongation for 45 s at 72 °C. The correlation coefficients (r2) of the standard curves were > 0.999. The amplification efficiencies (E) of sqr, soxB, and dsrA were 87.6, 90.3, and 83.6%, respectively.
Nucleotide sequence accession number
Sequence data are available in the NCBI Sequence Read Archive under accession number SRR3623345 for sqr, SRR3624939 for soxB, and SRR3624940 for dsrA.
Results
Chemical characteristics and functional gene content of the water
The concentrations of sulfide, sulfate, and sulfur in the water ranged from 0.53 to 3.12, 18.9 to 35.0, and 0.53 to 2.22 mg L−1, respectively; the content of sulfide and sulfur in the four sampling sites increased in the downstream direction, while that of sulfate decreased (Fig. 1). Abundance of sqr, soxB, and dsrA in the samples ranged from 1.2 to 8.1 × 104 copies mL−1, 1.2 to 3.0 × 105 copies mL−1, and 3.7 to 8.1 × 103 copies mL−1, respectively; in the sampling sites, sqr was most abundant in BXK; soxB, in NS; and dsrA, in TW (Fig. 2).
Diversity of sulfur oxidation genes in the Pearl River water
High-throughput sequencing generated an average of 42,937, 8364, and 47,045 filtered reads for sqr, soxB, and dsrA, respectively. The Shannon–Wiener estimator indicated that the three genes were enriched in the Pearl River water, with sqr, soxB, and dsrA abundant in NS, SS, and TW, respectively (Table 1). In the BLASTx analysis, 12,988 reads, accounting for 7.05–8.39% of the total sqr reads had no hits; 2157 reads, accounting for 0.77%–2.42% of the total soxB reads, had no hits; and 3040 reads, accounting for 0.70%–2.28% of the total dsrA reads, had no hits (data not shown).
The remaining sequences were assigned to taxonomic classifications and are shown in Fig. 3. Approximately, 46.92–59.96% of the total sqr reads, 50.79–74.77% of the total soxB reads, and 61.10–69.17% of the total dsrA reads were assigned to the uncultured or unclassified bacteria. The remaining sqr sequences were assigned to four classes, Nitrospira (2.34–4.58%), Alphaproteobacteria (3.06–7.35%), Betaproteobacteria (29.58–39.49%), and Gammaproteobacteria (2.76–8.02%). They covered 11 orders, 13 families, and 18 genera. The dominant genera were Gallionella (4.33–5.37%), Hydrogenophaga (2.22–4.76%), Limnohabitans (5.32–9.51%), Methylomonas (2.52–7.99%), Nitrospira (2.34–4.58%), Rhodoferax (0.91–19.26%), and Sulfuritalea (2.30–4.90%). The remaining soxB sequences were assigned to three classes, Alphaproteobacteria (0.51–37.54%), Betaproteobacteria (11.54–33.50%), and Gammaproteobacteria (0.07–0.12%). They covered 6 orders, 8 families, and 12 genera. The dominant genera were Dechloromonas (2.22–10.90%), Limnohabitans (1.73–11.41%), Paracoccus (0.10–4.79%), Sulfuritalea (0.88–4.36%), Sulfitobacter (0.31–30.39%), and Thiobacillus (0.84–3.14%). The remaining dsrA sequences were assigned to four classes, including Alphaproteobacteria (0.13–0.27%), Betaproteobacteria (29.31–37.66%), Gammaproteobacteria (0.31–0.91%), and Deltaproteobacteria (0.48–1.22%). They covered seven orders, eight families, and ten genera. The dominant genera were Sulfuritalea (15.70–23.41%), Sulfurisoma (4.99–10.32%), and Thiobacillus (1.97–6.02%).
Dominant SOB members in the Pearl River water
Among the sqr sequences, 27 OTUs showed a relative abundance of > 1%. Heat map analysis of these key OTUs revealed that OTU5726 represented sequences that were most abundant in TW (6.64%) and BXK (6.77%), and OTU15178 and OTU15918 represented sequences that were most abundant in SS (18.58%) and NS (3.73%), respectively (Fig. 4a). Phylogenetic analysis revealed that OTU5726 was closely related to uncultured bacteria; OTU15178, to the genus Rhodoferax; and OTU15918, to the genus Variovorax (Fig. 4a). Among the soxB sequences, 17 OTUs showed a relative abundance of > 1%. Heat map analysis of these key OTUs revealed that OTU6751, OTU9791, OTU11231, and OTU509 represented sequences that were most abundant in TW (10.23%), BXK (6.45%), SS (2.85%), and NS (16.26%), respectively (Fig. 4b). Phylogenetic analysis revealed that OTU6751 and OTU11231 were closely related to uncultured bacteria; OTU9791, to the genus Limnohabitans; and OTU509, to the genus Sulfitobacter (Fig. 4b). Among the dsrA sequences, 38 OTUs showed a relative abundance of > 1%. Heat map analysis of these key OTUs revealed that OTU5122, OTU1059, OTU3027, and OTU4024 represented sequences that were most abundant in TW (12.71%), BXK (13.80%), SS (4.99%), and NS (14.48%), respectively (Fig. 4c). Phylogenetic analysis revealed that these OTUs were closely related to uncultured bacteria (Fig. 4c).
Techniques for measuring body composition
Correlation of SOB communities with environmental factors
CCA suggested a high species–environmental correlation for the first two axes (53.1 and 25.9% for sqr; 60.5 and 36.7% for soxB; 47.6 and 30.2% for dsrA) (Fig. 5). The SS sample was divergent from the other samples along the CCA axis 2. TW and BXK showed similar values for soxB and dsrA, indicating that their bacterial communities were similar. The abundance of sqr, soxB, and dsrA sequences in NS correlated positively with the sulfide and sulfur content and negatively with sulfate content; this is contrary to the result in BXK and TW. In the SS sample, the abundance of most sqr and soxB sequences correlated negatively with sulfide and sulfate content and was independent of sulfur content; the abundance of most dsrA sequences correlated positively with sulfide content and negatively with sulfur content.
Discussion
Key enzyme system responsible for sulfur oxidation
The quantitative survey indicated that soxB was more abundant than sqr and dsrA in the Pearl River water, which suggested that Sox was probably the key enzyme that catalyzed sulfur oxidation in this environment. The Sox enzyme complex not only converts thiosulfate to sulfate without the formation of any free intermediate, but also oxidizes sulfide and sulfur by feeding HS− and S0 into the Sox pathway as appropriate intermediates via enzymatic or non-enzymatic conjugation to a carrier protein SoxY (Ghosh and Dam 2009). However, the Sqr and reverse Dsr mostly convert hydrogen sulfide to sulfur and intracellular sulfur to sulfate, respectively (Dahl et al. 2005; Ghosh and Dam 2009; Chan et al. 2009). The Sox system commonly controls sulfur oxidation in aerobic chemotrophic and anaerobic phototrophic Alphaproteobacteria and a shortened sox gene cluster was identified in the genomes of other chemotrophic or phototrophic bacteria (including colorless sulfur bacteria, green sulfur bacteria, purple sulfur bacteria, purple non-sulfur bacteria); this has given rise to the hypothesis about emergence of a common mechanism in SOB (Friedrich et al. 2001, 2005; Ghosh and Dam 2009). From the above analysis, we infer that the sulfur oxidation in the Pearl River water was mainly catalyzed by the Sox enzyme system.
SOB members that contribute to sulfur oxidation
High-throughput sequencing revealed that most of the SOB belonged to the classes Nitrospira, Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. sqr was predominantly expressed in the genera Gallionella, Hydrogenophaga, Limnohabitans, Methylomonas, Nitrospira, Rhodoferax, and Sulfuritalea; soxB, in Dechloromonas, Limnohabitans, Paracoccus, Sulfuritalea, Sulfitobacter, and Thiobacillus; and dsrA, in Sulfuritalea, Sulfurisoma, and Thiobacillus. Of these, Sulfuritalea and Thiobacillus were the predominant genera in the communities positive for all three functional genes; Limnohabitans and Hydrogenophaga, in communities positive for sqr and soxB; Gallionella, Methylomonas, Nitrospira, and Rhodoferax in communities positive for sqr; Dechloromonas, Paracoccus, and Sulfitobacter, in communities positive for soxB community; and Sulfurisoma, in communities positive for dsrA.
Sulfuritalea, an important genus of communities positive for sqr, soxB, and dsrA, is usually isolated from oxic or anoxic habitats and capable of facultative autotrophic growth by oxidation of thiosulfate, elemental sulfur, and hydrogen (Kojima and Fukui 2011, 2014). Kojima et al. (2014) have also identified Sulfuritalea as a major planktonic sulfur oxidizer in a stratified freshwater lake using aprA, dsrA, soxB, sqr, and 16S rRNA gene clone libraries. Furthermore, sulfur oxidation genes, such as sqr, sox operons soxXYZABEF, and dsrABEFHCMKLJOPNR, have been identified in the genome of Sulfuritalea (Watanabe et al. 2014). Thiobacillus, including the species Thiobacillus denitrificans, Thiobacillus aquaesulis, Thiobacillus thioparus, and Thiobacillus thiophilus, are typical SOB that are commonly found to be predominant in various environments (Koenig et al. 2005; Maestre et al. 2009; Luo et al. 2011). More than 50 genes (sqr, soxWXYZABEF, dsrABEFHCMKLJOPNR, and genes encoding sulfite dehydrogenase, APS reductase, ATP sulfurylase, rhodanese, etc.) associated with sulfur oxidation have been identified in the genome of Thiobacillus denitrificans (Beller et al. 2006). Bacteria of the genus Limnohabitans may have a prominent role in the freshwater ecosystem because of their high rates of substrate uptake and diverse metabolic pathways (Newton et al. 2011; Kasalický et al. 2013). A large number of Limnohabitans were found in diverse freshwater bodies and are predicted to be involved in the oxidation of reduced sulfur and nitrogen compounds (Kasalický et al. 2013; Herrmann et al. 2015). Although there is no direct evidence for their sulfur-oxidizing ability, genes associated with sulfur oxidation, including sqr (annotated as pyridine nucleotide-disulfide oxidoreductase) and the soxBXAZYDCR gene cluster, have been identified in the genome of Limnohabitans strains (Zeng et al. 2012). Bacteria from the genus Hydrogenophaga are facultative heterotrophs and are frequently detected in wastewater treatment systems (Cytryn et al. 2005; Chung et al. 2007). Several Hydrogenophaga species have been identified as being capable of oxidizing thiosulfate (Graff and Stubner 2003; Chung et al. 2007; Yoon et al. 2008).
Members of Gallionella belong to the group of organisms called “iron bacteria” owing to their ability to grow chemolithotrophically by oxidizing iron (Lütters-Czekalla 1990; Dworkin et al. 2006). Although Gallionella is commonly found in salt waters, marine bays, and acid mine waters (Dworkin et al. 2006), the diversity of Gallionella is evinced by their isolation from neutrophilic habitats, such as freshwaters (Wang et al. 2011; Emerson et al. 2013) and soils (Wang et al. 2009). The ability to oxidize sulfide and thiosulfate was observed in Gallionella ferruginea strain BD (Lütters-Czekalla 1990) and the sulfur oxidation genes, including sqr, tetH, and the dsrABEFHCMKLJOPN cluster, were found in an assembled genome of a Gallionella species (Bertin et al. 2011); however, neither growth on reduced sulfur compounds nor sulfur oxidation genes were observed in Gallionella capsiferriformans ES-2 (Emerson et al. 2013). In the communities positive for soxB, Dechloromonas species were predominant in samples from TW, BXK, and SS, while Sulfitobacter species predominated in samples from NS. The soxFRCDYZAXB gene cluster has been identified in the genome of Dechloromonas (Salinero et al. 2009); this cluster probably encodes a Sox system that couples sulfur oxidation with perchlorate reduction (Sahu et al. 2009). Most Sulfitobacter strains are able to oxidize sulfite (Sorokin 1995; Pukall et al. 1999; Park et al. 2007). Sulfurisoma, comprising only one taxonomically assigned species, Sulfurisoma sediminicola, was the second largest genus in the communities positive for dsrA. Sulfurisoma sediminicola is a facultative autotroph capable of oxidizing thiosulfate, elemental sulfur, and hydrogen; moreover, sqr, soxB, and dsrA genes have been detected in this strain (Kojima and Fukui 2014).
Remarkably, Nitrospira and Rhodoferax species were found to be predominant in the communities positive for sqr. Although Nitrospira are among the most diverse and widespread nitrifiers in natural ecosystems and biological wastewater treatment systems, they have hardly been studied and are mostly uncultured (Lücker et al. 2010). Although sqr have been identified in the genomes of Nitrospira defluvii, Nitrospira moscoviensis, and some other Nitrospira sp., no sulfur-oxidizing potential has been reported (Lücker et al. 2010; Koch et al. 2015; Speth et al. 2016). Similarly, no sulfur-oxidizing potential has been reported for the genus Rhodoferax; moreover, Rhodoferax ferrireducens, too, is unable to utilize sulfide or thiosulfate (Finneran et al. 2003). These discrepancies could be attributed to lateral gene transfer (LGT). In fact, many studies have concluded that LGT plays an important role in oxidative sulfur metabolism by facilitating the acquisition of sqr, sox, and dsr (Meyer et al. 2007; Gregersen et al. 2011; Kleiner et al. 2012). Theissen et al. (2003) have reported that Sqr is not a well-conserved protein and that its gene has undergone a few lateral transfers during evolution, making it difficult to discern its precise lineage by phylogenetic methods.
Conclusion
Quantitative assays revealed that soxB is more abundant than sqr and dsrA, indicating that the Sox enzyme system has more important effect on scavenging sulfur pollutants in the Pearl River than the SQR and reverse Dsr systems. High-throughput sequencing analyses suggested that SOB groups closely related to classes Nitrospira, Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria were dominants in the Pearl River. This study presented high diversity of SOB and abundance of functional sulfur oxidation genes in the Pearl River, which enable the ecosystem to scavenge the inorganic sulfur compounds by itself. This survey was conducted at the level of functional genes; further studies using meta-omics and identification of unknown groups are necessary to obtain insights into the role of SOB in sulfur oxidation in this ecosystem.
References
Beller HR, Chain PSG, Letain TE, Chakicherla A, Larimer FW, Richardson PM, Coleman MA, Wood AP, Kelly DP (2006) The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitrificans. J Bacteriol 188:1473–1488
Bertin PN, Heinrich-Salmeron A, Pelletier E, Goulhen-Chollet F, Arsene-Ploetze F, Gallien S, Lauga B, Casiot C, Calteau A, Vallenet D, Bonnefoy V, Bruneel O, Chane-Woon-Ming B, Cleiss-Arnold J, Duran R, Elbaz-Poulichet F, Fonknechten N, Giloteaux L, Halter D, Koechler S, Marchal M, Mornico D, Schaeffer C, Smith AA, Van Dorsselaer A, Weissenbach J, Médigue C, Le Paslier D (2011) Metabolic diversity among main microorganisms inside an arsenic-rich ecosystem revealed by meta- and proteo-genomics. ISME J 5:1735–1747
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chan LK, Morgan-Kiss RM, Hanson TE (2009) Functional analysis of three sulfide:quinine oxidoreductase homologs in Chlorobaculum tepidum. J Bacteriol 191:1026–1034
Chung BS, Ryu SH, Park M, Jeon Y, Chung YR, Jeon CO (2007) Hydrogenophaga caeni sp. nov., isolated from activated sludge. Int J Syst Evol Microbiol 57:1126–1130
Cytryn E, van Rijn J, Schramm A, Gieseke A, de Beer D, Minz D (2005) Identification of bacteria potentially responsible for oxic and anoxic sulfide oxidation in biofilters of a recirculating mariculture system. Appl Environ Microbiol 71:6134–6141
Dahl C, Engels S, Pott-Sperling AS, Schulte A, Sander J, Lubbe Y, Deuster O, Brune DC (2005) Novel genes of the dsr gene cluster and evidence for close interaction of Dsr proteins during sulfur oxidation in the phototrophic sulfur bacterium Allochromatium vinosum. J Bacteriol 187:1392–1404
Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (2006) The prokaryotes: volume 7 Proteobacteria: delta and epsilon subclasses; deeply rooting bacteria third edition. Springer, New York
Emerson D, Field EK, Chertkov O, Davenport KW, Goodwin L, Munk C, Nolan M, Woyke T (2013) Comparative genomics of freshwater Fe-oxidizing bacteria: implications for physiology, ecology, and systematic. Front Microbiol 4:1–17
Finneran KT, Johnsen CV, Lovely DP (2003) Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int J Syst Evol Microbiol 53:669–673
Fogo JK, Popowsky M (1949) Spectrophotometric determination of hydrogen sulfide. Analy Chem 21:732–734
Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J (2001) Oxidation of reduced inorganic sulfur compounds by bacteria: emergence of a common mechanism? Appl Environ Microbiol 67:2873–2882
Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fisher J (2005) Prokaryotic sulfur oxidation. Current Opin Microbiol 8:253–259
Ghosh W, Dam B (2009) Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol Rev 33:999–1043
Graff A, Stubner S (2003) Isolation and molecular characterization of thiosulfate-oxidizing bacteria from an Italian rice field soil. Syst Appl Mcirobiol 26:445–452
Gregersen LH, Bryant DA, Frigaard NU (2011) Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front Microbiol 2:116
Herrmann M, Rusznyák A, Akob DM, Schulze I, Opitz S, Totsche KU, Küsel K (2015) Large fractions of CO2-fixing microorganisms in pristine limestone aquifers appear to be involved in the oxidation of reduced sulfur and nitrogen compounds. Appl Environ Microbiol 81:2384–2394
Kasalický V, Jezbera J, Hahn MW, Šimek K (2013) The diversity of the Limnohabitans genus, an important group of freshwater Bacterioplankton, by characterization of 35 isolated strains. PLoS ONE 8:e58209
Kleiner M, Petersen JM, Dubilier N (2012) Convergent and divergent evolution of metabolism in sulfur-oxidizing symbionts and the role of horizontal gene transfer. Current Opinion Microbiol 15:621–631
Koch H, Lücker S, Albertsen M, Kitzinger K, Herbold C, Spieck E, Nielsen PH, Wagner M, Daims H (2015) Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc Natl Acad Sci USA 112:11371–11376
Koenig A, Zhang T, Liu LH, Fang HHP (2005) Microbial community and biochemistry process in autosulfurotrophic denitrifying biofilm. Chemosphere 58:1041–1047
Kojima H, Fukui M (2011) Sulfuritalea hydrogenivorans gen nov, sp. nov., a facultative autotroph isolated from a freshwater lake. Int J Syst Evol Microbiol 61:1651–1655
Kojima H, Fukui M (2014) Sulfuritalea sediminicola gen nov, sp. nov., a facultative autotroph isolated from a freshwater lake. Int J Syst Evol Microbiol 64:1587–1592
Kojima H, Watanabe T, Iwata T, Fukui M (2014) Identification of major planktonic sulfur oxidizers in stratified freshwater lake. PLoS ONE 9:e93877
Lücker S, Wagner M, Maixner F, Pelletier E, Koch H, Vacherie B, Rattei T, Damsté JS, Spieck E, Le Paslier D, Daims H (2010) A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad Sci USA 107:13479–13484
Luo JF, Lin WT, Guo Y (2011) Functional genes based analysis of sulfur-oxidizing bacteria community in sulfide removing bioreactor. Appl Microbiol Biotechnol 90:769–778
Lütters-Czekalla S (1990) Lithoautotrophic growth of the iron bacterium Gallionella ferruginea with thiosulfate or sulfide as energy source. Arch Microbiol 154:417–421
Maestre JP, Rovira R, Kinney KA, Lafuente J, Gabriel D (2009) Characterization of the bacterial community in a biotrickling filter treating high loads of H2S by molecular biology tools. Water Sci Technol 59:1331–1337
Meyer B, Imhoff JF, Kuever J (2007) Molecular analysis of the distribution and phylogeny of the soxB gene among sulfur-oxidizing bacteria-evolution of the Sox sulfur oxidation enzyme system. Environ Microbiol 9:2957–2977
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Park JR, Bae JW, Nam YD, Chang HW, Kwon HY, Quan ZX, Park YH (2007) Sulfitobacter litoralis sp. nov., a marine bacterium isolated from the East Sea, Korea. Int J Syst Evol Microbiol 57:692–695
Pukall R, Buntefuß D, Fruhling A, Rohde M, Kroppenstedt RM, Burghardt J, Lebaron P, Bernard L, Stackebrandt E (1999) Sulfitobacter mediterraneus sp. nov., a new sulfite-oxidizing member of the α-Proteobacteria. Int J Syst Evol Microbiol 49:513–519
Rother D, Henrich HJ, Quentmeier A, Bardischewsky F, Friedrich CG (2001) Novel genes of sox gene cluster, mutagenesis of the flavoprotein SoxF, and evidence for a general sulfur oxidizing system in Paracoccus pantotrophus GB17. J Bacteriol 183:4499–4508
Sahu AK, Conneely T, Nusslein KR, Ergas SJ (2009) Biological perchlorate reduction in packed bed reactors using elemental sulfur. Environ Sci Technol 43:4466–4471
Salinero KK, Keller K, Feil WS, Feil H, Trong S, Bartolo GD, Lapidus A (2009) Metabolic analysis of the soil microbe Dechloromonas aromatic str RCB: indications of a surprisingly complex lift-style and cryptic anaerobic pathways for aromatic degradation. BMC Genomic 10:351
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Sorokin DY (1995) Sulfitobacter pontiacus gen. nov., sp. nov—a new heterotrophic bacterium from the Black Sea, specialized on sulfite oxidation. Microbiology 64:295–305
Speth DR, In’t Zandt MH, Guerrero-Cruz S, Duthilh BE, Jetten MSM (2016) Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nat Commun 7:11172
Tabatabai MA, Bremner JM (1970) An alkaline oxidation method for determination of total sulfur in soils. Soil Sci Soc America J 34:62–65
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA 5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
ter Braak CJF, Smilauer P (2002) Canoco reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (version 45). Microcomputer Power, New York
Theissen U, Hoffmeister M, Grieshaber M, Martin W (2003) Single eubacterial origin of eukaryotic sulfide:quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol Biol Evol 20:1564–1574
Thomas F, Giblin AE, Cardon ZG, Sievert SM (2014) Rhizosphere heterogeneity shapes abundance and activity of sulfur-oxidizing bacteria in vegetated salt marsh sediments. Front Microbiol 5:309
Tian H, Gao P, Chen Z, Li Y, Li Y, Wang Y, Zhou J, Li G, Ma T (2017) Compositions and abundances of sulfate-reducing and sulfur-oxidizing microorganisms in water-flooded petroleum reservoirs with different temperatures in China. Front Microbiol 8:143
Tourna M, Maclean P, Condron L, O’Callaghan M, Wakelin SA (2014) Links between sulphur oxidation and sulphur-oxidising bacteria abundance and diversity in soil microcosms based on soxB functional gene analysis. FEMS Microbiol Ecol 3:538–549
Tourova TP, Slobodova NV, Bumazhkln BK, Kolganova TV, Muyzer G, Sorokin DY (2013) Analysis of community composition of sulfur-oxidizing bacteria in hypersaline and soda lakes using soxB as a functional molecular maker. FEMS Microbiol Ecol 84:280–289
Wang J, Muyzer G, Bodelier PLE, Laanbroek HJ (2009) Diversity of iron oxidizers in wetland soils revealed by novel 16S rRNA primers targeting Gallionella-related bacteria. ISME J 3:715–725
Wang J, Vollrath S, Behrends T, Bodelier PLE, Muyzer G, Meima-Franke M, Den Oudsten F, Van Cappellen P, Laanbroek HJ (2011) Distribution and diversity of Gallionella-like neutrophilic iron oxidizers in a tidal freshwater marsh. Appl Environ Microbiol 77:2337–2344
Watanabe T, Kojima H, Fukui M (2014) Complete genomes of freshwater sulfur oxidizers Sulfuritalea denitrificans skB26 and Sulfuritalea hydrogenivorans sk43H: genetic insights into the sulfur oxidation pathway of Betaproteobacteria. Syst Appl Microbiol 37:387–395
Yoon JH, Kang SJ, Ryu SH, Jeon CO, Oh TK (2008) Hydrogenophaga bisanensis sp. nov, isolated from wastewater of a textile dye works. Int J Syst Evol Microbiol 58:393–397
Zeng Y, Kasalický V, Šimek K, Koblížek M (2012) Genome sequences of two freshwater Betaproteobacterial isolates, Limnohabitans species strains Rim28 and Rim47, indicate their capabilities as both photoautotrophs and ammonia oxidizers. J Bacteriol 194:6302–6303
Zhao X, Zhang X, Li N, Shao S, Geng Y (2017) Decoupling economic growth from carbon dioxide emissions in China: a sectoral factor decomposition analysis. J Clean Product 142:3500–3516
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 21276099; 41301318; 41473072), the Specialized Research Found for the Doctoral Program of Higher Education of China (No. 20120172120045), and the Fundamental Research Funds for the Central Universities (No. 2015ZM171).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Luo, J., Tan, X., Liu, K. et al. Survey of sulfur-oxidizing bacterial community in the Pearl River water using soxB, sqr, and dsrA as molecular biomarkers. 3 Biotech 8, 73 (2018). https://doi.org/10.1007/s13205-017-1077-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-1077-y