Abstract
Bioethanol is an environmentally friendly alternative to petroleum energy sources. This study evaluated the effects of H2O, HCl, NaOH and FeCl3 pretreated rice husk feedstocks on the production of bioethanol. The pretreatments were carried out using water, 0.1 M HCl, NaOH and FeCl3 at 121 °C for 15 min, followed by simultaneous saccharification and fermentation (SSF) as well as separate hydrolysis and fermentation (SHF). The raw and pretreated lignocellulosic feedstocks were analyzed using Fourier transform infrared spectroscopy. Saccharification and fermentation were accomplished using Trichoderma reesei cellulase and Saccharomyces cerevisiae, respectively. The products obtained after saccharification and fermentation were collected and analyzed for reducing sugars and ethanol contents using 3,5-dinitrosalicylic acid and high-performance liquid chromatography, respectively. Enzyme hydrolysis of the FeCl3 and HCl treated samples resulted in hydrolysates containing 3.845 and 3.402 mg/ml glucose equivalent, respectively. In all pretreatments, SSF for each pretreatment produced more ethanol than the SHF method; the FeCl3 pretreatment gave the highest ethanol yield of 3.011 ± 0.034 and 3.802 ± 0.041% in the SHF and SSF methods, respectively. Utilization of FeCl3 pretreatment of rice husk is a potential option for bioethanol production in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, there is a rising demand for food and energy due to increasing human population; and this has stimulated research into the development of alternative energy sources that are sustainable, renewable, economically competitive, environmentally friendly and do not compete with human food sources. Alternatives to petroleum-derived fuels such as bioethanol are sought for to reduce the world’s dependence on non-renewable resources (Gray et al. 2006; Hahn-Hagerdal et al. 2007; Balat and Balat 2008). Significant efforts are being made to enhance the production of ethanol from lignocellulose feedstock and make this alternative, economically feasible and competitive with gasoline.
Currently, commercial bioethanol is derived from corn grains (starch) and sugarcane (sucrose) and these sources compete for fertile land as well as food production. Except that these food crops are modified to improve yield at very short periods, in the future, utilization of these food sources for ethanol production will further complicate the already existing food and energy crisis as human population continues to increase (de Fraiture et al. 2008; Koh and Ghazoul 2008). Therefore, utilization of lignocellulosic biomass is seen as an attractive feedstock for future supplies of ethanol considering its great availability, low cost and non-competition with human food sources (Kim and Dale 2004; Tilman et al. 2006; Balat et al. 2009).
Economic and environmental concerns are the major propelling factors that have made the search for alternatives to fossil fuels a subject of intensive research; demands for these alternative sources continue to increase and lignocellulosic plant biomass have been identified as promising renewable feedstock for biofuel production (Hannon et al. 2010; Kumar and Sahu 2013). Lignocellulosic biomass from plants is cheap and readily available. However, its components are difficult to ferment or degrade biologically depending on the nature and composition of a particular plant; but with carefully selected pretreatment methods, efficient enzymes for the saccharification and optimized fermentation conditions; the cellulose and hemicellulose components of lignocellulosic biomass can be efficiently converted to ethanol considering the large amounts of glucose monomers present in them (Ibeto et al. 2011; Bhagwat et al. 2015; Welker et al. 2015).
In Nigeria, rice is widely cultivated principally for its grains and its husks are abundantly available as waste and in few areas used as feeds for ruminant. Hence, rice husk are of little economic use presently; therefore, could be used as suitable lignocellulosic substrate for bioethanol production.
The present study focuses on the potentials of rice husk to produce bioethanol under different pretreatment and fermentation methods.
Materials and methods
Materials
Trichoderma reesei ATCC 26921 Cellulase was from Sigma Chemical Co., USA; bacteriological yeast extract powder and peptone were from Fisher Scientific UK. Other reagents were of high purity and analytical grade from reputable vendors.
Raw materials
Rice husk was collected from a rice mill located in Yola, Adamawa State, Nigeria. The husks obtained were dried and of uniform particle size, it was stored in plastic container and kept at room temperature (27 ± 2 °C) until use.
Pretreatment of lignocellulosic samples
Rice husk samples were subjected to different pretreatments at 10% w/v loading with water and 0.1 M of FeCl3, HCl, and NaOH in triplicates at 121 °C for 15 min, the samples were washed with tap water until a neutral pH was obtained for all treatment. The pretreated-washed samples were oven dried at 90 °C, overnight (12–18 h) and were used for further studies.
FTIR analysis of pre-treated and raw samples
Evaluation of the chemico-structural changes that occurred with the different pretreatments were carried out on a BUCK Scientific FTIR model 530. Two milligram of dried samples were mixed with 250 mg of dried KBr, pressed to pellets and scanned over the range 4000–600 cm−1 wavenumber, with a 4 cm−1 spectral resolution. The spectra (from the KBr pellets) were used to evaluate the chemico-structural changes that occurred with the different pretreatments (Himmelsbach et al. 2002; Pavia et al. 2005).
Organism used for fermentation and inoculum preparation
Commercial yeast (S. cerevisiae) was used for the bioethanol production. The yeast was maintained according to the method described by Ishola et al. (2013). Prior to use, a pre-culture of the S. cerevisiae used was grown on 1% (w/v) yeast extract, 2% (w/v) peptone, and 2% (w/v) glucose for 60 min and was washed four times at 8500 rpm using sterile normal saline.
Simultaneous saccharification and fermentation of pre-treated rice husk feedstock
The fermentation medium described by Sun and Tao (2010) consisting of 3 g/l of yeast extract; 0.25 g/l of urea; 0.25 g/l of calcium chloride; 0.25 g/l of magnesium sulfate and 2.5 g/l of potassium phosphate monobasic was adapted for this study. The samples were loaded at 10% w/v and where adjusted to pH 5, followed by sterilization at 121 °C for 15 min and were allowed to cool to room temperature. For enzymatic hydrolysis, Sigma aqueous cellulase enzyme solution from T. reesei ATCC 26921 with activity of ≥ 700 units/ml at a loading of 30 units/g of substrate was used for the hydrolysis and 2 ml of the S. cerevisiae with optical density 0.5 was simultaneously and aseptically transferred from the pre-culture to the fermentation media at 35 °C and 50 rpm in a shaker. Flasks were maintained in a shaker for 48 h and samples were collected to determine the amount of sugars and ethanol produced.
Separate hydrolysis and fermentation to ethanol
The samples were loaded at 10% w/v and adjusted to pH 5 for enzymatic hydrolysis. Cellulase from T. reesei ATCC 26921 aqueous enzyme solution of ≥ 700 units/ml at a loading of 30 units/g of substrate was used for the hydrolysis at temperature of 50 °C and 50 rpm in a shaker for 48 h. After the hydrolysis, samples were centrifuged at 8500 rpm for 20 min and supernatants were incorporated fermentation media components described by Sun and Tao (2010), sterilized at 121 °C for 15 min and were allowed to cool to room temperature. The samples were then subjected to fermentation using pre-cultured commercial active S. cerevisiae. Two milliliters of the S. cerevisiae with optical density 0.5 were used for each flask; conditions of fermentation were pH 5, temperature 35 °C, agitation 50 rpm for 48 h. The fermentation for each treatment was carried out in a 250-ml Erlenmeyer flask in triplicates. Samples were collected to determine the residual sugars and ethanol content.
Estimation and analysis of reducing sugars and ethanol produced
The sugars produced during enzyme hydrolysis were estimated using the 3,5-dinitrosalicylic acid (DNSA) method described by Miller (1959). For ethanol analysis, samples obtained after fermentation were centrifuged to separate the biomass from the product and filtered through a 0.22-μm membrane filter and then analyzed using the Agilent HPLC 1200 Series equipped with an auto injector and isocratic pump. HPLC analyses were carried out using the following conditions: column, HyperSil BDS C18 RP; 10 µl of sample was injected into the HPLC system. The mobile phase was 0.01 M sulphuric acid pH 2; at a flow rate of 1.0 ml/min and the detection was set at a wavelength of 254 nm.
Statistical analysis
The results obtained are expressed as mean ± standard error of mean (SEM) of three replicate experiments. Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests using graph pad prism 5. p values < 0.05 were considered significant.
Results and discussion
Appearance of the samples before and after pretreatment
Production of bioethanol from plant lignocellulosic/cellulosic feedstock is a practicable venture. However, there is a major challenge with the structural/compositional variability of different lignocellulose components of available feedstocks, as plant biomass contain lignin–carbohydrate complex that gives rise to complex intractable crystalline structure (Pu et al. 2013; Sorek et al. 2014). This crystalline structure of lignocellulose prevents cellulases from binding onto the cellulose surfaces to liberate sugars for biofuel production (Vermaas et al. 2015). The treatments of the rice husk using water, 0.1 M of FeCl3, HCl, and NaOH resulted in visible altered physical appearance and increased surface area compared to the untreated sample. In this form, when the biomass are exposed to the cellulase, they can be more easily degraded to produce sugar, as most of the pretreatment methods used are known to make the biomass more porous with increased surface area and easier for degradation. Figure 1 shows the physical appearance of the rice husks before and after various pretreatments.
Qualitative analysis of the chemico-structural changes in lignocellulosic biomass using FTIR is well known. The FTIR spectra of samples have been utilized in the investigation of the relative loss or changes in the key chemical components of samples in association with visible changes in structure (Himmelsbach et al. 2002). The structural/compositional changes which occurred as a result of the acid, base, water and FeCl3 pretreatment of the rice husk indicates that all the treatment resulted to varying forms of structural alterations of the lignocelluloses biomass compared to the raw sample (Fig. 2a). The FTIR analysis of the raw and pre-treated samples confirmed the cleavage of lignin-carbohydrate complex. Previous studies on lignocellulose biomass showed that the structure of lignin–carbohydrate complex can be identified by the FTIR spectrum with the frequencies of around 1600, 1509, 1464 and 1422 (Chen et al. 2011; Dai et al. 2015; Sun et al. 2003). Even though some slight differences were observed in the peak values attributed to lignin compared to values published in literature, prominent bands occurred at 1600–1410 cm−1 for the raw sample but were of lower intensity and slightly shifted positions in the treated samples. The raw (untreated) sample had a sharp and intense peak at 1541 and 1650 cm−1 (Fig. 2b, c), however, these were either lower or absent in the treated samples and can be related to the different experimental conditions to which the husk were treated.
The critical role played by pretreatment on the release of sugars and eventually improved yield and reduced cost have resulted into investigation of novel pretreatment materials such as FeCl3 (Chen et al. 2015), and NaOH/Urea (Dai et al. 2015); these pretreatments are needed to render the cellulose and other carbohydrates components in plant biomass accessible for enzymatic hydrolysis and ethanol fermentation, thereby improving ethanol yield in fermentation (Jönsson and Martín 2016).
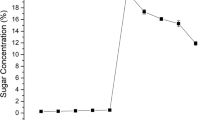
Reducing sugars obtained from pretreatment and enzymatic hydrolysis
The choice of pretreatment takes into account the sugar-release pattern and the compatibility/suitability of these sugars in the overall process of ethanol production. Any pretreatment with much yield of inhibitors capable of inhibiting cellulase activity or hindering the fermenting organism from growth is usually not considered suitable (Larissa et al. 2012). Table 1 shows the sugar content obtained from the rice husk biomass under SSF and SHF. HCl and FeCl3 pretreatments gave high reducing sugars on treatment with the T. reesei cellulase, but the yield obtained for the FeCl3 treatment was significantly (p < 0.05) higher compared to other treatments (Table 1). The difference between the amounts of sugar produced after the different pretreatments can be attributed to pretreatment and other by-products these pretreatments produce.
Ethanol obtained from fermentation media
Yeast (S. cerevisiae) has been historically used for second-generation bioethanol production because of its fermentative capacity and ethanol tolerance. The yield of ethanol obtained from different treatments using both simultaneous and independent saccharification and fermentation is shown in Table 2. The rice husk being non-edible and of low economic profile makes the utilization of the plant part of advantage in ethanol production.
The yields of ethanol from FeCl3 and NaOH pretreatments methods showed that these pretreatment methods on rice husk did not produce substances which were capable of inhibiting the yeast cells from converting the reducing sugars to ethanol; as FeCl3 treated sample were observed to have yielded the highest amount of reducing sugars compared to other treatments. Furthermore, analysis of the fermentation broth after fermentation indicates that most of the initially present reducing sugars were converted to ethanol via fermentation unlike the water treated samples which still have much reducing sugars left (0.270 ± 0.018 and 0.232 ± 0.048 mg/ml for SHF and SSF, respectively) after fermentation. The inhibition of enzymatic activities in the water treated fermenting media may be as a result of direct inhibition of catabolic enzymes, generation of reactive oxygen species, decreased intracellular pH, ATP depletion, toxic anion accumulation (Westman et al. 2014). The ethanol produced from water pretreatment was the lowest. However, its yield was comparable to that of the HCl treatment. The result of this water treatment could be advantageous to the industry as no additional chemicals are required and may also not require costly materials that are resistant to corrosion for the scale-up.
The SSF of samples resulted higher ethanol yield compared to the SHF for the same treatment (Table 2). The SSF process combines the saccharification of cellulose and fermentation of glucose which diminishes end-product sugar inhibition and yields. In all fermentation methods, extension of fermentation periods up to 72 h resulted to the production of acetic acid for FeCl3 and NaOH pretreatments.
Conclusion
In this study, four different thermochemical pretreatments were performed on rice husk with the aim of evaluating the potentials of each treatment for producing bioethanol and meeting the increasing demand of alternative sources of energy with and noncompetitive with human food sources. Significant differences in the sugar-release patterns and ethanol produced were observed, with FeCl3 and NaOH producing high quantities of ethanol. Optimization of the pretreatment, saccharification and fermentation condition will prove its potential and feasibility to use as a good cellulosic material for second-generation bioethanol production but evaluation of the costs for these options are also required.
References
Balat M, Balat H (2008) Recent trends in global production and utilization of bio-ethanol fuel. Appl Energy 86:2273–2282
Balat M, Balat H, Oz C (2009) Progress in bioethanol processing. Progr Energy Combust Sci 34:551–573
Bhagwat S, Ratnaparkhe S, Kumar A (2015) Biomass pre-treatment methods and their economic viability for efficient production of biofuel. Br Biotechnol J 8(2):1–17
Chen WH, Tu YJ, Sheen HK (2011) Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Appl Energy 88:2726–2734
Chen L, Chen R, Fu S (2015) FeCl3 pretreatment of three lignocellulosic biomass for ethanol production. ACS Sustain Chem Eng 3:1794–1800. https://doi.org/10.1021/acssuschemeng.5b00377
Dai Y, Si M, Chen Y, Zhang N, Zhou M, Liao Q, Shi D, Liu Y (2015) Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour Technol 198:725–731
de Fraiture C, Giordano M, Liao Y (2008) Biofuels and implications for agricultural water use: blue impacts of green energy. Water Policy 10(Supplement 1):67–81
Gray KA, Zhao L, Emptage M (2006) Bioethanol. Curr Opin Chem Biol 10:141–146
Hahn-Hagerdal B, Galbe M, Gorwa-Grauslund MF, Liden G, Zacchi G (2007) Bio-ethanol–the fuel of tomorrow from the residues of today. Trends Biotechnol 24(12):549–556
Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential. Biofuels 1(5):763–784
Himmelsbach DS, Khalili S, Akin DE (2002) The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J Sci Food Agric 82:685–696. https://doi.org/10.1002/jsfa.1090
Ibeto CN, Ofoefule AU, Agbo KE (2011) A global overview of biomass potentials for bioethanol production: a renewable alternative fuel. Trends Appl Sci Res 6:410–425
Ishola MM, Jahandideh A, Haidarian B (2013) Simultaneous saccharification, filtration and fermentation (SSFF): a novel method for bioethanol production from lignocellulosic biomass. Bioresour Technol 133:68–73
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residue. Biomass Bioenergy 26:361–375
Koh LP, Ghazoul J (2008) Biofuels, biodiversity, and people: understanding the conflicts and finding opportunities. Biol Conserv 141:2450–2460
Kumar K, Sahu O (2013) Hydrogen energy as advance renewable resource. Sustain Energy 1(2):32–37
Larissa C, Anuj KC, dos Santos Suzane, Milessi T, Felipe Antônio FA, Wagner L, Costa F, das Gracas Almeida Felipe M, da Silva S (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol. https://doi.org/10.1155/2012/989572
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Pavia DL, Lampman GM, Ktiz GS, Engel GR (2005) Introduction to organic laboratory techniques, a small scale approach, 2nd edn. Thomson Brooks/Cole, Belmont, CA
Pu Y, Hu F, Huang F, Davison BH, Ragauskas AJ (2013) Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol Biofuels 6(15):13. https://doi.org/10.1186/1754-6834-6-15
Sorek N, Yeats TH, Szemenyei H, Youngs H, Somerville CR (2014) The implications of lignocellulosic biomass chemical composition for the production of advanced biofuels. Bioscience 64:192–201
Sun WL, Tao WY (2010) Comparison of cell growth and ethanol productivity on different pretreatment of rice straw hemicellulose hydrolysate by using Candida shehatae CICC 1766. Afr J Microbiol 4(11):1105–1109
Sun JX, Sun XF, Sun RC, Paul F, Mark SB (2003) Inhomogeneities in the chemical structure of sugarcane bagasse lignin. J Agric Food Chem 51:6719–6725
Tilman D, Hill J, Lehman C (2006) Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314:1598–1600
Vermaas JV, Petridis L, Qi X, Schulz R, Lindner B, Smith JC (2015) Mechanism of lignin inhibition of enzymatic biomass deconstruction. Biotechnol Biofuels 8:217. https://doi.org/10.1186/s13068-015-0379-8
Welker CM, Balasubramanian VK, Petti C, Rai KM, DeBolt S, Mendu V (2015) Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies 8:7654–7676
Westman JO, Mapelli V, Taherzadeha MJ, Franzénb CJ (2014) Flocculation causes inhibitor tolerance in Saccharomyces cerevisiae for second-generation bioethanol production. Appl Environ Microbiol 80(22):6908–6918. https://doi.org/10.1128/AEM.01906-14
Acknowledgements
We are grateful to the departments of Petroleum Chemistry, and Natural and Environmental Sciences, American University of Nigeria Yola for providing all reagents and facilities used for this work.
Author information
Authors and Affiliations
Contributions
BOA, conceived, designed the study and critically revised the manuscript. JOM, performed analysis on all samples, interpreted the results and drafted the manuscript. Both authors approved the final version of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Madu, J.O., Agboola, B.O. Bioethanol production from rice husk using different pretreatments and fermentation conditions. 3 Biotech 8, 15 (2018). https://doi.org/10.1007/s13205-017-1033-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-1033-x