Abstract
Over the past decade, the increasing demand of vegetable oils for biodiesel production has highlighted the need for alternative oil feedstocks that do not compete with food production. In this context, the combined use of agro-industrial wastes and oleaginous microorganisms could be a promising strategy for sustainable biodiesel production. The present investigation involves the performance of the oleaginous yeast Wickerhamomyces anomalus strain EC28 to produce lipids from different agro-industrial wastewaters (i.e., deproteinized cheese whey, olive mill wastewater, and wastewaters from confectionary industries) and waste frying oils (i.e., waste oil from frying fish, waste oil from frying potato and waste oil from frying meat). Results indicated that this strain can adequately grow on agro-industrial wastewater-based media and produce substantial amounts of lipids [up to 24%, wt/wt in deproteinized cheese whey-based medium and olive mill wastewater-based medium (75%, v/v in water)] of similar fatty acid composition to that of the most commonly used vegetable oils in the biodiesel industry. However, the addition of frying oils to the culture media resulted in a significant decrease in total lipid content, probably due to excess of available nitrogen released from meat, fish, and potato into the frying oil. The estimated properties of the resulting biodiesels, such as SV (190.69–203.13), IV (61.77–88.32), CN (53.45–59.32), and CFPP (−0.54 to 10.4), are reported, for the first time, for W. anomalus and correlate well with specified standards. In conclusion, W. anomalus strain EC28, for which there is very limited amount of available information, might be regarded as a promising candidate for biodiesel production and additional efforts for process improvement should be envisaged.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decrease in oil reserves and gas fields around all over the world can justify the depth of studies investigating new energy sources. Bioenergy has received great attention in recent years as an alternative energy source due to increasing environmental concerns, as well as depletion of natural energy sources such as petroleum reserves. Currently, biodiesel is regarded as a promising alternative energy source as it is renewable, biodegradable, non-toxic, and has low negative environmental impacts (Ghanavati et al. 2015).
Various sources of lipids, including vegetable and animal fat-based oils, have been used as feedstocks for biodiesel production (Eguchi et al. 2015). Nevertheless, biodiesel synthesis from edible oils requires large amounts of arable lands and may affect the food supply chain (Wang et al. 2015). Recently, single cell oils (SCOs) obtained from oleaginous microorganisms have garnered increasing interest as suitable feedstocks for renewable biodiesel production due to the similarity of their fatty acid composition to that of vegetable oils currently used in biodiesel production, i.e., chain length and saturation degree (Bellou et al. 2016). On average, oleaginous microorganisms are able to accumulate large amounts of lipids reaching more than 20% of their biomass under suitable growth conditions (Nascimento et al. 2013). Among the tested oleaginous microorganisms, yeasts have several advantages features due to their higher lipid content, shorter life cycle, easier manipulation, and independence from season and climate factors (Ageitos et al. 2011).
Glucose and glucose rich substrates are the most common carbon sources used for growth and lipid production by oleaginous microorganisms. To reduce the cost of microbial lipid production, considerable efforts have been devoted to exploring new feedstocks for biodiesel production instead of glucose, such as waste frying oils (Eguchi et al. 2015), lignocellulosic biomass (Kumar et al. 2017), biodiesel-derived crude glycerol (Souza et al. 2016), and industrial wastes (Dourou et al. 2016).
Several oleaginous yeasts including Rhodosporidium toruloides (Xu et al. 2012), Rhodotorula graminis (Galafassi et al. 2012), Rhodotorula glutinis (Chi et al. 2011), Lipomyces starkeyi (Ageitos et al. 2011), Yarrowia lipolytica (Sestric et al. 2014), and Debaryomyces etchellsii (Arous et al. 2016) have shown great ability to produce lipids on a wide range of low-cost agro-industrial and agricultural wastes. In the current report, we describe, for the first time, low-cost lipid production by the oleaginous yeast Wickerhamomyces anomalus.
Wickerhamomyces anomalus, also known as Pichia anomala and Hansenula anomala, has been isolated from several habitats and is frequently associated with spoilage or processing of food and grain products such as beer, silage, baking, and dairy products. It is physiologically versatile being capable of growing on a wide range of carbon sources, at low pH, under high osmotic pressure and anaerobic conditions, while it shows low tolerance to ethanol and acetate (Passoth et al. 2006). On the other hand, this species was also applied as a food production organism as it has been used as a food-flavouring agent as well as a producer of food bioemulsifiers (Chen et al. 1998). In recent years, it has been used as a biocontrol agent against a variety of fungi in different habitats due to its ability to produce mycocin killer toxins (Aloui et al. 2015). It has been investigated for its cyanide-resistant alternative oxidase activity (Minagawa et al. 1990), lipase activity (Yalçın et al. 2013), and beta-glucosidase activity that plays a key role in wine fermentations (Swangkeaw et al. 2009). Several studies have reported the use of W. anomalus for biosurfactant and bioethanol production (Passoth et al. 2013; Dejwatthanakomol et al. 2016), and more recently, this strain has been described as having an oleaginous character (Souza et al. 2016).
The present study investigates biomass production and lipid accumulation potential of W. anomalus strain EC28 grown on glucose and on various low-cost feedstocks as carbon sources, i.e., agro-industrial wastewaters and waste frying oils. The profile of fatty acid methyl esters derived from transesterification of SCOs was determined and compared with well-established feedstocks for biodiesel production. The quality of the resulting biodiesels was evaluated according to the European Standard EN 14214.
Materials and methods
Agro-industrial wastewaters
Different hydrophilic and hydrophobic low-cost carbon sources were investigated for lipid production by W. anomalus EC28.
Oil wastes used in the present study [i.e., waste oil from frying fish (FFO), waste oil from frying potato (PFO) and waste oil from frying meat (MFO)] were obtained from local fast food restaurants.
Samples of fresh olive mill wastewater (OMW), wastewaters from sugar confectionary industries (WCI1 and WCI2), and cheese whey (DCW) were kindly supplied from different industries located in Sfax, Tunisia, i.e., traditional mills, two different sugar confectionary industries, and dairy processing industry, respectively. Prior to use, OMW, WCI1, and WCI2 samples were centrifuged at 8000 rpm for 20 min at 4 °C. Supernatants were further used for media preparation. Cheese whey samples were sterilized at 121 °C for protein coagulation and then centrifuged at 8000 rpm for 20 min. The deproteinized supernatant was collected and used for the preparation of growth media. Before inoculation, all media were subjected to sterilization by autoclaving at 121 °C for 20 min.
The chemical composition of the different agro-industrial wastewaters used in this study [i.e., deproteinized cheese whey (DCW), olive mill wastewater (OMW), and wastewaters from two confectionary factories (WCI1, WCI2)] is summarized in Table 1. pH values of all wastewaters were in the range of 4.1–6.9. Hence, the initial pH value of all media was adjusted to 6.0 ± 0.1 before sterilization to enhance cell growth and lipid accumulation. The total nitrogen concentration in the selected effluents, except for deproteinized cheese whey (1.9 g/L), was extremely low. WCI1 contains the highest level of total sugars concentration (91.5 g/L), while deproteinized cheese whey showed the highest reducing sugars concentration (32.1 g/L). All wastewaters were characterized by their high COD content (77.4–298.8 g/L) as a result of their high organic load. These effluents with high concentrations in total and reducing sugars, but low total nitrogen content may be promising media for SCO production.
Yeast strain and culture conditions
The yeast strain W. anomalus EC28 (Genbank accession number KP895595) was recently isolated from brine of naturally fermented black olives and maintained in the Laboratory of Enzyme Engineering and Microbiology on slants YPD agar (5 g/L Yeast Extract, 5 g/L Peptone, 10 g/L Glucose, and 20 g/L agar) at 4 °C. This strain was used throughout this study owing to its ability to grow and to accumulate acceptable amounts of lipids on a nitrogen limiting medium (Arous et al. 2017).
The growth kinetic of W. anomalus EC28 was performed in 250 mL Erlenmeyer Flasks containing 50 mL of nitrogen limiting medium (NLM) supplemented with glucose (30 g/L) and having the composition described in Arous et al. (2017).
Growth experiments in wastewater-based media were carried out in 250-mL Erlenmeyer Flasks containing 50 mL of the respective wastewaters supplemented with minerals of the following composition: KH2PO4, 7 g/L; Na2HPO4, 2 g/L; MgSO4, 1.5 g/L; CaCl2, 0.1 g/L; MnSO4, 0.0001 g/L; CuSO4, 0.0001 g/L; Co (NO3)3, 0.0001 g/L; ZnSO4, 0.001 g/L. 0.5 g/L of (NH4)2SO4 and yeast extract were used as nitrogen sources in all wastewaters, except for the deproteinized cheese whey-based medium.
Growth experiments conducted in the presence of oil wastes as glucose co-substrates were carried out in 250-mL Erlenmeyer flasks containing 50 mL of basal medium (BM) of the following composition: glucose, 10 g/L; yeast extract, 0.5 g/L; (NH4)2SO4, 0.5 g/L; KH2PO4, 12 g/L; Na2HPO4, 12 g/L; MgSO4, 1.5 g/L; CaCl2, 0.1 g/L; MnSO4, 0.0001 g/L; CuSO4, 0.0001 g/L; Co (NO3)3, 0.0001 g/L and ZnSO4, 0.001 g/L. To promote the dispersion of oil in water, each type of oil was mixed to tween 80 at 25% and autoclaved separately. Autoclaved oil was aseptically added to BM at a final concentration of 5 g/L.
The pH of all media was adjusted before sterilization (121 °C for 20 min) to 6.0 ± 0.1 with 1 M H2SO4 and 5 M NaOH solutions. After sterilization (at 121 °C for 20 min), the flasks were inoculated with 1 mL of a mid-exponential pre-culture on YPD medium containing 4 × 108 cells. The cultures were then incubated in a rotary shaker at 180 rpm and 28 °C for 96 h. Cells were visualized after staining with Nile red fluorescence as described by Arous et al. (2017).
Analytical methods
Chemical oxygen demand (COD) and total nitrogen content (Kjeldahl) of the above-mentioned wastewaters were determined according to Standard Methods (APHA 1998). Total sugars concentration was determined by the phenol–sulfuric acid method described by DuBois et al. (1956), while reducing sugars concentration was estimated by DNS (3,5-dinitrosalicylic acid) method and was expressed as glucose equivalent (Miller 1959).
The yeast biomass was separated by centrifugation, washed with distilled water three times and then dried in an oven at 80 °C until a constant weight. Dry biomass was determined as dry weight per volume and the supernatant was used to determine total sugars content as above. Total cellular lipids were extracted from powdered biomass with chloroform: methanol 2:1 (v/v) for 24 h (Folch et al. 1957). Afterwards, the solvent was evaporated and total lipids were determined gravimetrically to calculate lipid yield (L, g/L) according to the following equation:
where WL is the weight of lipid extracted (g) and V is the working medium volume (mL).
Lipids were transesterified to fatty acid methyl esters (FAME) using CH3 − Na+ and CH3OH/HCl according to the AFNOR (1984) method. FAME were analyzed by gas chromatography (Shimadzu, GC 17A). The GC was equipped with flame ionization detector (FID) and a capillary column (50 m × 0.32 mm, 0.5 mm, PERICHROM Sarl, France). Helium was used as carrier gas (at a flow rate 1.13 mL per min). Oven temperature was set to 100 °C with a rate increase of 30 °C per min until to reach the temperature of 150 °C. The temperature was then increased to 190 °C (at 10 °C per min) and maintained for 14 min before increased (at 5 °C per min) to 255 °C and then held at this temperature for 10 min. The injector and detector temperatures were set to 255 and 270 °C, respectively. Standards of FAME were used for the identification and quantification of fatty acids in the lipids.
Estimation of biodiesel properties using FAME data
The FAME profile was used to estimate some important properties of the biodiesel produced from W. anomalus strain EC28 grown on various low-cost raw materials. Chemical biodiesel properties like cetane number (CN), iodine value (IV), saponification value (SV), -chain saturated factor (LCSF), and cold-filter plugging point (CFPP) were calculated using previously derived equations (Nascimento et al. 2013).
The value of the CN was estimated using both the IV value as well as the SV and was determined according to the following equation:
The SV and IV of the biodiesel were, respectively, calculated from Eqs. (3) and (4), where D is the number of double bonds, M is the FAME molecular mass, and P is the percentage of each FAME component:
Estimation of the cold-filter plugging point (CFPP) was performed as follows [Eq. (6)]. The first step was to estimate the long-chain saturated factor (LCSF) using Eq. (5) and then to substitute the value of this parameter into Eq. (6):
Statistical analysis of data
All experiments in this work were performed in triplicate, and mean values were presented. Experimental data were statistically analyzed using the one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test using IBM SPSS Statistics 21 software package.
Results and discussion
Cell growth and lipid production by W. anomalus strain EC28 on glucose-based medium
Lipid accumulation in oleaginous microorganisms typically occurs under nitrogen-limited conditions with high concentrations of sugars or similarly metabolized compounds (Bellou et al. 2016). In this study, experiments were carried out in batch shake flask cultures on nitrogen-limited media in which glucose was used as the sole carbon source at 30 g/L. Kinetics of growth (X, g/L), lipid accumulation (L/X, %), and glucose consumption (glucose-S, g/L) by W. anomalus under nitrogen-limited conditions are depicted in Fig. 1.
Kinetics of total cell mass (X, g/L), lipids in dry biomass (L/X, %), remaining substrate (glucose-S, g/L) of W. anomalus EC28 cultivated on nitrogen limiting conditions. Inset light microscopy (left panel) and Nile red fluorescence microscopy (right panel) images of 96-h-old cells under ×100 oil immersion objective
During growth phase, biomass increased rapidly and achieved 8 g/L within 50 h of incubation, while 29.2 g/L of glucose were consumed simultaneously by the yeast cells within this period. After approximately 80 h of fermentation, cell growth ceased, while glucose was smoothly assimilated and converted into storage lipids (lipogenic phase). After complete depletion of the nitrogen source (data not shown), an important increase in the intracellular lipid content occurred from a mass fraction of 7.2% of lipids on dry cell mass to 16.5%. After 120 h of fermentation, a significant decrease in lipid production was noticed from 12.1% wt/wt to 9.2% wt/wt. The data reviewed indicate that initiation of lipid degradation occurred immediately after carbon starvation in external medium. According to Papanikolaou et al. (2002), fatty acid breakdown in yeasts takes place via the β-oxidation pathway under carbon limiting conditions.
Cell growth and lipid production by W. anomalus on agro-industrial wastewaters-based media
The lipid yield, fatty acid composition, and degree of unsaturation are closely related to the type and concentration of carbon source (Amaretti et al. 2010). Accordingly, in the present study, we investigated the effect of the above-mentioned agro-industrial wastewaters [i.e., deproteinized cheese whey (DCW), olive mill wastewater (OMW), and wastewaters from two confectionary factories (WCI1, WCI2)], on cell growth, lipid yield, and sugar consumption by W. anomalus (Table 2).
Cheese whey was used as nitrogen and carbon source, since W. anomalus was able to assimilate lactose and galactose as sole carbon sources (Arous et al. 2017). After 96-h incubation period, around 2.6-g/L biomass containing non-negligible lipid quantities (24%, wt/wt) was produced due to the conversion of lactose into biomass and SCO, while 23.3 g/L of total sugars were consumed (Table 2). According to Domingues et al. (2010), the ability of yeasts to metabolize lactose results from the presence of lactose permease and β-galactosidase that breaks down lactose to glucose and galactose. In the present study, experiments were conducted on DCW supplemented with minerals, in which no external addition of carbon or nitrogen source was performed. Therefore, the existing proteins were the nitrogen source, while the existing lactose and proteins were simultaneous carbon sources for W. anomalus. Considering that lactose is composed of around 40% wt/wt carbon, whereas proteins of 40% wt/wt carbon and 7% wt/wt nitrogen, the initial C/N molar ratio of the cheese whey was about 50 mol/mol; thus, cheese whey may be considered as a nitrogen-limited medium (Vamvakaki et al. 2010). The use of DCW as a substrate for SCO production was properly investigated and some encouraging results have been obtained. For instance, Taskin et al. (2015) reported lipid content of 58% (wt/wt) during growth of Yarrowia lipolytica on lactose enriched deproteinized whey-based medium in batch shake flask cultures, while Castanha et al. (2013) obtained SCO production of 13.1% (wt/wt) when Cryptococcus laurentii was cultivated on DCW.
To evaluate cell growth and lipid synthesis performances of W. anomalus in OMW, cultures were carried out in increasing concentrations of OMW: 25, 50, 75, and 100% (v/v) in distilled water under nitrogen limiting conditions. W. anomalus was able to grow in all tested OMW concentrations. Maximum biomass yield was achieved at 25 and 50% OMW (4.1 and 4.5 g/L, respectively) after 96-h incubation period. However, higher concentrations of OMW negatively affected biomass production due to the high content of phenolic compounds (only 2.6 g/L of biomass). The highest lipid content was observed at 75 and 100% OMW, i.e., 23 and 19.5%, wt/wt, respectively (statistically significant at p ≤ 0.05), despite the high content of phenolic compounds. Owing to its richness in soluble and insoluble carbohydrates, OMW should be considered as a suitable substrate for SCO production. The use of OMW as a rich organic substrate for lipid production was properly investigated by Yousuf et al. (2010) for a strain of Lipomyces starkeyi which was able to accumulate up to 29.9% wt/wt of lipids on OMW, while Arous et al. (2016) reported a lipid content of 17.9% wt/wt during growth of D. etchellsii on OMW (25%, v/v in water) after 48 h of fermentation.
Two wastewaters (WCI1 and WCI2) from two different confectionary industries were tested for SCO production. WCI 2 was mainly composed of sucrose, while WCI1 contained a wide range of soluble carbohydrates (i.e., glucose, sucrose, fructose, and lactose). Maximum biomass values of 5.7 and 5.5 g/L were obtained on WCI1 and WCI2, respectively. However, WCI2 medium containing a mixture of sugars was found to be more suitable for SCO production by W. anomalus as maximum lipid accumulation was 17.4% wt/wt compared to 5.4% wt/wt obtained in sucrose-rich substrate (WCI1). However, in some cases, higher lipid accumulation occurred in media composed of wastewaters from confectionary industries. For instance, Ling et al. (2013) tested milk candy wastewater for the cultivation of a strain of Rhodosporidium toruloides and obtained a biomass concentration of 4.4 g/L containing significant quantities of reserve lipids (51.7%, wt/wt).
Among the tested wastewater-based media, deproteinized cheese whey-based medium and olive mill wastewater-based medium (75%, v/v in water) supported the highest lipid accumulation, i.e., up to 24 and 23%, wt/wt, respectively (statistically significant at p ≤ 0.05). These amounts are slightly lower than those determined by Souza et al. (2016) in W. anomalus strain CCMA 0358 (30%, wt/wt lipids) when grown on glycerol (100 g/L) under nitrogen-limitation. The differences in lipid production abilities are primarily attributed to the microbial physiology, nutrient limitation, and environmental conditions.
Even though lipid yield was low (only up to 1 g/L), all wastewaters met the general criteria for SCO production. There was no evidence for the presence of any inhibitory substances in the selected agro-industrial wastewaters. Nevertheless, to reach a financially viable stage, the lipid production needs to be significantly increased.
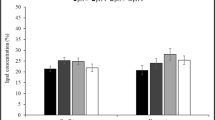
The fatty acid composition of lipids produced by W. anomalus showed high amounts of oleic (33.6–50%), palmitic (17.5–34.7%), and linoleic (14.8–21.6%) acids (Fig. 2). As reported by Zhu et al. (2013), fatty acids indicated for good biodiesel properties include C14:0, C16:0, C16:1, C18:0, C18:2, and mainly C18:1. Since W. anomalus lipids contain a high level of monounsaturated fatty acids (oleic acid); they might be regarded as an excellent source for biodiesel production. Mittelbach and Remschmidt (2004) suggested that oleic acid is considered ideal for biodiesel as it provides better cold flow properties without losses to oxidative degradation. In the same line, several studies showed the predominance of oleic acid in microbial lipids derived from oleaginous yeasts (Arous et al. 2017). Based on European Biodiesel Standards EN14214 for vehicle use, the amount of Linolenic acid (18:3) ought to be limited to a maximum of 12%, which is in good agreement with that found in W. anomalus lipids (only 0.5–1.7%) (Knothe et al. 2005).
FAME composition of transesterified microbial lipids after 96 h of cultivation with W. anomalus EC28 on different agro-industrial wastewaters. DCW deproteinized cheese whey, WCI1 and WCI2 wastewaters from two confectionary industries, OMW olive mill wastewater. Others: C10:0, C12:0, C14:0, and C14:1
The oil profile of W. anomalus grown on different cheap media revealed no significant change in proportion of the major fatty acids when compared to that obtained in glucose-based media (Arous et al. 2017). This suggested that the cheap raw materials could be used without any compromise on the quality of the oil produced by yeasts.
Cell growth and lipid production by W. anomalus using different oil wastes as glucose co-substrates
Several fatty materials have been used as substrates for oleaginous microorganisms such as vegetable oils, fatty esters, soap-stocks, pure free-fatty acids, industrial fats composed of free-fatty acids of animal or vegetable origin, and crude fish oils (Montet et al. 1985; Aggelis and Sourdis 1997; Guo and Ota 2000).
In this study, different oil wastes were used as glucose co-substrates [i.e., waste oil from frying fish (FFO), waste oil from frying potato (PFO), and waste oil from frying meat (MFO)] for growth and lipid accumulation by W. anomalus.
As shown in Table 3, the addition of frying oils to the culture media resulted in a significant decrease in total lipid content, while total biomass yield remains almost the same. SCO production was the highest, i.e., 14.6%, wt/wt when the control medium (10 g/L of glucose) was used (p < 0.05), followed by potato frying oil (11.9%, wt/wt), fish frying oil (10.8%, wt/wt), and meat frying oil (10.1%, wt/wt). Lipid accumulation in oleaginous yeasts requires excessive amounts of sugars (or resembling media components such as glycerols) with low concentrations of nitrogen in the media. However, oil wastes used in the present study contain high nitrogen amounts (estimated at 1.8, 4.9, and 5.5 g/L for the PFO, FFO, and MFO, respectively) owing to the release of nitrogen from potato, fish, and meat into the frying oil, which resulted in a significant decrease of the lipid content when compared to that obtained with glucose as the sole carbon source. Similarly, El Bialy et al. (2011) showed a significant decrease in total lipid content of a strain of Yarrowia lipolytica when oil wastes for frying meat, chicken, and fish were used as carbon sources. In the same line, Papanikolaou and Aggelis (2011) reported that, in many cases, the amounts of lipids produced through the de novo lipid accumulation pathway are higher than those produced on media in which hydrophobic materials are added as substrates (or co-substrates).
The fatty acid profile of the lipids produced by W. anomalus cultivated in different oil wastes before and after fermentation was determined, and the results are depicted in Table 4. In all cases, a variety of long-chain fatty acids were found in W. anomalus lipids with predominance of oleic acid (49.38–51.89%) followed by linoleic acid (18.33–19.02%) and palmitic acid (17.23–19.10%). However, the composition of the initial oil wastes showed higher amounts of linoleic acid (37.37–49.3%) and lower concentrations of oleic acid (26.13–34.32%) (statistically significant at p ≤ 0.05), which may suggest the tendency of this strain to dissimilate polyunsaturated fatty acids for growth and maintenance and to accumulate the monounsaturated ones. According to several authors, the oleaginous yeasts growing on different hydrophobic substrates (e.g., oils, fats, and free-fatty acids) are able to accumulate and simultaneously to modify the fatty acid composition of the employed fatty material used as carbon source (Papanikolaou et al. 2001). The fatty acid composition of the cellular lipids is controlled by the specific rate of incorporation of substrate aliphatic chains inside the cells as well as the intracellular modification of fatty acids defined by the enzymatic arsenal of the microorganism (Papanikolaou et al. 2001).
Physical properties of the resulting biodiesels
Main biodiesel properties that are directly influenced by the FAME profile are the cetane number (CN), iodine value (IV), and the cold-filter plugging point (CFPP). Cetane number (CN) is widely used as biodiesel quality parameter related to the ignition quality of a diesel fuel. For most diesel engines, a value above 40–50 is considered acceptable (Knothe et al. 2005). The IV refers to the tendency of the biodiesel to react with oxygen at ambient temperature. It depends on both the number and position of the double bonds in the FAMEs. High number of double bonds decreases the stability of fuel. The maximum IV accepted in the European Standards EN 14214 is 120 I2/100 g. The CFPP is one of the representative parameters, typically used for the prediction of the biodiesel behavior at low temperatures (Knothe et al. 2005).
A mathematical estimate of the FAMEs derived from W. anomalus lipids was performed following the same methodology used for evaluating algal and vegetable biodiesels and compared with the predicted/estimated values reported for biodiesel obtained from other oleaginous yeasts and vegetable oils (Table 5). Biodiesels derived from W. anomalus were found to have good estimated CN values (53.45–59.32), as well as an appropriate degree of unsaturation, as the estimated IV satisfied the limits imposed by EN 14214 standards. Since the cold-filter plugging point depends on climate conditions of each country, the EN 14214 standard does not specify a low-temperature parameter in its list of specifications. In the present study, the estimated CFPP were lower than those found in many vegetable oils (except biodiesels produced in DCW-based medium) such as palm (10 °C) and a wide range of oleaginous yeasts (Table 5).
Conclusion
The oleaginous yeast, W. anomalus EC28, exhibited a good ability for lipid accumulation when grown on various agro-industrial wastewaters (up to 24%, wt/wt in dry biomass), while the use of oil wastes as glucose co-substrates was not adequate to support yeast growth and SCO production. The predicted biodiesel properties based on FAME composition of the lipids of W. anomalus EC28 grown on glucose and on low-cost raw substrates were found to lie within the range specified by international biodiesel standard specifications, which may suggest that the cheap raw materials used in this study could be used without any compromise on the quality of the oil produced by yeasts. For economic feasibility, the lipid production should be further improved by optimizing the substrate C/N ratio and co-fermentation of wastes of different C/N characteristics. Owing to its ability to grow at low pH and to produce antimicrobial agents, W. anomalus EC28 may be subsequently cultivated in low-cost non-sterile conditions which can further increase the economic viability of SCO production process.
References
AFNOR (1984) Recueil des normes françaises des corps gras, grains oléagineux et produits dérives, 3rd edn. Association Française pour normalisation, Paris, p 95
Ageitos JM, Vallejo JA, Veiga-CrespoP Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227
Aggelis G, Sourdis J (1997) Prediction of lipid accumulation-degradation in oleaginous micro-organisms growing on vegetable oils. Antonie Van Leeuwenhoek 72:159–165
Aloui H, Licciardello F, Khwaldia K, Hamdi M, Restuccia C (2015) Physical properties and antifungal activity of bioactive films containing Wickerhamomyces anomalus killer yeast and their application for preservation of oranges and control of postharvest green mold caused by Penicillium digitatum. Int J Food Microbiol 200:22–30
Amaretti A, Raimondi S, Sala M, Roncaglia L, Lucia M, Leonardi A, Rossi M (2010) Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb Cell Fact 9:73–78
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Arous F, Frikha F, Triantaphyllidou I-E, Aggelis G, Mechichi T, Nasri M (2016) Potential utilization of agro-industrial wastewaters for lipid production by the oleaginous yeast Debaryomyces etchellsii. J Clean Prod 133:899–909
Arous F, Azabou S, Triantaphyllidou I-E, Aggelis G, Jaouani A, Nasri M, Mechichi T (2017) Newly isolated yeasts from Tunisian microhabitats: lipid accumulation and fatty acid composition. Eng Life Sci 17:226–236
Bellou S, Triantaphyllidou I-E, Mizerakis P, Aggelis G (2016) High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J Biochem 234:116–126
Castanha RF, Salgado de Morais LA, Mariano AP, Monteiro RTR (2013) Comparison of two lipid extraction methods produced by yeast in cheese whey. Braz Arch Biol Technol 56:629–636
Chen HL, Su HP, Lin CW (1998) Characterization of yeast cultures for a flavoring agent in a yoghurt-type product. J Food Sci 63:897–900
Chi Z, Zheng Y, Jiang A, Chen S (2011) Lipid production by culturing oleaginous yeast and algae with food waste and municipal wastewater in an integrated process. App Biochem Biotechnol 165:442–453
Dejwatthanakomol C, Anuntagool J, Morikawa M, Thaniyavarn J (2016) Production of biosurfactant by Wickerhamomyces anomalus PY189 and its application in lemongrass oil encapsulation. Sci Asia 42:252–258
Domingues L, Guimarães PMR, Oliveira C (2010) Metabolic engineering of Saccharomyces cerevisiae for lactose/whey fermentation. Bioeng Bugs 1:164–171
Dourou M, Kancelista A, Juszczyk P, Sarris D, Bellou S, Triantaphyllidou I-E, Rywinska A, Papanikolaou S, Aggelis G (2016) Bioconversion of olive mill wastewater into high-added value products. J Clean Prod 139:957–969
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Eguchi S, Kagawa S, Okamoto S (2015) Environmental and economic performance of a biodiesel plant using waste cooking oil. J Clean Prod 101:245–250
El Bialy H, Gomaa OM, Azab KS (2011) Conversion of oil waste to evaluable fatty acids using Oleaginous yeast. World J Microb Biot 27:2791–2798
Folch J, Lees M, Sloan-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C (2012) Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresources Technol 111:398–403
Ghanavati H, Nahvi I, Karimi K (2015) Organic fraction of municipal solid waste as a suitable feedstock for the production of lipid by oleaginous yeast Cryptococcus aerius. Waste Manage 38:141–148
Guo X, Ota Y (2000) Incorporation of eicosapentaenoic and docosahexaenoic acids by a yeast (FO726A). J Appl Microbiol 89:107–115
Knothe G, Van Gerpen JH, Krahl J (2005) The Biodiesel Handbook. AOCS press Champaign, Illinois
Kumar D, Singh B, Korstad J (2017) Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew Sust Energ Rev 73:654–671
Ling J, Nip S, Shim H (2013) Production of microbial lipid from food industry wastewater by Rhodosporidium toruloides. In: Xu Q (ed) Proceedings of the 2013 international conference on material science and environmental engineering-2013. DEStech Publications, USA, pp 171–177
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Minagawa N, Sakajo S, Komiyama T, Yoshimoto A (1990) Essential role of ferrous iron in cyanide-resistant respiration in Hansenula anomala. FEBS Lett 267:114–116
Mittelbach M, Remschmidt C (2004) Biodiesel: the comprehensive handbook. Martin Mittelbach
Montet D, Ratomahenina R, Galzy P, Pina M, Graille J (1985) A study of the influence of the growth media on the fatty acid composition in Candida lipolytica diddens and lodder. Biotechnol Lett 7:733–736
Munch G, Sestric R, Sparling R, Levin DB, Cicek N (2015) Lipid production in the under-characterized oleaginous yeasts, Rhodosporidium babjevae and Rhodosporidium diobovatum, from biodiesel-derived waste glycerol. Bioresour Technol 185:49–55
Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, de Souza CO, Vich DV, de Carvalho GC, Nascimento MA (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg Res 6:1–13
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Papanikolaou S, Chevalot I, Komaitis M, Aggelis G, Marc I (2001) Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 80:215–224
Papanikolaou S, Chevalot I, Komaitis M, Marc I, Aggelis G (2002) Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl Microbiol Biot 58:308–312
Passoth V, Fredlund E, Druvefors UA, Schnurer J (2006) Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res 6:3–13
Passoth V, Tabassum MR, Nair HA, Olstorpe M, Tiukova I, Ståhlberg J (2013) Enhanced ethanol production from wheat straw by integrated storage and pre-treatment (ISP). Enzyme Microb Tech 52:105–110
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Pérez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Sestric R, Munch G, Cicek N, Sparling R, Levin DB (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient-limited conditions. Bioresour Technol 164:41–46
Souza KST, Ramos CL, Schwan RF, Dias DR (2016) Lipid production by yeasts grown on crude glycerol from biodiesel industry. Prep Biochem Biotechnol 47:357–363
Swangkeaw J, Vichitphan S, Butzke CE, Vichitphan K (2009) The characterization of a novel Pichia anomala beta-glucosidase with potentially aroma-enhancing capabilities in wine. Ann Microbiol 59:335–343
Taskin M, Saghafian A, Aydogan MN, Arslan NP (2015) Microbial lipid production by cold-adapted oleaginous yeast Yarrowia lipolytica B9 in non-sterile whey medium. Biofuel Bioprod Bioorg 9:595–605
Vamvakaki AN, Kandarakis I, Kaminarides S, Komaitis M, Papanikolaou S (2010) Cheese whey as a renewable substrate for microbial lipid and biomass production by Zygomycetes. Eng Life Sci 10:348–360
Wang Y, Gong Z, Yang X, Shen H, Wang Q, Wang J, Zhao ZK (2015) Microbial lipid production from pectin-derived carbohydrates by oleaginous yeasts. Process Biochem 50:1097–1102
Xu J, Zhao X, Wang W, Du W, Liu D (2012) Microbial conversion of biodiesel by product glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem Eng J 65:30–36
Yalçın HT, Çorbacı C, Uçar FB (2013) Molecular characterization and lipase profiling of the yeasts isolated from environments contaminated with petroleum. J Basic Microb 00:1–8
Yousuf A, Sannino F, Addorisio V, Pirozzi D (2010) Microbial conversion of olive mill waste waters into lipids suitable for biodiesel production. J Agric Food Chem 58:8630–8635
Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z (2013) Nutritional removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302
Acknowledgements
Financial support has been provided by the Ministry of higher education and scientific research-Tunisia. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Arous, F., Atitallah, I.B., Nasri, M. et al. A sustainable use of low-cost raw substrates for biodiesel production by the oleaginous yeast Wickerhamomyces anomalus . 3 Biotech 7, 268 (2017). https://doi.org/10.1007/s13205-017-0903-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0903-6