Abstract
The work is devoted to the investigation of the electrophysical properties of nanopowder systems based on zirconia in a compacted form with varying degree of system hydration. The dependence of conductivity and activation energy of conductivity on the amount of moisture in the system was studied. It is shown that the value of the total impedance of the system decreases by 55 times as the amount of moisture in the system increases from minimum to maximum. The main contribution to conductivity is made by diffusion processes in the space between nanoparticles. The possibility of using this effect to characterize the thermodynamic environmental conditions, in which the system is located, is shown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Nanopowders based on zirconia have a great practical and scientific interest due to the unique combination of electrical, mechanical, optical, and chemical properties. One of advantages of porous disperses systems produced by a pressure or a thermal influence based on nanopowders compared with coarse-grained analogues is relatively high part of phase boundaries The surface layer of nanoparticles has special properties that are not characteristic of bulk materials due to the free energy localized at the heterophase boundary. Zirconia is a wide-gap dielectric (bandgap = 3.5–5 eV) with a relatively high dielectric constant (ε = 25), due to which the electric fields on the surface of ZrO2 nanoparticles can reach threshold values for field electron emission even with a slight change in external conditions. In particular, an increase in atmospheric humidity (Strekalovsky et al. 1987), accompanied by the high chemical activity of the surface of ZrO2 materials by the adsorption of water molecules, leads to a change in its charge state (Subhoni et al. 2018; Doroshkevich et al. 2017a,b) and in the extreme case, pronounced electron emission (Doroshkevich et al. 2019; Lyubchyk et al. 2017). Thus, the high density of surface energy in the bulk of nanostructured systems greatly increases their sensitivity to changes in external conditions (Meng et al. 2015), which creates the prerequisites for the development of long-range sensor systems (Vasiliev 2012). However, the most important feature of nanoscale objects is thermodynamic instability, which significantly reduces the activation thresholds of processes in, for example, sensor systems, that is, the thermodynamic parameters of nanopowder systems that are in dynamic equilibrium with the external environment, respectively, change with high speed even with minor changes in the thermodynamic parameters of the latter. Of course, these changes entail changes in the charge state of the surface of nanoparticles and the dispersed system as a whole (Kuyyadi and Mahaveer 2008).
Considering the availability on the surface of nanoparticle of ionic atmospheres of adsorption origin with a work function different from the work function of electron from nanoparticles material, it is clear that nanoparticle has properties of functional heterojunction. Changes in external conditions will lead to a shift of the adsorption equilibrium, and, as a consequence, changes of electrical properties of heterojunction. Thus, the measurement of electrical properties of nanopowder system can be used for the characterization of thermodynamic conditions of environment in which the latter is located. For example, porous nanostructured dispersed medium can be used for the accurate determination of changes in atmospheric pressure, ambient humidity, or temperature. Taking into account a high specific surface area of interfacial boundaries, the sensitivity of such sensor will be significantly higher than those using planar structures.
Thus, nanostructured media based on ZrO2 are extremely interesting as functional materials for electronics, involving energy exchange with the external environment.
Investigation of the electrical properties of a nanopowders based on ZrO2 in a compacted state using electrochemical impedance spectroscopy (EIS) from the dependence on the degree of its hydration was the aim of this work.
Methods
As an object of investigation, a nanopowder of composition ZrO2 + 3 mol% Y2O3 compacted in the form of tablets (diameter 15 mm, thickness 1.5 mm, m = 1 g) was used. The technology of nanopowder obtaining by the method of co-precipitation from chloride raw material is identical to Konstantinova et al. (2011). Y2O3 was added as stabilizing impurity in the crystal lattice of zirconia to eliminate volumetric changes in the crystalline lattice, which occur during phase transformation. Cations of Y2O3 form a solid substitution solution in the zirconium lattice. The addition of 3 %mol Y2O3 stabilizes the tetragonal phase of polymorphic modification of zirconia. A further increase in the amount of yttrium oxide leads to the existence of a two-phase region of the tetragonal and cubic phases. Sintering conditions of the nanopowder are 700 °C for 2 h. According to the TEM data, the size of nanoparticles is 19 nm. Samples of nanopowders for TEM research were prepared using the method of ultrasonic spraying (at a frequency of 22 kHz) of a powder suspension in ethyl alcohol on a carbon replica. From the nanopowder using preliminary uniaxial pressure (Pcomp = 40 MPa), samples in the form of tablets were formed; after that, the tablets (compact sets) were sealed by high hydrostatic pressure (HHP, 500 MPa) using UVD-2 unit. Carbon electrodes were deposited on opposite faces of the tablets. Estimation of porosity of samples was carried out using hydrostatic weighing method.
The study of electrical properties of nanopowder compact sets was carried out in the climatic chamber with the possibility of controlling the relative humidity level inside a wide range (20–95% of relative humidity) and maintaining it at a constant level. Impedance measurements were carried out in an automatic mode using a precision virtual meter-analyzer of impedance parameters 2B-1 (Doroshkevych et al. 2012; Agamalov et al. 1993,2004). The frequency dependences of imaginary ImZ and real ReZ components of complex impedance Z were obtained. Voltage amplitude of the exciting sinusoidal signal U = 50 mV, and the range of measured frequencies was 1 Hz–1 MHz. Approximation of the model and experimental impedance spectra was performed using the EIS Spectrum Analyzer (EIS Spectrum Analyser 2020) program.
Measurements of electrical properties were carried out cyclically. Moisture adsorbed from the atmosphere was removed from the tablet in a furnace (T = 120 °C, 1 h). The impedance of the dried tablet is infinitely large and it is not fixed using the impedance spectroscopy technique. Each measurement of the impedance of the tablets was carried out in the climatic chamber with a certain level of humidity after preliminary aging the tablet in the chamber for 1 h. After that, the cycle was repeated with a different value of relative humidity η in the chamber. Humidity values were 95% RH (23,865 ppm V); 80% (200,021 ppm V); 70% (17,475 ppm V); 60% (14,941 ppm V); 50% (12,420 ppm V); 40% (9911 ppmV).
Results and discussion
The study of the morphology of the nanoparticle compact sets using TEM and SEM methods
The results of investigation of the initial ZrO2 + 3 mol%Y2O3 (700 °C, 2 h) nanopowder by TEM method are shown in Fig. 1. The powder is relatively well distributed spatially (Fig. 1a), the particles were individualized or formed small aggregates (50–70 nm) (Fig. 1b).
The SEM images of the compacted tablets at 2000 magnification (Fig. 2a) have a homogeneous microstructure. With a higher magnification (20,000), it can be seen that the microstructure of the sample is relatively loose. Particles after compaction are aggregated. Aggregates have a rounded shape with an average size up to 1 µm. Samples contain pores that facilitate penetration of moisture into bulk. The porosity for compact sets at a given pressure is about 47%.
Change in mass of samples during adsorption
The relative content of water accumulated by the samples at different relative ambient humidity, Δm = (mmax− m0)/m0·100%, where m0 is the mass of the dried sample, mmax is the maximum mass of the sample after saturation, is shown in Table 1. It also shows the calculated values of the number of water molecules in the sample Nm after saturation of the latter to the mass Δm calculated by the equation:
where NA = 6.02·1023 [1/mol] is the Avogadro number; m0 [g] is the mass of the dried sample; \(M_{{{\text{H}}_{{2}} {\text{O}}}}\) [g/mol] is the molar mass of the water molecule; \(M_{{{\text{H}}_{{2}} {\text{O}}}}\) = 18 g/mol.
EIS investigation of electrical properties of compact sets under humidification
The dependences of the impedance components in the form of hodographs are shown in Fig. 3. All hodographs have a similar shape. The hodograph consists of two regions with different geometries: from a fragment of a circle α in the high-frequency region and a straight-line region β in the low-frequency region.
Spectra of the electrochemical impedance of sealed nanopowders with composition ZrO2 + 3 mol% Y2O3 at different relative humidity of the surrounding air: 1—95%; 2—80%; 3—70%; 4—60%; 5—50%; 6—40% (a) The equivalent electrical circuit of the measured object (b). The arrow shows the direction of increase in the frequency of the exciting signal
The frequency sweep of the excitation sinusoidal signal causes a resonant electrical response of certain structural elements presented in the compact set, that is, the inverse sorted values τ = 2π/ω (ω is the angular frequency of the sinusoidal signal), sorted by ascending, considering symmetry of the system regarding to the electrical properties, are the reaction coordinate.
In general case, a sample under study is spatially inhomogeneous and its electrical properties change along the reaction coordinate. As a rule, electrically inhomogeneous systems consist of inner homogeneous layers with constant parameters. Each layer is assigned a separate link of the electrical circuit with specific parameters, for example, a parallel RC circuit with a characteristic time constant τ = RC, that is, structural elements of the system with characteristic spatial parameters correspond to time-ordered segments on the reaction axe (Boukamp 2004), and hodograph reflects the relationship between temporal and spatial characteristics of the system under study. The equivalent electrical circuit of the measured compact set modeled using the hodograph is shown in Fig. 3b. The hodograph is approximated by two series-connected parallel RC chains with different time constants. The R1C1 chain characterizes the semicircle α located in the high-frequency region of the spectrum. The semicircle corresponds to short relaxation times τhf = R1C1 and the capacitive nature of the conductivity (Abdalaa et al. 2010; Fleig 2002). The R2C2 chain characterizes the conductivity of the low-frequency straight line segment β. The beam corresponds to large proper relaxation times R2C2 = τlf and the diffusion nature of conductivity (Boukamp 2004; Abdalaa et al. 2010; Fleig 2002).
As can be seen, the center of the semicircle is shifted upward relative to the abscissa axis. On an equivalent circuit, this impedance feature is taken into account by replacing capacitor C with a constant phase element (CPE). Impedance of CPE is described by the formula: ZCPE (iω) = P−1(iω)−n, where P is the coefficient of proportionality, and n is the exponent characterizing the phase shift. For n = 1 – ε, where 0 < ε < (0.1–0.2), CPE gives the impedance of a pure or distributed capacitive element (Jorcina et al. 2006; Pajkossy 2005; Macdonald 1987).
Thus, the analysis of the data shows that according to the electrical properties, the material under study is a system consisting of at least two areas differing in the reaction time of the structural elements to the excitation signal and the type of conductivity.
There are only two phases in the compact set, and it is known that the material of zirconia nanoparticles is a wide-gap dielectric (Zaynullina and Zhukov 2001). Thus, a part of a spectrum with a capacitive nature of conductivity α characterizes the dielectric volume of nanoparticles (Fig. 4b), and the straight line part β (Fig. 4b) corresponds to interparticle space. The structure of the sample is represented in the form shown in Fig. 4. Such structure is typical for systems with liquid electrolyte, for example, for cells of lithium-ion batteries (Gao et al. 2015).
Geometric interpretation of the conductivity of the nanopowder system (a); the relationship of spatial (r) and temporal (τ) coordinates with the hodograph shape (b); model representation of the system under study in accordance with the real spatial distribution of phases (c), where 1—the volume of the dielectric nanoparticles; 2—ion-conducting dispersion medium; 3—electrode; r is the radius vector; τ is the characteristic reaction time (time constant) of structural elements
As shown in (Blumenthal 1958), the main amounts of the adsorption ionic atmospheres are ions of the dissociated water molecules. That is, a change in the humidity of the ambient air will change the electrical properties of sealed nanopowder.
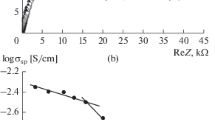
Calculated values of the parameters of equivalent circuit and own relaxation times τ of the sample under study with increasing air relative humidity from 40 to 95% show that the relaxation time decreases by three orders of magnitude (from 2.73 × 10–02 to 5.69 × 10–05 s), and the total resistance value of equivalent circuit by 55 times (from 2.06 MΩ to 37 kΩ). As can be seen from Fig. 5a (curve 2) and Fig. 5b, changes in total resistance and own relaxation times are determined by the impedance of the adsorption layer.
In the space between the particles, the active component of conductivity is prevails, and in the volume of the material of nanoparticles—capacitive type, therefore the impedance of the system Z is represented as the sum of the reactive and active components of the resistance, as described in the following formula:
where R is the active component of the total impedance, \(R = U \cdot I^{{^{ - 1} }} = U \cdot (\frac{N \cdot e}{t})^{{^{ - 1} }}\); XC is the reactive component of the total impedance, \(X_{C} = - (\omega \cdot C)^{{^{ - 1} }}\); N is the quantity of free charge carriers; e is the electron charge; ω is the excitation angular frequency (ω = 2π/t); C is the electric capacity, \(C = \frac{{\varepsilon \varepsilon_{0} S}}{l}\); ε is the relative dielectric constant of zirconia; ε0 is the dielectric constant; S is the tablet cross-sectional area; l is the tablet thickness.
As shown in Wolkenstein (1963), Kiselev and Krylov (1979), and Alekseenko and Volkova (2000), the approaching of a neutral molecule to a surface of semiconductors leads to the appearance of an impurity localized energy level in a bandgap. The authors of Gao et al. (2015) showed that for zirconia this level is an acceptor. In this case, the adsorption center is ionized due to its own donor surface-localized electronic states (dangling bonds); chemical bond between the adsorbate and the surface active center is formed.
The difference between the work function of electron from the nanoparticle material and the ionization energy of adsorbate molecules is such that the electron density is shifted toward the adsorption layer. The ionization potential of the OH group is AOH = IOH = 13.08 eV (Tang and Thomas 2015), and the work function of electron for zirconia is AZrO2 = 3 ÷ 5 eV (Ciuparu et al. 2000; Foster et al. 2001). Thus, as the water molecules adsorb, the surface begins to charge negatively, and in the bulk of nanoparticles near the surface, the Debye layer (Gao et al. 2015) is increased:
where n+ is the concentration of free charge carriers; k is the Boltzmann constant; T is the temperature.
This layer is a space charge region (SCR). Negatively charged ions in the gas adsorption layer and positively charged holes in the surface region of the nanoparticles form an electrical capacitance, the value of which determines the reactive conductivity of the dispersed phase volume (ZrO2 + 3 mol% Y2O3 nanoparticles). The active conductivity in the space between the particles is caused by physically sorbed diffuse layer that formed to compensate the charge of potential-forming layer (Efremov 1971).
As the adsorption of water molecules from the atmosphere on the surface of the particles increases, the surface charge and, as a result, the concentration of positive N+ ions in the diffuse layer and n+ holes in the SCR is increased too. In this case, the thickness L decreases. Consequently, the capacitance C is increased and the reactance XC is decreased. An increase in the specific concentration of free charge carriers N+ in the volume between particles leads to a decrease of the active resistance R. The total impedance of the system Z also decreases.
Quantitative estimates of the activation parameter of conductivity initiated by adsorption
Dependences on moisture values of all three resistances are shown in Fig. 5a. For the reactive component of the volume impedance, calculations were performed for a frequency of 3 kHz. According to the equivalent circuit in Fig. 3b, this frequency is located in the region of own relaxation times of the structural elements localized in the volume of the nanoparticle (chain R1C1).
The obtained dependencies are approximated by an exponential function:
where η is the relative humidity 0 < η < 1, ηmax = 1.
In this system, processes with a strong dependence on changes in the number of adsorbed molecules are implemented during adsorption.
The generalized equation for the full impedance, taking into account the thermal and adsorption components (Introduction to the temperature-humidity relationship 2020), is written as
where Z0 is the volume resistance at high temperatures and humidity; Ea1 is the temperature component of activation energy; R is the universal gas constant; T is the temperature; Ea2 is the activation component depending on humidity.
Such a representation is within the framework of the theory of an activated complex (Glasstone et al. 1941). The tangent of the angle of inclination of the straight line to the positive direction of the x-axis on the graph lnZ = f (η)−1 allows, up to a constant, to calculate the activation component of the processes initiated in the system by adsorbed molecules. Changes in the total impedance of the system Z and its components R and XC are written as
where XC1, R1, and Z1 are the impedance values, respectively, of the volume of nanoparticles, dispersion medium, and total impedance of the system at η = 1.
Using formula in Doroshkevych et al. (2012), we proceed to the description of the activation parameter of the ith component of conductivity:
where l is the tablet thickness, S is the tablet cross-sectional area, and Ri is the resistance of the ith component.
For conductivity curves, the angle tangent will be negative, the and exponential coefficients in this case will be α' = − α, β' = − β, γ' = − γ. By equalizing the coefficients at the exponentials in Eqs. (10)–(12), we obtain the dependences on humidity for the activation parameter of the physical processes initiated by adsorption for the system under investigation: Eaz, EaR, EaXc in terms of kT (13)–(15):
The dependences of the activation parameter of conductivity on humidity are shown in Fig. 6. The activation of processes initiated by adsorption decreases according to a hyperbolic law during humidity increasing.
The total activation parameter of physical processes initiated by adsorption in a material at a temperature of 300 K is an order of magnitude less than the activation energy of thermal diffusion conductivity in bulk materials based on ZrO2 (0.6–0.8 eV) (Kiselev and Krylov 1979; Zavodinsky 2004; Glinchuk and Bykov 2006). The main contribution to conductivity is made by diffusion processes in the space between nanoparticles. The impedance of the ionic atmosphere is an order of magnitude less than the impedance of the material in the volume of nanoparticles, which is proportional to the activation parameter of conductivity initiated by adsorption; the activation parameter of conductivity initiated by adsorption in the volume of nanoparticles Eax is 1.8–1.9 times greater than the activation parameter of conductivity initiated by adsorption of the dispersion medium EaR. Adsorption is an external physicochemical factor affecting the surface of a solid in a nanoscale state, significantly reducing the activation energy of physical processes in it that increase the electrical conductivity.
Conclusions
The electrical properties of nanopowder based on zirconia in a compacted state under conditions of adsorption of atmospheric moisture were investigated by the method of EIS. It is shown that moisture adsorption of almost an order of magnitude reduces the activation of electrical conductivity both in the volume of nanoparticles and in interparticle space. Adsorption should be taken into account at calculating the activation energy of physical and chemical processes occurring on a surface of oxide nanoparticles.
Based on the analysis of the dynamics of increase in conductivity with increasing of degree of hydration of the system, it concluded that the ZrO2 crystal has hole conductivity. A decrease in the active resistance R indicates an increase in the concentration of free charge carriers in the space between the particles and, as a result, on the surface of the particles. The magnitude of the reactive resistance of XC is inversely proportional to the concentration of free charge carriers, but in the volume of nanoparticles near the surface. A simultaneous decrease in the active resistance on the side of the adsorption layer and on the reactive side, on the side of the nanoparticle volume, indicates an increase in the concentration of free charge carriers on both sides of the heterophase boundary. Based on the difference work function material heterojunction ΔA, the volume of material near the surface of the nanoparticles is positively charged, thus, increasing the concentration during adsorption of own free charge carriers and the carrier holes.
Abbreviations
- HHP:
-
High hydrostatic pressure
- EIS:
-
Electrochemical impedance spectroscopy
- CPE:
-
Constant phase element
- SCR:
-
Space charge region
References
Abdalaa P, Custob G, Lamasa D (2010) Enhanced ionic transport in grained scandia-stabilized zirconia ceramics. J Power Sour 195:3402–3406
Agamalov YuZ, Bobylev DF, Kneller VY (2004) Virtual meters-analyzers of impedance parameters. Sens Syst 5:14–18
Agamalov Y, Bobylev D, Kneller V (1993) PC-based instrument for impedance measurement. In: Proc. 4 IMECO TC-4 Symp. BEMECO, IBRA-BIRA, Brussels, pp 405–412
Alekseenko VI, Volkova GK (2000) Adsorption mechanism of phase transformation of stabilized zirconium dioxide. J Tech Phys 70:57–62
Blumenthal WB (1958) The chemical behavior of zirconium. Princeton, New Jersey
Boukamp B (2004) Electrochemical impedance spectroscopy in solid state ionics: recent advances. Solid State Ion 169:65–73
Ciuparu D, Ensuque A, Shafeev G, Bozon-Verduraz F (2000) Synthesis and apparent bandgap of nanophase zirconia. J Mater Sc Lett 19:931–933
Doroshkevich AS, Askerov EB, Lyubchyk AI, Shylo AV (2019) Direct transformation of the energy of adsorption of water molecules in electricity on the surface of zirconia nanoparticles. Appl Nanosci. https://doi.org/10.1007/s13204-019-00979-6
Doroshkevich AS, Logunov AI, Shylo AV, Lyubchyk AI (2017b) Integrated research of HHP-sealed nanostructured system based on ZrO2 in the aspect of sensor applications. High-Press Phys Technol 27(3):18–31
Doroshkevich AS, Askerov EB, Nabiev AA (2017) Electrical properties of hydrated nanopowder systems based on zirconia. In: Transactions of a scientific seminar in memory of I.L. Khodakovsky, Dubna, pp 70–75
Doroshkevych OS, Shylo AV, Saprukina OV, Danilenko IA, Konstantinova TE, Ahkozov LA (2012) Impedance spectroscopy of concentrated zirconia nanopowder dispersed systems experimental technique. World J Condens Matter Phys 2:1–9
EIS Spectrum Analyser (2020). https://www.abc.chemistry.bsu.by/vi/analyser/
Efremov IF (1971) Periodic colloidal structures. The Chemistry, Leningrad
Fleig J (2002) The grain boundary impedance of random microstructures: numerical simulations and implications for the analysis of experimental data. Solid State Ion 150:181–193
Foster AS, Sulimov VB, Lopez Gejo F, Shluger AL, Nieminen RM (2001) Phys Rev B 64:224108
Gao P, Zhang C, Wen G (2015) Equivalent circuit. J Power Sour 294:67–74
Glasstone S, Laidler KJ, Eyring H (1941) The theory of rate processes. McGraw-Hill Book Co., New York
Glinchuk MD, Bykov PI (2006) Hilcher B (2006) Features of the ionic conductivity of oxygen in oxide nanoceramics. Phys Solid State 48:2079–2084
Introduction to the temperature-humidity relationship (2020). http://www.weibull.com/hotwire/issue50/relbasics50.htm
Jorcina J, Orazemb M, Peґbèrea N, Tribolletc B (2006) CPE analysis by local electrochemical impedance spectroscopy. Electrochem Acta 51:1473–1479
Kiselev VF, Krylov OV (1979) Electronic phenomena in adsorption and catalysis on semiconductors and dielectrics. The Science, Moscow
Konstantinova TE, Danilenko IA, Glazunova VA, Volkova GK, Gorban OA (2011) J Nanopart Res 13:4015
Kuyyadi PB, Mahaveer KJ (2008) Sol–gel derived TiO2:ZrO2 multilayer thin films for humidity sensing application. Sens Actuators B 128:407–413
Lyubchyk A, Águas H, Fortunato E, Martins R (2017) Experimental evidence for chemo-electronic conversion of water adsorption on the surface of nanosized yttria-stabilized zirconia. In: Proceedings of international conference nanomeeting physics, chemistry and application of nanostructures, pp 257–263. https://doi.org/10.1142/9789813224537_0059
Macdonald JR (1987) Impedance spectroscopy: emphasizes solid materials and systems. Wiley, New York
Meng F, Hou N, Jin Z, Sun B, Li W, Xiao X, Wang C (2015) Li M (2015) Sub-ppb detection of acetone using Au-modified flower-like hierarchical ZnO structures. Sens Actuators B Chem 219:209–217
Pajkossy T (2005) Impedance spectroscopy at the interface of the aqueous and aqueous solutions—surface roughness, CPE and related issues. Solid State Ion 176:1997–2003
Strekalovsky VN, Polezhaev YuM, Palguyev SF (1987) Oxides with impurity disorder. Nauka, Moscow
Subhoni M, Kholmurodov K, Doroshkevich A, Askerov E, Yamamoto T (2018) Density functional theory calculations of the water interactions with ZrO2 nanoparticles Y2O3 doped. J Phys Conf Ser 994:012013. https://doi.org/10.1088/1742-6596/994/1/012013
Tang H, Thomas M (2015) Introduction to plasmas and plasma dynamics. magnetic fusion and space physics. Academic Press, New York
Vasiliev AA (2012) Malyshev VV (2012) Sensors for the ultra-fast monitoring of explosive gas concentrations. Procedia Eng 47:224–227. https://doi.org/10.1016/j.proeng.2012.09.124
Wolkenstein F (1963) The electron theory of catalysis. Mc Millan, New York
Zavodinsky (2004) About the mechanism of ionic conductivity in stabilized cubic zirconia. Phys of the Solid State 46:441–445
Zaynullina VN, Zhukov VP (2001) Effect of defect clustering and transport properties of oxide and fluoride ionic conductors with fluorite structure. Quantum Chem Approach Solid State Phys 43:1619–1631
Acknowledgements
The authors are thankful to the H2020-MSCA-RISE-2015 Program project no. 691010 HUNTER, H2020-MSCA-RISE-2019 Program project no. 871284 SSHARE, and RO-JINR Project nos. 323 and 322/15.05.2018 items 70 and 71.
Author information
Authors and Affiliations
Contributions
Artem Shylo conceived and designed the experiments, and wrote the manuscript. Aleksandr Doroshkevich produced the main idea for writing this manuscript and participated in the writing of the manuscript. Andriy Lyubchyk participated in the data analysis in the writing of the manuscript. Yuri Bacherikov participated in the discussion of the article and editing the final version. Maria Balasoiu participated in the writing of the manuscript. Tetyana Konstantinova participated in the data analysis and revised the final edition of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shylo, A., Doroshkevich, A., Lyubchyk, A. et al. Electrophysical properties of hydrated porous dispersed system based on zirconia nanopowders. Appl Nanosci 10, 4395–4402 (2020). https://doi.org/10.1007/s13204-020-01471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01471-2