Abstract
Development of resilient and efficient composite materials that exhibit substantial antimicrobial activity, ample photocatalytic ability and considerable capacity for drug delivery is highly desirable. The present study demonstrates the incorporation of selenium (Se) in transition metals (vanadium-V, niobium-Nb, and tantalum-Ta) via standard solid-state hydrothermal reaction method. Generated nanostructures (V:Se, Nb:Se, Ta:Se) were characterized using various techniques. XRD analysis confirmed the presence of monoclinic phase compounds while crystallite size (~ 13.5–26.2 nm) was calculated using Debye–Scherrer equation. FESEM was used to examine surface morphologies of V:Se (rod-like and disc-shaped) and of Nb and Ta selenides (irregular agglomerated nanospheres). Compositional and functional group analysis was undertaken through FTIR spectroscopy and EDS coupled with SEM, respectively. Raman spectroscopy was carried out to ascertain the bending and stretching vibrational modes in samples. Optical properties were investigated through UV–Vis spectroscopy and the PL emission spectra were acquired to evaluate the charge separation and recombination behavior of compounds. Thermal stability of designed composites was measured using TGA/DSC. Potential uses of the prepared nanoparticles were explored in multiple fields including photocatalysis, antimicrobial and drug delivery systems. Methylene blue dye was degraded under 400–700 nm wavelength in the presence of synthesized wide band gap composites as photocatalysts. The selenium–metal particles were subjected as antimicrobial agents against gram (−ve) and gram (+ve) bacteria and experimental results suggested this material to be an effective antibiotic. Furthermore, synthesized nanocomposites were examined as carrier of anticancer drug (DOX) and their drug delivery profile was also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In comparison with long and eminent history of the study of metal sulfides, research on metal selenide and telluride chemistry did not attract worldwide attention until the last decade of nineteenth century (Roof and Kolis 1993; Coucouvanis et al. 1989). This was due to the unavailability of convenient synthetic routes required to produce heavy metal chalcogenides compared to metal sulfides. However, with the advancement in synthesis methodologies, chemistry of transition metal selenides and tellurides has improved at a rapid pace. Selenium, a nutritional element in human body, is found in a number of proteins (DuBois 1989; Stadtman 1990). Selenide compounds of different transition metals (e.g., ZnSe, FeSe2, HgSe, CuSe, Cu3Se2, CdSe, and MoSe2) have fascinated researchers owing to their significant potential for use in industrial applications, such as semiconductors, IR detection, laser diodes, photovoltaic (Bube 1990) electronics, catalysis, energy storage devices, medical diagnostics, photodetective devices and photocatalysis (Ivanova et al. 2019; Grayfer et al. 2017; Yousefi 2014).
Rapid industrialization and population growth are the major causes of energy shortage and environmental contamination, two of the most critical global challenges that exist nowadays. Synthetic dyes are used extensively in plastic, textile, leather, paper, glass, food and pharmaceutical industries and release non-degradable organic compounds, which have adverse effects on the environment and cause serious health risks due to its toxicity (Saleem et al. 2018; Reza and Kurny 2017). To remove dye effluents, various methodologies, such as coagulation, ultrafiltration, adsorption, biodegradation, photochemical and electrochemical treatment, have been widely adopted (Ghosh et al. 2016; Ren et al. 2014). Among these, one of the most feasible techniques is photocatalysis which completely degrades various dyes in a safe manner (Kruefu 2017; Wan et al. 2013). Many transition metal selenides (where transition metal is V, Nb, Ta and chalcogen = S, Se and Te) with wide band gap are active photocatalysts due to high electrical conductivity provided by these metals (Ulusoy Ghobadi et al. 2017). VSe2 carries high charge density (Yadav and Rastogi 2010) and is reported in the literature as a potential candidate for photocatalysis (He et al. 2017), supercapacitors (Marri et al. 2017), and lithium-ion and sodium-ion batteries (Yang and Zhang 2017).
In the medical field, many attempts have been made to prepare metal-chalcogenide antibacterial agents in an effort to overcome the serious problem of drug-resistant bacteria (Shahmoradi 2019). To assess the antibacterial activity, numerous bioassays, such as disc diffusion, broth dilution and well diffusion, are generally used. However, disc diffusion scheme has proved superior to others owing to its simplicity, low cost, ability to test a number of microbes and antimicrobial agents and easy interpretation of results (Balouiri 2016). Metal-Se system has a broad range of biological applications, such as in silver- and gold-based nanoagents, as they have been widely reported (Ahmad et al. 2020; Galdiero et al. 2011). Similarly cancer, a complicated disease of various types, is still one of the most prevalent health problems at a global level (Naz et al. 2018; Fan et al. 2016). Although a number of cancer therapies, such as surgery, radiotherapy, immunotherapy, chemotherapy and photodynamic therapy (Afzal et al. 2020; Deng and Zhang 2013; Cao et al. 2012), have been developed, its treatment is still a big challenge for researchers and medical experts. Limitations of chemotherapy involve non-targeted distribution of drug, incapability to differentiate between healthy and cancerous cells and poor control over drug release (Nafees et al. 2019). Therefore, utmost caution is required during targeted delivery of chemotherapeutic drugs to the affected tumor cells. Use of nanomaterials as a drug carrier is a relatively safe, more efficient and convenient method for drug delivery (Afzal et al. 2020; Fan et al. 2018) because nanoparticles show better thermal, electrical, magnetic and optical properties compared to the bulk system.
In this work, binary transition metal selenides (V3Se4), (Nb2Se3, Nb2Se9) and (TaSe3, Ta2Se3) were prepared through highly effective, simple and low-cost solid state hydrothermal method. Isolated products were fully characterized via elemental analysis, FTIR, Raman, UV–Vis spectroscopy and TGA. Moreover, materials were investigated for photocatalytic response, antimicrobial activity and besides this, nanocomposites were loaded with anticancer drug [Doxorubicin (Fig. 1)] and their drug release profile, in vitro, was also explored.
Experimental part
Chemicals
Vanadium pentoxide, V2O5 (> 99.5%), niobium pentoxide, Nb2O5 (99.99%), tantalum pentoxide, Ta2O5 (99%), Selenium, Se (≥ 99.5%), nitric acid, HNO3 (65%) and methylene blue (MB) were purchased from Sigma-Aldrich. Hydrochloric acid, HCl (37%) was obtained from Riedel–de Haen. Nutrient agar was acquired from Merck, while anticancer doxorubicin HCl (DOX) injection [Novartis Pharma (Pakistan) Limited, Karachi, 74000] was used for drug loading.
Synthesis of binary metal selenides
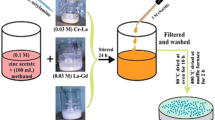
The process conditions and composition of binary selenides of transition metals (V, Nb and Ta) formed via standard solid state hydrothermal synthesis are summarized in Table 1. Precursor metal oxides M2O5: V2O5 (m.p: 690 °C), Nb2O5 (m.p: 1512 °C) and Ta2O5 (m.p: 1872 °C) were mixed with chalcogen selenium Se powder (m.p: 221 °C) in various stoichiometric ratios. To ensure the best reaction activity and homogeneity, mixture was ground thoroughly with pestle and mortar. Excess HCl was added as a solvent to provide reaction medium. The resultant mixture was placed in a muffle furnace to heat at 550 °C for 48 h (at 50 °C/h) and was subsequently cooled to room temperature at 10 °C/h before taking it out of the furnace. Schematic diagram of solid state reaction is shown in Fig. 2.
Photocatalytic activity process
The photocatalytic activity of prepared binary compounds (transition metal selenides) was studied by monitoring the degradation rate of methylene blue (10 mg/l) in aqueous solution. Stock solution of MB (5 mg/500 ml) was placed in dark for 30 min. A mercury lamp (400 W) as visible light source was employed in wavelength range of 400–700 nm. Typically, a photocatalyst (10 mg) was added in 60 ml MB solution and exposed to Hg lamp under vigorous stirring. The mixture was stirred in dark (5 min) to attain equilibrium between methylene blue and synthesized composite before illumination. After specific time intervals (20 min) 5 ml suspension was withdrawn for recording UV–Vis absorption to evaluate dye concentration. Gradual decrease in the concentration of dye at λmax = 665 nm represents the photodegradation efficiency of nanoparticles (NPs) as shown in Eq. 1

Antimicrobial activity
Disc diffusion assay was employed to investigate the antimicrobial potential of synthesized products against pathogenic microbial strains (Escherichia coli and Staphylococcus aureus). Sterilized Whatman filter paper was used to prepare the disc. Nutrient agar of certain composition [(g/l): (meat extract-1; peptone-5; yeast extract-2; sodium chloride-5 and agar-20)] was placed in autoclave for 30 min. Bacterial culture (107 cells/ml) was spread over agar plates and different dilutions (1 mg/ml, 0.5 mg/ml and 0.25 mg/ml) were used to evaluate the bactericidal potential of synthesized composites using deionized water (DIW) as negative control. Growth of microorganisms was noted after overnight incubation at 37 °C. The diameter of inhibition zone was measured by meter scale (Image J software). Experiments were repeated thrice to check the reliability and reproducibility of the results.
Drug loading and releasing
Drug loading
Reference solution of DOX in DIW (200 μg/ml) was prepared and mixed with 1 ml dilution of each synthesized product (10 mg/ml) individually. For proper incorporation of medicine into NPs, the said mixtures were subjected to sonication for 30 min. Afterwards, samples were left for 24 h in complete darkness followed by ultra-centrifugation at 15,000 rpm. To evaluate the accumulation of doxorubicin into nanomaterials (loading efficiency), supernatant was collected and characterized by measuring absorbance (at 480 nm). Loading efficiency (LE) and loading capacity (LC) of drug were calculated by Eqs. (2 and 3), respectively (Afzal et al. 2020)
where DOXi and DOXf are the initial concentrations (μg) of the drug and the unbound drug remained in supernatant, respectively, and Drugcarrier is the total amount (mg) of nanoparticles.
Drug release behavior
To investigate drug release behavior, the acquired centrifuged pellet of DOX loaded on nanomaterials was added in 100 ml buffer solution (pH = 7). Upon moderate agitation, drug started to release in buffer solution and concentration response was checked by withdrawing 3 ml of aliquots at various time intervals (1, 2, 3, 4, 5, 6, 24 and 48 h) and analyzed through UV spectrophotometer. Meanwhile, each time 3 ml of fresh buffer solution was added to maintain the level at 100 ml. Percentage of drug release was calculated by following equation (Prasad 2017)
where Dt is the drug released by nanomaterials at given interval while Do is the total amount of drug loaded on nanoparticles.
Characterizations
To ascertain the crystallinity and phase constitution of prepared compounds, X-ray diffraction was performed with PANalytical X’Pert PRO model with Cu Kα radiation (λ = 0.154 nm) for 2θ variation from 20° to 80° at a rate of 2.0°/min with a step size of 0.02°. Information regarding surface morphology, particle size and chemical composition were obtained using JSM-6460LV field emission scanning electron microscope (FESEM) coupled with EDX spectrometer. To detect the presence of functional groups, Fourier transform infrared spectroscopy (FTIR) was used with PerkinElmer spectrometer between 4000 and 400/cm. UV–Vis spectrophotometer (GENESYS 10S) was employed to study the optical characteristics and drug loading and releasing behavior of nanohybrids. Raman studies were supported with a diode laser emitting at 532 nm. Thermal stability of prepared products was determined via differential scanning calorimetry and thermogravimetric analysis (DSC/TGA) using model No. 600 TA instrument. For photocatalytic activity, NPs were subjected to Vis light (400–700 nm) using mercury lamp (400 W) as simulated light source in photocatalytic reactor under vigorous stirring for 5 h. Photoluminescence (PL) measurements of samples were carried out with spectrofluorometer (JASCO, FP-8300).
Results and discussion
XRD data of powdered samples S1, S2 and S3 synthesized by hydrothermal method is presented in Fig. 3. The diffraction peaks (▲) observed at 2θ = (26.3, 34.3, 41.4, 51.4, 55. 6 and 61.2)° correspond to (220), (310), (− 240), (130), (330), (620) planes, respectively. These peaks can be ascribed to monoclinic crystal structure of binary V3Se4 as (JCPDS 01-087-2431) (Gunning et al. 2017). Rest of the peaks (Δ) at (21.6, 26.3, 30.9, 41.4, 47.2 and 51.4)° are for unreacted V2O5 according to JCPDs card (00-041-1426) (Kruefu 2017). XRD pattern of S2 depicts the formation of two binary compounds of Niobium selenides in addition to unreacted Nb2O5 (*) hexagonal crystals with resolved peaks at 2θ = (22.6, 28.1, 36.7 and 56.1)° as reported in JCPDS card No. 28-0317 (Joya and Ortega 2017). Appearance of peaks (■) at 2θ = (28.1, 36. 3, 45.8, 47.3, 50.7, 55.0 and 56.2)° were attributed to (200), (− 203), (104), (− 1 14), (310), (120) and (105) diffraction planes by JCPDS card no. 01-089-2335, which designate the formation of monoclinic phase of Nb2Se3 (Ivanova et al. 2019). Characteristic peaks (□) at 2θ = (22.3, 23.3, 24.8, 28.1, 28.8, 35.0 and 36.3)° are well assigned to (hkl) planes for anorthic phase of Nb2Se9 (JCPDS: 33-0968) (Agyapong-Fordjour et al. 2019). In S3 peaks (●) at 2θ = (28.2, 46.5, 49.6, 55.4, 58.4, 63.6 and 70.5)° due to diffraction along (011), (212), (− 312), (204), (312), (221) and (320) planes can be attributed to monoclinic crystals of Ta2Se3 which matched well with the reference JCPDS file no. 01-089-2336 (Kadijk et al. 1968). Additional peaks (○) are indexed for monoclinic TaSe3 phase according to JCPDS file: 18-1310 (Wu et al. 2011). Moreover, unreacted Ta2O5 (⊕) with diffraction peaks (overlap with other peaks) at = 22.8°, 28.4°, 36.7°, 46.7°, 55.7°, 63.9° are ascribed to orthorhombic phase according to (JCPDS 025-0922) (Krishnaprasanth and Seetha 2018). Crystallite sizes: 26.2, 21.2, and 13.5 nm corresponding to S1, S2 and S3, respectively, were computed by utilizing the Debye–Scherrer formula [D = kλ/β cos θ; where k = 0.9, λ (lambda) = 0.154 nm and β (beta) is full width half maximum of given peak].
The surface morphology and average particle size of samples prepared at 550 °C were scrutinized by FESEM Fig. 4a–c. Micrograph of sample (a) possesses rod-like and disc-shaped microcrystals with a length of 26.2 nm. In the case of b- and c-agglomerated irregular nanospheres with an average diameter of 13–23 nm are observed. It can be seen from insets that the mixture of various phases is observed in samples indicating the formation of different products. Since the samples were ground manually before solid state reaction, it resulted in the formation of non-uniform sized particles, which may be responsible for different reactions in solid state reaction. Further elemental composition was also analyzed by energy-dispersive X-ray spectroscopy (EDX) (Fig. 4d–f). The peaks in EDX spectra confirmed the presence of all constituent elements (V, Nb, Ta and Se) and absence of other distinct peaks except carbon which came from the supporting grid revealed the phase purity of consequent products. Resultant material composition and elemental confirmation based on XRD and EDS, respectively, are summarized in Table 1.
FTIR spectra measurements of S1, S2 and S3 were performed in the range of 400–4000/cm to categorize the functional group and chemical bond of compounds (Fig. 5a). A strong broad band at 3436/cm and 1629/cm correspond to the stretching frequency of O–H group. Significant transmission peaks around 600–759/cm belong to characteristic stretching vibrations of Se–O bond (Sivakumar et al. 2006). Stretching at 1023/cm indicates the terminal Se=O vibrations (S1) (Brisdon and Ogden 1987). Vibrations in the region of 700–900/cm reveal the presence of vanadyl unit attributed to terminal V–O bond in S1 (Sivakumar et al. 2006), NbO4 tetrahedral unit in S2 (Kabalci and Gökçe 2014) and Ta–O–Ta bond in S3 (Chennakesavulu and Reddy 2015). Figure 5b depicts the Raman spectra of prepared composites in the range of 50–1050/cm. Spectra may be described in terms of three bands: one below 400/cm, second between 400 and 830/cm and the third region beyond 830/cm. Raman shifts observed that the above 830/cm are assigned to stretching of terminal Se–O bond. A broad band between 400 and 830/cm is due to Se–O bridging stretching (Brassington et al. 1987). In the case of S1, characteristic bands at 143/cm, 397/cm and 996/cm are attributed to V–O–V, V–O and terminal V=O stretching vibrations, respectively (Abd-Alghafour et al. 2016; Kang et al. 2012). Four major vibrational modes of Nb2O5 octahedron, bending of Nb–O–Nb linkage, stretching of Nb–O bond (Kong et al. 2016; Palatnikov et al. 2012) and NbO6 unit (Gao et al. 2011) are observed at 125, 234, 685 and 985/cm (S2). Spectrum S3 shows band in the region of 200–400/cm due to Ta–O bond vibrations. Absorbance between 400 and 830/cm and at 928/cm corresponds to Ta2O5 moiety and to stretching mode of O=Ta–O–H bond (Chennakesavulu and Ramanjaneya Reddy 2015).
UV–Vis spectroscopy was deployed to investigate the optical characteristics of synthesized materials (Fig. 6a). Spectra displayed characteristic absorption bands in UV region of 285–293 nm accompanied by red shift in S1 and S2. The direct band gap energies can be assessed by plotting a graph between (αhυ)2 vs photon energy (hυ) as shown in Fig. 6b. The x intercept of extra plotted linear fits give the estimated band gap energy 3.87, 3.82 and 3.95 eV for S1, S2 and S3, respectively, and depicts the decrease in band gap energy with absorption in longer wavelength region (Junaid et al. 2019).
The fluorescence emission spectra were performed to illustrate the charge separation behavior and inhibition of recombination of electrons and holes generated as a result of photo irradiation in synthesized nanocomposites as shown in Fig. 7. Peaks located in the range of 300–350 nm are ascribed to the recombination of excitons due to near band-edge emission (NBE) or blue emission. Meanwhile, peak positioned at 500 nm is called green emission or deep level emission (DLE) which indicates the presence of defects, vacancies and interstitial spaces in solids (Polat et al. 2014; Shabannia 2018; Zhu 2006). As all formed products are wide band gap materials, the longer wavelength emission at 500 nm was attributed to shallow vacancy trap level just below the conduction bands of the material (Song and Gao 2007).
To further study the thermal stability and structural integrity of nanocomposites, DSC/TGA was used in air atmosphere from 20 to 1000 °C. Figure 8a–c show the TGA curves of S1, S2 and S3 as a function of temperature. No distinct region for water removal is observed which guarantees the absence of moisture in compounds as a result of high temperature annealing at 550 °C. Weight gain in (a) and (b) is due to oxidation of unreacted Se into selenium dioxide SeO2 (Eq. 5) (House 2008). In Fig. 8a, first degradation step is associated with the melting of V2O5 (m.p: 690 °C) (Lebourgeois et al. 2007). From DSC curve, a sharp endothermic peak confirmed this phase change. The TGA curves for V:Se (a) and Nb:Se (b) reveal weight loss at 750–1000 °C with an endothermic dip at around 750–780 °C in respective DSC plots. Experimental weight loss of 4.5% (S1) and 14% (S2) can be attributed to the release of SeO2 (Vlaev et al. 2007; Cao et al. 2015; Johnston et al. 2010). In Fig. 8c, two steps degradation observed as 1st step reveals the decomposition of low temperature phase of TaSe3 at 250–600 °C into diselenide and selenium as suggested by Kikkawa et al. (1982; Hayashi et al. 1987). Possible reactions are mentioned in Eqs. 6 and 7.
The second step degradation indicates weight loss of 26% from 650 to 1000 °C which can be ascribed to the volatility of elemental Se (Govindraju 2017) as the reported boiling point of Se is 685 °C
The photocatalytic potential of the metal selenide nanocomposite, for the reduction of MB, was conducted under mercury lamp. When metal selenide nanocomposite is illuminated by light, electrons in valence band (VB) excite to the conduction band (CB) creating holes in valence bond. These electrons and holes interact with other molecules in aqueous solution and play important role in photocatalytic process as shown in Fig. 9. The generated hydroxyl ions and superoxide anions significantly reduce methylene blue to leucomethylene blue (Eq. 1). Several semiconductor materials have been reported for their photocatalytic applications. Among them are transition metal oxides such as TiO2, ZnO, CuO, WO3; metal sulfides: Bi2S3, ZnS and Se-doped semiconductors, such as Bi2S3 (Song et al. 2011); mixed metal oxides: Cu2S/TiO2, CuO–SnO2, WO3/ZnO, CdxZn1″XO, ZnInV (ZIV) (Arora 2016). In the present study, a promising approach is adopted to investigate the effect of Se doping into metal oxides. Se doping led to structural changes by narrowing the band gap due to the incorporation of defects within the forbidden gaps which results in the enhancement of photocatalysis process (Talukdar and Dutta 2016). However, metal selenides exhibit significant degradation of dye as a function of time.
The observed dye degradation of prepared samples is shown in Fig. 10a which showed that S1 has higher potential of MB degradation compared to rest of the samples. The % degradation of MB was calculated by the formula given below:
here Co is the initial absorbance and Ct is the absorbance at time t. Percentage degradation of MB was 15, 12.5 and 4% for sample S1, S2 and S3, respectively, in 80 min as shown in Fig. 10b. Pseudo-first order kinetics (Zhang et al. 2014) can be used to estimate the photocatalytic performance of selenium-doped nanocomposite quantitatively; using the following expression
here Co is the concentration of the MB at zero time and k represents rate constant. The Fig. 10c depicts the rate constants achieved from the absorbance curve. It is 0.97257/min for S1, 0.95619/min for S2 and 0.80843/min for S3. Sample S1 demonstrates significant catalytic potential over MB degradation with an increased rate constant.
Performance of photocatalyst depends upon its stability and recycling capacity. The present work also demonstrates the recyclability and constancy of the prepared composites as it is important to plan an efficient photocatalyst that is also cost-effective. The stability of S1 nanocomposite, that showed the best photocatalytic performance among other samples, was investigated by allowing the performed experiment to stay for 48 h. After 48 h, same results were reproduced as degradation was still in its previous state, which indicated the stability of synthesized photocatalyst. To test the reusability of photocatalyst, 3 mg of S1 was recycled by repeating the reduction process four times. Figure 10d shows that photocatalyst is reusable and also sustains the photocatalytic activity for four consecutive cycles. Moreover, the weight of photocatalyst before and after recycling process was measured. A slight weight loss of catalyst ranging from 3 mg (before) to 1.5 mg (after four cycles) was detected including ~ 5% standard deviation. Considering these results, we believe that S1 nanocatalyst is relatively stable during the reactions and is an efficient photocatalyst that can remove organic dyes from water relatively quickly. Further, its stability, reusability and enhanced activity under sunlight adds to lessen the cost of water treatment (Arshad et al. 2019).
The highest photocatalytic activity was observed for Se-doped V2O5, S1 (molar ratio of V:Se was 1:4) photocatalyst due to good crystallinity (as visualized in SEM image) and surface area of sample (Arora 2016). The mechanism of improvement in photocatalytic activity due to Se doping could be understood by considering that doping yields nanostructured composites containing Se+2 ions. Upon the irradiation of Se-doped NPs, Se+2 ions scavenge excitons and inhibit the recombination of electron–hole (e−/h+), as a result promoting photo-oxidation efficiency. However, excess Se2+ ions are responsible for the blockage of many photocatalytic centers and subsequently reduce their activity as was observed in S2 and S3 (e.g., molar ratio of Nb/Ta:Se was greater than S1) (Song et al. 2011).
Disc diffusion method was employed to confirm the bactericidal sensitivity of selenium-doped transition metals as shown in Fig. 11. Inhibition zones recorded for samples S1, S2 and S3 against gram-positive and negative bacterial strains ranged from 1.8 to 31 mm (Table 2). However, maximum zone of inhibition was observed in sample S1 for D4 concentration (1 mg/ml) against E. coli (31 mm) and S. aureus (21 mm). At D3 concentration (0.5 mg/ml), zone of inhibition was only recorded for S. aureus (1.8 mm) in sample S1 and S3. The results of inhibitory activity of synthesized NPs on microorganisms show that in low concentrations, nanoparticles show decreased bactericidal potential. This might be due to the fact that a few nanoparticles were available to directly interact with microbial cell wall; while, D1 and D2 did not affect bacterial growth. Graphs clearly depict that D4 concentration of sample S1 and S3 represent strong antibacterial activity compared to other concentrations as shown in Fig. 11. A number of mechanisms for antimicrobial activity have been proposed; however, exact reason is still not fully understood (Arshad et al. 2017). These mechanisms could be categorized into three classes: (1) physical process, including the adsorption of NPs on the surface of microbe cells; (2) biological phenomena comprising interaction of NPs with bacterial cell wall through ions or via cellular protein; (3) chemical process leading to the formation of reactive species or due to the release of toxic ionic species from NPs (Dutta 2014). In this study, opposite charges present on synthesized compounds (metal cation) and bacterial cell wall (anion) might be the possible reason for significant antimicrobial capability demonstrated by the samples (Stoimenov et al. 2002). Se-doped metal oxide NPs did not exhibit complete killing of bacteria. This reduced bactericidal effect is attributed to the additional micronutrient ability of Se for bacterial growth (Dutta 2014).
Compounds containing transition metal are promising candidates and are extensively studied for drug delivery owning to their reversible redox properties and charged state (Yan et al. 2016; Staff et al. 2012). DOX incorporation onto NPs depends upon the neural form of drug penetration through lipid bilayer, where it meets the acidic environment and becomes protonated, charged species, and thus remains bound with drug carrier. It is suggested that transition metal interacts with DOX as shown in Eq. 10
In aqueous medium, transition metal cations exist as complex hydrated ions as determined in Eq. 11:
The hydrated metal cation behaves as weak acid and dissociate as depicted in Eq. 12:
M(H2O)5(OH)+1 ion probably coordinates with the hydroxyl group of DOX at C-11 or C-6 (Fig. 1) thus promoting the retention of drug in nanocomposites (Abraham et al. 2002). Observed UV–Vis spectra (Fig. 12a) for the incorporation of DOX into synthesized composites showed the maximum loading of medicine in S1 and least in S3 over a period of 24 h. Figure 12b depicts the drug releasing profile in a neutral medium (pH = 7). Cumulative drug release % for S2 is double (30%) as compared to S1 (15%) and only 5% drug is released by S3 after 6 h. After 48 h, percentage of drug release was found to be 68%, 17% and only 7–8% for S2, S1 and S3, respectively. Partial detachment of metal–DOX interaction results in the release of medicine from loaded NPs. Thus, it may be interpreted that S1 (V:Se) behaves as an efficient drug carrier due to maximum loading and S2 showed maximum release rate compared to other samples. Loading efficiency (LE) calculated for S1, S2 and S3 was found to be 96.4, 15.7 and 12%, respectively. LC value (4 μg/mg) for S1, (2.4 μg/mg) for S2 and (1.4 μg/mg) for S3 are very small compared to LE (Fig. 12c) since very small weight ratio of DOX to nanoparticles was used.
Conclusions
In the present study, selenides of transition metals (V, Nb, Ta) were successfully synthesized by adopting solid state hydrothermal route. XRD patterns revealed monoclinic crystal structure with average crystallite size (~ 13–26 nm) showing rod-like and disc-shaped morphology of vanadium selenide and agglomerated nanospheres of Nb and Ta selenides as examined by FESEM. One of the reasons why uniform nanoparticle distribution, unique materials composition and effective interaction of V–Se, Nb–Se, Ta–Se and Se–O was observed with SEM, XRD and FTIR/Raman, respectively, is due to the increased degree of mixing of the V/Nb/Ta–O and Se–Se domains at the molecular level during hydrothermal reaction, which enhances the formation of composites. The calculated bandgap energies 3.87, 3.82 and 3.95 eV revealed the products as wide band gap semiconductors and promising photocatalysts under light irradiation. The PL spectra show the capability of NPs, exhibiting enhanced charge separation and suppression in electron hole recombination rate. Thermal stability was assessed via DSC–TGA measurements and all compounds were found stable at high temperature. Furthermore, prepared composites showed significant antimicrobial activity with 21–31 mm and 23–24 mm inhibition zones at 1 mg/ml concentration, respectively, indicating their suitable performance as an antibiotic. Besides this, NPs were loaded to anticancer drug (DOX) to test their loading and releasing efficacy. Loading efficiencies, 96.4% of S1, 15.7% of S2 and 12% of S3 were observed. Releasing rate showed reverse behavior from loading efficiency as S1 attained moderate release rate, e.g., only 15% in 48 h. On the other hand, S3 released about 68–70% drug in the same span of time. On the bases of our findings it is believed that metal (V/Nb/Ta) selenides are a class of compounds with significant applicability in photocatalysis, drug delivery systems and antimicrobial effectiveness.
References
Abd-Alghafour NM, Ahmed NM, Hassan Z (2016) Fabrication and characterization of V2O5 nanorods based metal–semiconductor–metal photodetector. Sens Actuators A Phys 250:250–257
Abraham SA, Edwards K, Karlsson G, MacIntosh S, Mayer LD, McKenzie C, Bally MB (2002) Formation of transition metal–doxorubicin complexes inside liposomes. Biochim Biophys Acta (BBA) Biomemb 1565(1):41–54
Afzal H, Ikram M, Ali S, Shahzadi A, Aqeel M, Haider A, Imran M, Ali S (2020) Enhanced drug efficiency of doped ZnO–GO (graphene oxide) nanocomposites, a new gateway in drug delivery systems (DDSs). Mater Res Express 7:015405
Agyapong-Fordjour FO, Oh S, Lee J, Chae S, Choi KH, Choi SH, Boandoh S et al (2019) One-dimensional single-chain Nb2Se9 as efficient electrocatalyst for hydrogen evolution reaction. ACS Appl Energy Mater 2(8):5785–5792
Ahmad W, Shams S, Ahmad A, Wei Y, Yuan Q, Khan AU, Khan MS, Rahman AU, Iqbal M (2020) Synthesis of selenium–silver nanostructures with enhanced antibacterial, photocatalytic and antioxidant activities. Appl Nanosci 10:1191–1204. https://doi.org/10.1007/s13204-019-01213-z
Arora AK, Jaswal VS, Singh K, Singh R (2016) Applications of metal/mixed metal oxides as photocatalyst (a review). Orient J Chem 32(4):2035
Arshad A, Iqbal J, Mansoor Q (2017) NiO-nanoflakes grafted graphene: an excellent photocatalyst and a novel nanomaterial for achieving complete pathogen control. Nanoscale 9(42):16321–16328
Arshad A, Iqbal J, Mansoor Q (2019) Graphene/Fe3O4 nanocomposite: solar light driven Fenton like reaction for decontamination of water and inhibition of bacterial growth. Appl Surf Sci 474:57–65
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6(2):71–79
Brassington NJ, Edwards HGM, Fawcett V (1987) The vibrational Raman spectra of selenium trioxide ag. Spectrochim Acta Part A 43(3):451–454
Brisdon AK, Ogden JS (1987) Matrix isolation and mass spectrometric studies on selenium trioxide vapour: the characterization of monomeric SeO3. J Mol Struct 157(1–3):141–153
Bube RH (1990) Materials for photovoltaics. Annu Rev Mater Sci 20(1):19–50
Cao H, Phan H, Yang L-X (2012) Improved chemotherapy for hepatocellular carcinoma. Anticancer Res 32(4):1379–1386
Cao X-L, Chun-Li Hu, Kong F, Mao J-G (2015) Explorations of new SHG materials in the alkali-metal–Nb5+–selenite system. Inorg Chem 54(22):10978–10984
Chennakesavulu K, Reddy GR (2015) Synthesis and characterization of carbon microtube/tantalum oxide composites and their photocatalytic activity under visible irradiation. RSC Adv 5(69):56391–56400
Coucouvanis D, Toupadakis A, Koo S-M, Hadjikyriacou A (1989) An inorganic functional group approach to the systematic synthesis and reactivity studies of binuclear Mo/S and Mo/S/O complexes. Polyhedron 8(13–14):1705–1716
Deng Y, Zhang H (2013) The synergistic effect and mechanism of doxorubicin-ZnO nanocomplexes as a multimodal agent integrating diverse anticancer therapeutics. Int J Nanomed 8:1835
DuBois MR (1989) Catalytic applications of transition-metal complexes with sulfide ligands. Chem Rev 89(1):1–9
Dutta RK, Nenavathu BP, Talukdar S (2014) Anomalous antibacterial activity and dye degradation by selenium doped ZnO nanoparticles. Colloids Surf B Biointerf 114:218–224
Fan Li, Jin B, Zhang S, Song C, Li Q (2016) Stimuli-free programmable drug release for combination chemo-therapy. Nanoscale 8(25):12553–12559
Fan J, Wang S, Sun W, Guo S, Kang Y, Jianjun Du, Peng X (2018) Anticancer drug delivery systems based on inorganic nanocarriers with fluorescent tracers. AIChE J 64(3):835–859
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M (2011) Silver nanoparticles as potential antiviral agents. Molecules 16(10):8894–8918
Gao B, Jijiang Fu, Huo K, Zhang W, Xie Y, Chu PK (2011) Quasi-aligned Ag–Nb2O5 nanobelt arrays with enhanced photocatalytic and antibacterial activities. J Am Ceram Soc 94(8):2330–2338
Ghosh A, Kulsi C, Banerjee D, Mondal A (2016) Galvanic synthesis of Cu2−XSe thin films and their photocatalytic and thermoelectric properties. Appl Surf Sci 369:525–534
Govindraju SJ (2017) Synthesis and characterization of hybrid nanocomposites using polyvinylcarbazole and metal selenides to demonstrate photovoltaic properties. PhD diss.
Grayfer ED, Kozlova MN, Fedorov VE (2017) Colloidal 2D nanosheets of MoS2 and other transition metal dichalcogenides through liquid-phase exfoliation. Adv Coll Interface Sci 245:40–61
Gunning NS, Dankwort T, Falmbigl M, Ross U, Mitchson G, Hamann DM, Lotnyk A, Kienle L, Johnson DC (2017) Expanding the concept of van der waals heterostructures to interwoven 3D structures. Chem Mater 29(19):8292–8298
Hayashi K, Kawamura A, Komai K (1987) Control of phase transition in TaSe3. Mater Res Bull 22(10):1341–1345
He S, Lin H, Qin L, Mao Z, He H, Li Y, Li Q (2017) Synthesis, stability, and intrinsic photocatalytic properties of vanadium diselenide. J Mater Chem A 5(5):2163–2171
House JE (2008) Inorganic chemistry. Academic Press, Berlin (ISBN 978-0-12-356786-4)
Ivanova MN, Enyashin AN, Grayfer ED, Fedorov VE (2019) Theoretical and experimental comparative study of the stability and phase transformations of sesquichalcogenides M2Q3 (M = Nb, Mo; Q = S, Se). Phys Chem Chem Phys 21(3):1454–1463
Johnston KE, Tang CC, Parker JE, Knight KS, Lightfoot P, Ashbrook SE (2010) The polar phase of NaNbO3: a combined study by powder diffraction, solid-state NMR, and first-principles calculations. J Am Chem Soc 132(25):8732–8746
Joya MR, Barba Ortega JJ, Raba Paez AM, da Silva Filho JG, Cavalcante Freire PT (2017) Synthesis and characterization of nano-particles of niobium pentoxide with orthorhombic symmetry. Metals 7(4):142
Junaid M, Imran M, Ikram M, Naz M, Aqeel M, Afzal H, Majeed H, Ali S (2019) The study of Fe-doped CdS nanoparticle-assisted photocatalytic degradation of organic dye in wastewater. Appl Nanosci 9:1593–1602. https://doi.org/10.1007/s13204-018-0933-3
Kabalci I, Gökçe H (2014) Investigation of infrared and Raman spectra of TeO2Nb2O5TiO2 glasses. Acta Phys Pol A 4(125):877–881
Kadijk F, Huisman R, Jellinek F (1968) The crystal structures of niobium (III) selenide and tantalum (III) selenide. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem 24(8):1102–1106
Kang M, Oh E, Kim I, Kim SW, Ryu J-W, Kim Y-G (2012) Optical characteristics of amorphous V2O5 thin films colored by an excimer laser. Curr Appl Phys 12(2):489–493
Kikkawa S, Shinya K, Koizumi M (1982) The selenide systems of niobium and tantalum. J Solid State Chem 41(3):323–328
Kong L, Zhang C, Wang J, Qiao W, Ling L, Long D (2016) Nanoarchitectured Nb2O5 hollow, Nb2O5@ carbon and NbO2@ carbon core-shell microspheres for ultrahigh-rate intercalation pseudocapacitors. Sci Rep 6:21177
Krishnaprasanth A, Seetha M (2018) Solvent free synthesis of Ta2O5 nanoparticles and their photocatalytic properties. AIP Adv 8(5):055017
Kruefu V, Sintuya H, Pookmanee P, Phanichphant S (2017) Visible light photocatalytic degradation of methylene blue using V2O5 nanoparticles. In: 6th international conference on developments in engineering and technology (ICDET-2017), vol 10
Lebourgeois R, Duguey S, Ganne J-P, Heintz J-M (2007) Influence of V2O5 on the magnetic properties of nickel–zinc–copper ferrites. J Magn Magn Mater 312(2):328–330
Marri SR, Ratha S, Rout CS, Behera JN (2017) 3D cuboidal vanadium diselenide embedded reduced graphene oxide hybrid structures with enhanced supercapacitor properties. Chem Commun 53(1):228–231
Nafees M, Butt AR, Ali S, Haider A, Butt MR, Shamoon M, Haider J, Shahzadi I, Ijaz M, Ikram M (2019) Metal oxide nanoparticles for cellular response, anti-cancer drugs loading and adsorption kinetics. Nanosci Nanotechnol Lett 11(4):470–479
Naz M, Qureshi MZ, Shahbaz A, Haider A, Ikram M, Nafees M, Shahzadi A et al (2018) Bio-inspired synthesis of silver nanoparticles: anticancer drug carrier, catalytic and bactericidal potential. Nanosci Nanotechnol Lett 10(7):889–899
Palatnikov M, Shcherbina O, Sidorov N, Bormanis K (2012) The structure of niobium and tantalum oxides processed by concentrated light flux. Ukrain J Phys Opt 13(4):207–214
Polat İ, Yılmaz S, Altın İ, Bacaksız E, Sökmen M (2014) The influence of Cu-doping on structural, optical and photocatalytic properties of ZnO nanorods. Mater Chem Phys 148(3):528–532
Prasad D (2017) Nanoparticulate drug delivery systems, in vitro drug release test methods. Int J Pharm Bio Sci 8:103–119
Ren Lu, Li Y, Hou J, Zhao X, Pan C (2014) Preparation and enhanced photocatalytic activity of TiO2 nanocrystals with internal pores. ACS Appl Mater Interfaces 6(3):1608–1615
Reza KM, Kurny ASW, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7(4):1569–1578
Roof LC, Kolis JW (1993) New developments in the coordination chemistry of inorganic selenide and telluride ligands. Chem Rev 93(3):1037–1080
Saleem A, Imran M, Shahzadi A, Junaid M, Majeed H, Rafiq A, Shahzadi I, Ikram M, Naz M, Ali S (2018) Drastic improvement in catalytic, optical and visible-light photocatalytic behavior of cobalt and nickel doped TiO2 nanopowder. Mater Res Express 6(1):015003
Shabannia R (2018) Structural, optical and electrical properties of Cu-doped ZnO nanorods grown on flexible polyethylene terephthalate substrate. Iran J Sci Technol Trans A Sci 42(4):2349–2353
Shahmoradi Y, Souri D, Khorshidi M (2019) Glass-ceramic nanoparticles in the Ag2O–TeO2–V2O5 system: antibacterial and bactericidal potential, their structural and extended XRD analysis by using Williamson–Smallman approach. Ceram Int 45(5):6459–6466
Sivakumar T, Ok KM, Halasyamani PS (2006) Synthesis, structure, and characterization of novel two-and three-dimensional vanadates: Ba2.5(VO2)3(SeO3)4·H2O and La(VO2)3(TeO6) ·3H2O. Inorg Chem 45(9):3602–3605
Song X, Gao L (2007) Synthesis, characterization, and optical properties of well-defined N-doped, hollow silica/titania hybrid microspheres. Langmuir 23(23):11850–11856
Song L, Chen C, Zhang S (2011) Preparation and photocatalytic activity of visible light-sensitive selenium-doped bismuth sulfide. Powder Technol 207(1–3):170–174
Stadtman TC (1990) Selenium biochemistry. Annu Rev Biochem 59(1):111–127
Staff RH, Gallei M, Mazurowski M, Rehahn M, Berger R, Landfester K, Crespy D (2012) Patchy nanocapsules of poly (vinylferrocene)-based block copolymers for redox-responsive release. ACS Nano 6(10):9042–9049
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18(17):6679–6686
Talukdar S, Dutta RK (2016) A mechanistic approach for superoxide radicals and singlet oxygen mediated enhanced photocatalytic dye degradation by selenium doped ZnS nanoparticles. RSC Adv 6(2):928–936
Ulusoy Ghobadi TG, Patil B, Karadas F, Okyay AK, Yilmaz E (2017) Catalytic properties of vanadium diselenide: a comprehensive study on its electrocatalytic performance in alkaline, neutral, and acidic media. ACS Omega 2(11):8319–8329
Vlaev LT, Georgieva VG, Genieva SD (2007) Products and kinetics of non-isothermal decomposition of vanadium (IV) oxide compounds. J Therm Anal Calorim 88(3):805–812
Wan X, Yuan M, Tie S-L, Lan S (2013) Effects of catalyst characters on the photocatalytic activity and process of NiO nanoparticles in the degradation of methylene blue. Appl Surf Sci 277:40–46
Wu X, Tao Y, Li L, Zhai T, Bando Y, Golberg D (2011) Preparation and field-emission of TaSe2 nanobelt quasi-arrays, and electrical transport of its individual nanobelt. J Nanosci Nanotechnol 11(11):10123–10129
Yadav CS, Rastogi AK (2010) Electronic transport and specific heat of 1T-V Se2. Solid State Commun 150(13–14):648–651
Yan Yi, Zhang J, Ren L, Tang C (2016) Metal-containing and related polymers for biomedical applications. Chem Soc Rev 45(19):5232–5263
Yang X, Zhang Z (2017) Carbon-coated vanadium selenide as anode for lithium-ion batteries and sodium-ion batteries with enhanced electrochemical performance. Mater Lett 189:152–155
Yousefi R (2014) Metal-selenide nanostructures: growth and properties. Metal Chalcogenide Nanostruct Renew Energy Appl. https://doi.org/10.1002/9781119008934.ch4
Zhang L, Li H, Liu Y, Tian Z, Yang B, Sun Z, Yan S (2014) Adsorption-photocatalytic degradation of methyl orange over a facile one-step hydrothermally synthesized TiO2/ZnO–NH2–RGO nanocomposite. RSC Adv 4:48703–48711
Zhu H, Zheng Z, Gao X, Huang Y, Yan Z, Zou J, Yin H et al (2006) Structural evolution in a hydrothermal reaction between Nb2O5 and NaOH solution: from Nb2O5 grains to microporous Na2Nb2O6.2/3H2O fibers and NaNbO3 cubes. J Am Chem Soc 128(7):2373–2384
Acknowledgements
Authors are obliged to the higher education commission (HEC) Pakistan, SRGP-21-1669 and for financial support through indigenous PhD fellowship program. For the provision of experimental lab, we pay our gratitude to PCSIR Labs, complex Lahore. Support provided by the Research Institute, King Fahd University of Petroleum & Minerals, Dhahran, is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest were declared by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altaf, S., Ajaz, H., Imran, M. et al. Synthesis and characterization of binary selenides of transition metals to investigate its photocatalytic, antimicrobial and anticancer efficacy. Appl Nanosci 10, 2113–2127 (2020). https://doi.org/10.1007/s13204-020-01350-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-020-01350-w