Abstract
The ability of fullerene to form stable π → π-complexes was studied by quantum-chemical methods and the fluorescence quenching of reference dye (thiochrome). The correlation between the theoretically calculated and experimentally obtained energy of the π → π-complexes was determined. The energy of the stack interaction between fullerene and some biological active compounds, derivatives of 1,3-oxazoles, containing donor/acceptor substituents was estimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical and chemical, biological and pharmacological properties of fullerenes as nanoparticles have found applications in the clinical practice, in particular due to their antioxidant properties, possibility to inactivate free radicals, reduce oxidative stress, lipid peroxidation and neuronal membrane destruction (Li et al. 2008; Jain 2005; Kovtun et al. 2007). Fullerenes are potential carriers of drugs and radioactive labels: in the cavity during the synthesis process the drugs, radioactive particles can be placed (for the direct influence of diseased cells); they are used as a transporters for drugs because the "packaged" form of C60 will not cause toxic effects on the body and as a safe X-ray contrast agents in radiodiagnostics (Najam-ul-Haq et al. 2007; Movchan 2007). Studies have shown that C60 derivatives do not cause good or malignant neoplasms in animals after 2 months from the start of treatment, so fullerene is considered to have a very low level of cytotoxicity (Satoh and Takayanagi 2006). It has been experimentally proven that the human body can produce antibodies to fullerenes. The property of these specific anti-fullerene antibodies adsorbed on the surface of fullerene enable them to be used as cellular probes in immunology and to better investigate the function of the body's immune system (Ixhaky and Pecht 1998; Veetil and Ye 2007; Prato et al. 2008).
A number of studies have shown that fullerenes can attach molecules and fragments of DNA without altering their biological properties. By providing DNA/fullerene-nanoparticles with cytotropic properties they can serve as directional vectors in the body. These vectors are more efficient than the similar vectors studied earlier (Jensen et al. 1996; Nakamura and Isobe 2003). By a similar principle, fullerene molecules are used as vehicles for pharmacological drugs. Addition of the special chemical groups and substances to a fullerene molecule provides solubility in polar or non-polar compounds, sensivity to different organs, tissues and cells, and other properties. This allows applying fullerenes as biosensors that report changes in the body (Veetil and Ye 2007; Harrison and Atala 2007). Numerical applications of fullerene C60 in pharmacology are due to extraordinary high acceptor property of its collective π-electron system.
The purpose of our work was to study the mechanism of interaction of some conjugated molecules, primarly, substituted 1,3-oxazoles as far as these compounds exhibit a wide range of biological activities, including cytotoxicity (Sasse et al. 2002), immune suppression (Lawrence et al. 2001), antibacterial and antiviral activities (Borst 1999; Cameron et al. 2002; Rodnina et al. 1999; Johnson and Johnson 1993); then they have shown themselves as powerful scaffold in drug design, especially, highly active anticancer agents (Liu et al. 2009; Chen et al. 2011; Kachaeva et al. 2018; Meervelt et al. 1995).
Materials
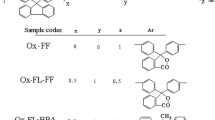
The molecules of fullerene C60 1, tiochrome dye 2 and some biological active 1,3-oxazole derivatives 3–8, are presented in Fig. 1.
Here, R1 is conjugated substituent; it can essentially affect on the donor/acceptor properties of main oxazole cycle. We will consider influence of the donor substituents 6, 7 acceptor substitution 8 and ambivalent phenyl residue 4; these compounds were studied as the cancer chemotherapeutic agents (Kachaeva et al. 2019a and refers therein).
Methodology
Materials
Fullerene 1. Previously it was proved that the fullerene with its spherical constitution is a strong acceptor (Pavlenko et al. 2018). In our work we used fullerene as a reference molecule for investigation π-stack interaction with the studied compounds 2–8. Water-soluble form of fullerenes C60, pyrrolidine tris-acid ethyl ester, purity 97%, was purchased from Sigma Aldrich.
Thiochrome 2. Previously thiochrome was used in biochemical analysis as a substance for confirming the structure of thiamine (Parkhomenko et al. 2012). We consider the thiochrome as a detector the complexes formation (with fullerene and 1,3-oxazoles), it has good water solubility, is not toxic for biological systems, and possesses intensive fluorescence at 443 nm (Edwards et al. 2017; Yu et al. 2015 and refers therein).
The synthesis of the 1,3-oxazole derivatives (3–8) and their physical, chemical and pharmacological properties are described in (Kachaeva et al. 2019a, b and refers therein).
Spectral measurements
The steady-state absorption and fluorescence spectra were received only for the compounds 1 and 2. The steady-state absorption spectra were recorded with the use of Analytic Jena Specord 210 Plus UV/Visible spectrophotometer at room temperature in the spectral range from 190 to 1100 nm. Fluorescence spectra were obtained with the use of single-beam Agilent Cary Eclipse fluorescence spectrophotometer (equipped with xenon lamp as excitation light source) from 200–900 nm using quartz cuvette for fluorescence (1 cm path length). Concentration of the dyes in dimethylsulfoxide (DMSO) solutions was CM = 1·10–6 for the absorption and for fluorescence spectra.
Quantum-chemical calculations
The main characteristics of the complexes (optimized molecular geometry, charge distribution, energies and shapes of molecular orbitals) and the binding energy were calculated by DFT/6–31 (d, p)/wB97XD method in GAUSSIAN 03 package (Frisch et al. 2003). Initial distance between both components of the complexes was chosen as 3.4 Å and after that procedure of geometry optimization was performed.
Results and discussion
Quantitative quantum-chemical estimation of acceptor property of fullerene and other investigated molecules
Similarly, to conjugated molecule, the donor/acceptor property of the fullerene can be quantitatively estimated by its topological index φ0, which directly connected with the relative position of the frontier levels of the conjugated molecules (Obernikhina et al. 2019) by the formulas (1):
where α is energy of the so-called non-binding MOs; Δ = εLUMO−εHOMO is energy gap; εLUMO is the energy of the lowest unoccupied molecular orbital, εHOMO is the energy of the highest occupied MO. The value α corresponds to the disposition of the frontier levels when the donor and acceptor properties are mutually balanced: φ0 = 0.5, i.e., the energy gap is disposed symmetrically in respect to the non-binding level α. Therefore, the shift of the energy gap up (and hence increase of the parameter φ0 > 0.5) indicates on the predominately donor properties of the conjugated molecules. If the parameter φ0 < 0.5 and the energy gap is shifted down, then the molecule is predominately acceptor. The calculated values φ0 for the fullerene 1 and other compounds 2–8 studied are collected in Table 1.
The calculations show that fullerene (as a conjugated molecule) is high acceptor, while the tiochrome dye is a donor molecule. The donor/acceptor index φ0 of the substituted azoles depends directly on the residue R and exceeds the topological index φ0 of fullerene. Therefore, we could suppose that fullerene should form the stable π–π-complex’ with the conjugated molecules 2–8.
Stacking interaction in π-complexes with fullerene C60
First, let us consider the complex of fullerene with tiochrome as a dye. Since the dye 2 exhibits the appreciable absorption and fluorescence in UV and visible spectral region (Edwards et al. 2017; Yu et al. 2015 and ref.), this enables to study the interaction between two the conjugated molecules not only by quantum-chemically, but also spectrally.
The complex stability depends on the geometrical conformity or complementarity of both components. The optimization shows that the dye 2 is planar and in the complex with fullerene is oriented parallel to 6-membered cycle of C60 (Fig. 2).
Obtained distance between the components in the complex is 3.4 Å, it corresponds to the thickness of π-electron systems (the same as it is between bases in DNA helix (Zaenger 1984) or the intermolecular distance in the polymethine dyes aggregates (Shapiro 2006).
Therefore, it can be stated that stability of the π–electron complexes can be quantitatively estimated by the binding energies of π-complexes obtained from the quantum-chemical calculations.
Quantum-chemical calculations of binding energies of π-complexes
It can be supposed that both π–electron affinity and charge distribution should influence the stability of the π–electron complexes. Generally, according to the perturbation theory (Dewar 1969), the interaction between π–electron systems A and B is connected with the relative positions of the molecular levels of both molecules as well as the overlapping of their π-systems. It can be estimated by formulas (2);
where εi and εj are MO energies; Ciμ and Cjν are MO coefficients; nieces i,μ ∈ system A, while indices j,ν ∈ system B; first two sums run the all levels, second two sums run all atom in the corresponding system.
Here, the binding Ebinding was estimated as the difference of the total energies of the complex its components:
where Ecompl is the energy of optimized complex, while Ecomp1,2 are energies of both optimized components.
Figure 3 presents electron density distribution in the complex [Fullerene–Tiochrome], the calculated energies are collected in Table 2.
Interaction of π–electron systems of both components of the complex causes shift of the molecular frontier levels, including HOMO and LUMO (the orbitals take part in the first electron transition: S0 → S1 ⇒ HOMO → LUMO >). Transformation of the frontier and nearest MOs in the complex shows Fig. 4.
It was established that the highest occupied level in the fullerene is quintuply degenerated, while the lowest vacant level is triply degenerated because of its symmetry (Edwards et al. 2017). Whereas in the dye, the frontier levels are not degenerated, the energy gap of dye is larger than the gap in the fullerene. The Fig. 4 shows the HOMO in dye molecule is disposed higher than the degenerated occupied levels of C60. It can be supposed that the most effective interaction occurs for the highest occupied orbitals of components. The degeneration of the MOs on fullerene component is removed and HOMO in the complex is located predominantly in the dye molecule, while the HOMO-1 is located in the fullerene. Thus, the complexation can cause changes in the spectra of the dye that can be observed experimentally. Besides, the relative positions of the frontier levels show that there occur a specific electron transitions from the HOMO of the dye to the vacant orbitals of the fullerene, so-called charge-transfer transitions (CT-transitions) (Valeur 2002). The transition should lead to the effective fluorescence quenching. The calculated data for separate compounds compared to the data of complex [Fullerene–Tiochrome] are collected in Table 2.
The obtained binding energies are close to similar to the typical the energies of π-complexes of some biologically active molecules (Schulz et al. 1979).
Spectral measurements of complex formation [Fullerene–Tiochrome].
Also, we have performed the spectral study of complex [Fullerene–Tiochrome] formation by spectrophotometric titration when the fluorescence quenching is registered. This method is based on the spectral change upon variation of the component concentrations in the solution. Then, the analysis of measured spectroscopic results enables determination of the complexing parameter (energy of π-complex stability) (Beck et al. 1990).
Tiochrome molecule is considered as a fluorophore, while fullerene is a quencher. The fluorescence quenching is described by Stern—Volmer formulas (Lackowicz 2006). Figure 5a presents the spectral data of fluorescence quenching of Tiochrome with constant concentration 5 × 10−6 M by the fullerene C60 with the variable concentration. One can see that the regular fluorescence quenching is observed upon increasing of the fullerene quantity in solution. The linear relationship between the intensity and fullerene concentration is obtained (Fig. 5b) that points on the same mechanism (or path) of the complexing.
The mathematical analysis of obtained spectral titration data [by Stern—Volmer formulas (Lackowicz 2006)] gives the [Fullerene–Tiochrome] complex formation energy: − 10.07 kcal/mole; this value is close to the experimental data obtained for the protein complexing (Schulz et al. 1979).
Quantum-chemical investigation of binding energies of π-complex [Fullerene–Azole]
Let us consider similar to previous one complex [Pharmacophore–Bio-Molecule] that can be formed under effective interaction of biological active drugs.
As an example of the simplest complexation between one 1,3-oxazole molecule and one fullerene molecule, we will consider the dependence of the binding energy of some oxazole derivatives 3–8 on their chemical constitution; the fullerene molecule should be as a reference acceptor molecule.
It was established (Kachaeva et al. 2019a,b,c) that the anticancer effect of the substituted 1,3-oxazole depends appreciable on the donor/acceprot properties of conjugated residue R in position 5. So, compounds with the substituents with high acceptor properties in position 5 (compounds 5 and 8) have demonstrated the appreciable higher level of the inhibition of the cell growth, whereas the analogous 1,3-oxazole derivatives 6, 7 which contain the donor substituents [-N(CH3)2, -SCH3] at position 5 do not practically affect cell growth. This fact was connected with the influence of the donor ability on the stable complex formation [Pharmacophore–Bio-Molecule] (Kachaeva et al. 2019a).
The performed calculations (Table 1) show that topological index φ0 for the oxazole derivatives 3–9 exceeds this parameter for the fullerene. The frontier levels in the considered molecules are shifted higher compared to the non-binding level energy (Fermi level, α), Fig. 6.
It can be supposed the compounds should form stable complexes with the acceptor fullerene. The calculated binding energies of the optimized complex compounds 3–8 [Fullerene–Azole] are collected in Table 3.
In should be noted that the calculated binding energies are close to the value obtained spectrally for the complex [Fullerene–Tiochrome] (see above). Also, one can see that there is no direct correlation between the binding energy, E, and difference in the parameters φ0 for the fullerene and heterocycles. Careful analysis shows that some spatial hindrances can occur in the molecular complex because of the bulk branched constitution of the introduced substituent in the position 5 in compounds 5 and 8, especially, when R = SO2CH3 and PO(OCH3)2.
Also, all oxazole derivatives are polarized molecules; their dipoles are diversely oriented on the fullerene surface. So, the dipole moments of the molecules 3, 4, 6, 7 are parallel to the 6-membered cycle of the C60, while the dipoles in the compound 5 and 8 are not. In dipole–dipole approach, the discrepancy in the mutual orientation of the moments, undoubtedly, should influence the binding energy. Table 4 shows the changes of dipole moments upon going from the individual molecules 3–8 to their complex with fullerene.
One can notice that complexing is accompanied by some change of the total dipole momentum; this effect is evidently caused by the electron density transfer between the complex components and depends on the donor or acceptor nature of the substituents in the 1,3-oxazole molecule.
Besides, the varying of the substituent nature is accompanied by regular changing of the frontier level positions and hence of the topological index φ0. The calculated values φ0 of the substituted oxazoles 3–6 are collected in Table 3. The 1,3-oxazole with three donor methyl (-CH3) groups (compounds 3) are weak donors (φ0 > 0.5). The enhancement of the π-electron system upon introduction of the ambivalent phenyl group, 4, dereasts the donor properties. On the contrary, the strong donor groups N(CH3)2 and SCH3 (5, 6) causes the parameter φ0 to increase. The introducing of the acceptor groups, SO2Me and PO(OMe)2, (7, 8) leads to essential decreasing of the index φ0, in comparison with the initial trimethyl substituted oxazole 3. Consequently, the topological parameters of all 1,3-oxazoles studied exceed the correspond index of the acceptor fullerene; the one can suppose that the 1,3-oxazoles should generate relative stable complex’ with the reference fullerene molecules.
We could optimistically assume that the similar trend should exist upon the generation of the complexes with the amino acids (peptides in the proteins) or nucleic bases. This could explain the dependence of biological effectivity of the pharmacophore molecules derivatives of the 1,3-oxazole.
Conclusion
A quantum-chemical model is proposed for analysis of the stack interaction with a calculation of the stability energy for the π → π-complexes, which correlates with experimental data. Thus, the fullerene molecule as a strong acceptor can be used as a convenient reference model for the estimation of dependence of stability π → π-complexes, including complexes with biological active compounds, for example, with the aromatic rests of the amino acids (phenyl-alanine, tryptophan etc.) or nucleic bases.
References
Beck MT, Nagypál I (1990) Chemistry of complex equilibria. XII, 402 S. Akadémiai Kiadó, Budapest Pappband, Ft 830. https://doi.org/10.1002/prac.19913330133
Borst P (1999) Multidrug resistance: a solvable problem? Ann Oncol 10:162–164. https://doi.org/10.1093/annonc/10.suppl4.S162
Cameron DM, Thompson J, March PE, Dahlberg AE (2002) Initiation factor IF2, thiostrepton and micrococcin prevent the binding of elongation factor G to the Escherichia coli ribosome. J Mol Biol 319:27–35. https://doi.org/10.1016/S0022-2836(02)00235-8
Chen J, Li C-M, Wang J, Ahn S et al (2011) Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization. Bioorg Med Chem 19:4782–4795. https://doi.org/10.1016/j.bmc.2011.06.084
Dewar MJS (1969) The molecular orbital theory of organic chemistry. McGraw Hill, New York, p 484
Edwards KA, Tu-Maung N, Cheng K et al (2017) Thiamine assays-advances, challenges, and caveats. Chem Open 6(2):178–191. https://doi.org/10.1002/open.201600160
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al (2003) GAUSSIAN03; revision B.05. Gaussian, Inc., Pittsburgh
Harrison BS, Atala A (2007) Carbon nanotube applications for tissue engineering. Biomaterials 28:344–353. https://doi.org/10.1016/j.biomaterials.2006.07.044
Ixhaky D, Pecht I (1998) What else can the immune system recognize? Proc Natl Acad Sci USA 95:11509–11510. https://doi.org/10.1073/pnas.95.20.11509
Jain KK (2005) The role of nanobiotechnology in drug discovery. Drug Discov Today 10(21):1435–1442. https://doi.org/10.1016/S1359-6446(05)03573-7
Jensen AW, Wilson SR, Schuster DI (1996) Biological applications of fullerenes. Bioorg Med Chem 4(6):767–779. https://doi.org/10.1016/0968-0896(96)00081-8
Johnson AW (1993) Ylides and ymines of phosphores. Wiley, New York, pp 1–99
Kachaeva MV, Hodyna DM, Semenyuta IV et al (2018) Design, synthesis and evaluation of novel sulfonamides as potential anticancer agents. Comput Biol Chem 74:294–303. https://doi.org/10.1016/j.compbiolchem.2018.04.006
Kachaeva MV, Obernikhina NV, Veligina ES et al (2019a) Estimation of biological affinity of nitrogen-containing conjugated heterocyclic pharmacophores. Chem Heterocycl Compd 55(4/5):448–454. https://doi.org/10.1007/s10593-019-02478-6
Kachaeva MV, Pilyo SG, Zhirnov VV, Brovarets VS (2019b) Synthesis, characterization, and in vitro anticancer evaluation of 2-substituted 5-arylsulfonyl-1,3-oxazole-4-carbonitriles. Med Chem Res 28(1):71–80. https://doi.org/10.1007/s00044-018-2265-y
Kachaeva MV, Hodyna DM, Obernikhina NV et al (2019c) Dependence of the anticancer activity of 1,3-oxazole derivatives on the donor/acceptor nature of his substitues. J Heterocycl Chem 56(10):1–13. https://doi.org/10.1002/jhet.3711
Kovtun GO, Zhila RS, Kamenev TM (2007) Disruption of chains in the oxidation reaction of organic compounds with fullerene C60. Rep Natl Acad Sci Ukr 9:117–120
Lackowicz JR (2006) Instrumentation for fluorescence spectroscopy. In: Principles of fluorescence spectroscopy, 3rd edn. Springer, Boston, pp 27–61. https://doi.org/10.1007/978-0-387-46312-4_2
Lawrence DS, Copper JE, Smith CD (2001) Structure–activity studies of substituted quinoxalinones as multiple-drug-resistance antagonists. J Med Chem 44:594–601. https://doi.org/10.1021/jm000282d
Li N, Xia T, Nel AE (2008) The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 44:1689–1699. https://doi.org/10.1016/j.freeradbiomed.2008.01.028
Liu X, Bai L, Pan C, Song B, Zhu H (2009) Novel 5-methyl-2-[(un)substituted phenyl]-4-{4,5-dihydro- 3-[(un)substituted phenyl]-5-(1,2,3,4-tetrahydroisoquinoline-2-yl)pyrazol-1-yl}-oxazole derivatives: synthesis and anticancer activity. Chin J Chem 27(10):1957–1961. https://doi.org/10.1002/cjoc.200990329
Meervelt L, Schuerman GS, Brovarets VS (1995) Structure and properties of phosphonium ylides-betaines, derivatives of 2-phenyl-2-oxazolin-5-one and its thio- and seleno-analogues. Tetrahedron 51:1471–1482. https://doi.org/10.1016/0040-4020(94)01041-W
Movchan BA (2007) Élektronno-luchevaya nanotekhnolohyya y novye materyaly v medytsyne—pervye shahy. Visnyk farmakolohiyi i farmatsiyi 12:5–13
Najam-ul-Haq M, Rainer M, Szabo Z et al (2007) Role of carbon nano-materials in the analysis of biological materials by laser desorption/ionization-mass spectrometry. Biochem Biophys Methods. 70:319–328. https://doi.org/10.1016/j.jbbm.2006.11.004
Nakamura E, Isobe H (2003) Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res 36(11):807–815
Obernikhina N, Kachaeva M, Shchodryi V et al (2019) Topological index of conjugated heterocyclic compounds as their donor/acceptor parameter. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2018.1538056
Parkhomenko YuM, Stepuro II, Donchenko GV, Stsiapura VI (2012) Oxidized derivatives of thiamine: formation, properties, biological role. Ukr Biochem J 84(6):5–24
Pavlenko EL, Sendiuk VA, Brusentsov VA et al (2018) Quantum-chemical study of acceptor properties of fullerene and its bridge derivatives. Nanosist Nanomater Nanotechnol 16(2):389–401
Prato M, Kostarelos K, Bianco A (2008) Functionalized carbon nantubes in drug design and discovery. Acc Chem Res 41(1):60–68. https://doi.org/10.1021/ar700089b
Rodnina MV, Savelsbergh A, Matassova NB et al (1999) Thiostrepton inhibits the turnover but not the GTPase of elongation factor G on the ribosome. Proc Natl Acad Sci USA 96:9586–9590. https://doi.org/10.1073/pnas.96.17.9586
Sasse F, Steinmetz H, Schupp T et al (2002) Immunosuppressive cyclic peptides from myxobacteria I. Production, isolation, physico-chemical and biological properties. J Antibiot 55:543–551. https://doi.org/10.7164/antibiotics.55.543
Satoh M, Takayanagi I (2006) Pharmacological studies on fullerene (C60), a novel carbon allotrope, and its derivatives. Pharmacol Sci 100:513–518. https://doi.org/10.1254/jphs.CPJ06002X
Schulz GE, Schirmer RHP (1979) Principles of protein structure, Springer Nature. Springer, New York. https://doi.org/10.1007/978-1-4612-6137-7
Shapiro BI (2006) Molecular assemblies of polymethine dyes. Russ Chem Rev 75(5):433–456. https://doi.org/10.1070/RC2006v075n05ABEH001208
Valeur B (2002) Molecular. In: Zander C, Keller RA, Enderlein J (eds) Fluorescence. Principles and applications. Single-molecule detection in solution methods and applications, vol VI. Wiley-VCH Verlag GmbH, Weinheim, pp 46–103
Veetil JV, Ye K (2007) Development of immunosensors using carbon nanotubes. Biotechnol Prog 23(3):517–531. https://doi.org/10.1021/bp0602395
Yu Y, Ching YJ, Tan YN (2015) A simple and fast fluorimetric method for thiamine (vitamin B1) detection by Au3+-mediated formation of thiochrome. Austin J Biosens Bioelectron 1(1):1004–1006
Zaenger W (1984) Principles of nucleic acid structure. Springer, Berlin, p 584
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obernikhina, N., Zhuravlova, M., Kachkovsky, O. et al. Stability of fullerene complexes with oxazoles as biologically active compounds. Appl Nanosci 10, 1345–1353 (2020). https://doi.org/10.1007/s13204-019-01225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01225-9