Abstract
In this investigation, polyaniline@CuCr2O4 nanocomposites possessing diverse mass proportions of polyaniline (PANI): CuCr2O4 were fabricated. The photocatalytic action of the diverse synthesized specimens throughout the photocatalytic decadence of acridine orange beneath Vis light illumination was elucidated. Consolidating polyaniline to CuCr2O4 nanospheres diminishes their specific surface areas from 100 to 90 m2/g. TEM micrographs acknowledged that both CuCr2O4 and polyaniline@CuCr2O4 nanocomposites have arisen as compatible nanospherical. It has been conducted that incorporating PANI to CuCr2O4 photo-catalyst did not alter the nanocomposites structure of the base CuCr2O4. Furthermore, the microscopic content loading of PANI onto the surface of CuCr2O4 makes it hard to be clearly monitored. The photocatalytic disintegration of acridine orange dye underneath Vis light illumination practicing 0.06% polyaniline/CuCr2O4 nanocomposites has reached 100% after 40 min adopting 2.0 g/L photo-catalyst dosage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the contemporary society, as long as there is population growth and expanding diverse industries there is an enlarged interest regarding environmental deterioration. Accordingly, many reviews have been assigned to expand highly adequate environmental degeneration approaches (Reddy et al. 2016; Rani et al. 2017; Haghighat Mamaghani et al. 2017; Szczepanik 2017). One of the most extensive compounds that engender environmental pollution is acridine orange (AO) dye. Acridine orange is a compound that could be utilized for diverse purposes like dyeing, lithography, leather manufacturing and printing ink (Xie et al. 2000). In addition, acridine orange (AO) dye has diverse biological applications (Girish et al. 2017). Simple methods of wastewater treatment failed to remediate acridine orange (AO) dye. Actually, the existence of this dye in wastewater poses a contemplative issue as it represents a major origin of aquatic pollution (Faisal et al. 2007). And so, assisting efficient design to remove this pollutant from aquatic environment has become an issue of great concern. Discrete regimes have been devoted to remediate this dye from environment including; advanced oxidation, electrochemical treatment, photolysis, chemical precipitation, chemical oxidation, etc. (Peng and Fan 2005). As long as it has great efficacy, advanced oxidation has been developed as one of the most meaningful approaches for dye deportation from wastewater. Obviously, advanced oxidation technique depends primarily on generation of active hydroxyl radicals by the aid of numerous manners like TiO2-interfered photocatalysis and O3/H2O2/UV, H2O2/UV, H2O2/Vis photolysis. Photo-catalysis, especially that of heterogeneous kind, has become one of the most relevant techniques to treat wastewater as it could effectively degrade pollutants beneath mild conditions and low-cost expenses generating harmless compounds (Szczepanik 2017; Chen and Cao 2005; Faisal et al. 2019). The heterogeneous photocatalyst should acquire so low bandgap energy (less than 3.0 eV) that it could absorb solar energy and hence it would be adopted in diverse applications (Wang et al. 2017). Nevertheless, the well-known photocatalysts (ZnS, ZnO, and TiO2) that absorb only in UV zone are noticeable as impoverished photo-catalyst beneath Vis light irradiation (Girish et al. 2017; Faisal et al. 2007; Peng and Fan 2005). Therefore, several attempts have been performed to design efficient photocatalysts in the Vis zone (Wang et al. 2017; An et al. 2016; Meng and Zhang 2016; Sohabi et al. 2017; Aljahdali et al. 2018; Sobahi et al. 2018; Baoum et al. 2018; Sobahi and Amin 2019a, b). Among the recently designed photocatalysts for various purposes like super-capacitors, photocatalytic destruction of dyes, thermal decomposition reactions, and photocatalytic hydrogen evolution is copper chromite (CuCr2O4) photo-catalyst that acquires narrow bandgap energy (Yan et al. 2009; Yuan et al. 2014; Acharyya et al. 2015a, b, c; Paul et al. 2015; Sanoop et al. 2015; Mageshwari et al. 2015; Ma et al. 2016). Numerous successful approaches have been investigated to fabricate CuCr2O4 like; sol–gel (Lee et al. 2016), solid-state (Arboleda et al. 2014), co-precipitation (Delmon 2007) and hydrothermal (George and Sugunan 2008) regimes. Some features restrain the capability CuCr2O4 to be adopted in various applications like its limited light absorption. Thus, the challenge is the development of an efficient strategy to boost the photocatalytic activity of CuCr2O4 by modifying its efficiency to absorb light. Polyaniline (PANI) has been devoted to the photocatalytic operations as a result of its amazing aspects like its great capability to absorb Vis light, the exclusive transporting characteristics of its electrons and holes, its low price and the ease methods of its synthesis in comparison to decorating rare metals. Diverse reviews concluded that inclusion of PANI to photo-catalyst could boost the migration efficacy of charge transporters amid photo-catalyst and PANI. Many attempts have been achieved for constructing efficient nanocomposite decorated with polyaniline for example; PANI/BiVO4 (Shang et al. 2009), PANI/TiO2 (Radoicic et al. 2017), PANI/ZnO (Zhang et al. 2009), PANI/BiOCl (Wang et al. 2013), Ag3PO4@PANI (Liu et al. 2017) and PANI@CdS (Zhang and Zhu 2010). And so, CuCr2O4 could be modified via doping with PANI and the modification may involve extension of its light absorption zone as well as enhancement of the separation of the charge carriers which certainly leads to improved photocatalytic action. In this investigation, PANI/CuCr2O4 nanocomposite of excellent capability towards Vis light absorption has been established. The photocatalytic activity of the fabricated nanocomposite has been assessed via destruction of acridine orange dye beneath Vis light. The interface interaction amid CuCr2O4 and PANI might be the reason for the boosted photocatalytic destruction performance over PANI/CuCr2O4 nanocomposite. In addition, long-wavelength of illuminated light has been adopted to assure that inclusion of PANI broadens the light absorption zone with great efficacy.
Materials and experimental designs
Materials
All adopted chemicals in this examination were of analytical grade and were utilized as received. Aniline, absolute ethanol, ammonium persulphate and citric acid of about 99.5% purity were utilized. In addition, highly pure (96%) sodium hydroxide was applied. Besides, two different nitrate salts (copper nitrate tri-hydrate and chromium nitrate nonahydrate) and tetrahydrofuran (THF) were handled.
Photocatalysts Fabrication
Fabrication of PANI
The polymerization process at mild temperature has been addressed to attain PANI. Deionized water (80 mL) was utilized to dissolute definite quantity of citric acid and the resultant mix was agitated for about 20 min. After that, aniline (0.5 mL) was introduced to the previously prepared solution and the resultant mixture was agitated for about 0.5 h. Then, 20 mL of ammonium persulfate solution (0.25 M) was incorporated dropwise to the produced mix and the attained solution was agitated for 24 h. The temperature all over the investigation was kept between 0–5 °C. After all, the specimen was gathered, washed with ethanol many intervals and then left to dry for 6 h at 60 °C. Polyaniline (PANI) powder of green color has been received.
Fabrication of CuCr2O4 nanospherical
Deionized water (50 mL) was utilized to dissolute chromium nitrate nonahydrate (15.4 g) and copper nitrate tri-hydrate (5.54 g) to receive 1 M solution of the analogous nitrates. The resultant solution was intermixed with deionized water together with 1 M sodium hydroxide solution (50 mL) at 60 °C. pH was adjusted to be 7 amid precipitation. After reaction completion, the precipitate was left in the mother liquor for 5 h. After that, the produced system was dried at 90 °C and the yield was fired for 5 h at 500 °C to receive CuCr2O4 nanospherical.
Fabrication of CuCr2O4 nanocomposite decorated with PANI
CuCr2O4 photocatalysts decorated with PANI were designed at ambient temperature. CuCr2O4 powder (2.0 g) was introduced to 0.22 g L−1 PANI/THF solution (0.75 mL) and the resultant mix was dissolved in ethyl alcohol (30 mL). After that, 2 M sodium hydroxide (15 mL) was incorporated dropwise to the former solution under constant agitation that extended for 4 h. The attained solution was washed at numerous intervals with deionized water and left to dry for 24 h at 60 °C. Evidently, the gathered specimen of PANI/CuCr2O4 was designated as 0.02% PANI/CuCr2O4 nanocomposite (mass proportion of PANI: CuCr2O4 = 0.02%). In a similar way, different PANI/CuCr2O4 nanocomposites acquiring diverse mass proportions of PANI:CuCr2O4 as 0.04%, 0.06% and 0.0.08% were fabricated and designated as 0.04% PANI/CuCr2O4, 0.06% PANI/CuCr2O4 and 0.08% PANI/CuCr2O4 nanocomposites, respectively.
Characterization
XRD appliance was applied to interpret the crystalline phases of the fabricated specimens. Whereas, FTIR spectrometer was appropriated to register the transmittance mode of the FTIR spectra at room temperature. For X-ray photoelectron spectroscopy (XPS) assessments, a Thermo Scientific spectrometer was supported. In addition, transmission electron microscopy (TEM) of JEOL kind was devoted to analyzing the morphology as well as the architecture of the diverse fabricated specimens. Chromatech appliance of Nova 2000 kind was practiced to interpret the textural aspects of the formulated specimens. Moreover, bandgap magnitudes were assessed (estimated from diffuse reflectance spectra) via covering UV–Vis–NIR spectrophotometer. The fluorescence spectrophotometer (Shimadzu model) was supported to record the photoluminescence emission spectra (PL). Finally, Zahner Zennium electrochemical workstation was affirmed to register the transient photocurrent measurements.
Photocatalytic Examination
In this examination, an acridine orange (AO) dye was chosen as a typical example of pollutant to test its photocatalytic destruction over CuCr2O4 nanocomposite decorated with polyaniline. A concentration of 100 ppm corresponding to the degraded dye has been chosen and a Xe-arc lamp, that emits UV–Vis radiation, was selected to be the light source. In the photocatalytic investigation, we allow only the Vis light to be absorbed by the tested photocatalyst and so, UV light was prevented from absorption via utilization UV cut filter. 0.5 L of the tested dye (100 ppm) was blended with nanocomposite (CuCr2O4 decorated with polyaniline) and the system was continuously pumped by air to assure the existence of oxygen all over the reaction. The mix was continuously agitated for 0.5 h in dark to attain equilibrium of adsorption/desorption of the tested dye solution prior to irradiation. After that, the previous system was subjected to irradiation via the mentioned light source for 1 h. after definite intervals, 3 mL of suspension was extracted from the reaction medium. Centrifugation was performed to the withdrawn solution to detach the solid. Hence, UV/Vis/NIR spectrophotometer was utilized to record the absorbance of the diverse specimens at 492 nm.
Results and discussion
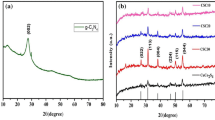
XRD spectra were implemented to diagnose the crystallinity and phase composition of the fabricated neat CuCr2O4 as well as PANI@CuCr2O4 nanocomposite specimens (Fig. 1). XRD patterns of Fig. 1 reveal the presence of (112) (211) (202) (224) and (303) crystal planes correspond to neat CuCr2O4 (JCPDS 05-0657). On the other hand, no prescribed peaks of PANI were registered in the diffractograms of Fig. 1. This observation could be attributed to the very limited amount of the applied PANI in the nanocomposite. Furthermore, alteration of the intensities correlated to some diffractograms as a result of boosting the PANI dose has been observed. This alteration in intensity might be associated with the particle size reduction of CuCr2O4 nanospherical as the result of enlargement of its degree of dispersion (Zhang et al. 2009).
The occupation CuCr2O4 by PANI in the PANI@CuCr2O4 nanocomposite might be confirmed through establishing FTIR analyses. Figure 2 exhibits FTIR spectra data of PANI, neat CuCr2O4 as well as PANI@CuCr2O4 specimens. It is clear that the FTIR spectrum of neat CuCr2O4 exhibits a strong absorption peak at 522 cm−1, this peak is associated with the stretching mode of the Cu–O bond (Zhao et al. 2017). Whereas, FTIR spectrum of PANI displays numerous absorption peaks located at 1584, 1496, 1415, 1300 cm−1, 1104 and 617 cm−1. Evidently, existence of quinoid and benzenoid stretching rings could be verified via the appearance of the peaks located at 1584, 1496 and 1415 cm−1. Whereas, stretching vibration mode of C–N corresponding to benzenoid unit might be documented through the emerging of the band located at 1300 cm−1. Furthermore, the bands found at 1104 cm−1 and 617 cm−1 could be associated with C–H bending vibration mode (Zhang et al. 2009; Tanwar et al. 2017). On the other hand, FTIR spectrum of PANI@CuCr2O4 nanocomposite displays two distinct bands at 1104 and 617 cm−1 in addition to extra peaks similar to those of pure CuCr2O4 photocatalyst. The appearance of distinct absorption peak at 1104 cm−1 is associated with the stretching vibration mode of CAN in the benzenoid unit in PANI, whereas, the absorption peak located at 617 cm−1 could be correlated to the bending vibration mode of CAH. These findings confirm strongly the habitation of PANI in the fabricated PANI@CuCr2O4 nanocomposite. Obviously, the occurrence of extra peaks in FTIR spectrum of PANI@CuCr2O4 similar to those of pure CuCr2O4 affirms the inclusion of PANI to CuCr2O4 to fabricate PANI@CuCr2O4 does not alter the skeleton of CuCr2O4.
XPS spectra of 0.06% PANI@CuCr2O4 nanocomposite are displayed in Fig. 3. Figure 3a confirms that PANI@CuCr2O4 nanocomposite specimen enclose Cu, Cr, O, C and N elements within its structure. Whereas, Fig. 3b displays two characteristic spectral peaks located at 935.2 and 954.0 eV and might be correlated to the Cu2p high-resolution. In fact, Cu2p3/2 and Cu2p1/2 could be verified via the appearance of these peaks (at 935.2 and 954.0 eV, respectively). This finding reflects the oxidation state of Cu2+ in 0.06% PANI@CuCr2O4 nanocomposite. On the other hand, Fig. 3 C displays two distinct spectral peaks located at 574.5 and 583.9 eV that could be associated with Cr2p3/2 and Cr2p1/2, respectively. This observation confirms the oxidation state of Cr3+ in 0.06% PANI@CuCr2O4 nanocomposite. Furthermore, the confirmation of O 1 s existence could be achieved via the peak located at 532.5 eV that reflects the oxidation state of O2− in 0.06% PANI@CuCr2O4 nanocomposite (Fig. 3d). Finally, the presence of polyaniline in the fabricated nanocomposite could be confirmed via the appearance of the peaks located at 400 and 284.7 eV for N 1 s and C 1 s (Fig. 3e, f, respectively).
Figure 4 exhibits TEM images of pure CuCr2O4 as well as PANI@CuCr2O4 specimens. It is elucidated from the data of Fig. 4 that the dominated shape in all illustrated morphologies is the nanospherical morphology. Furthermore, it is noticed that the inclusion of PANI to CuCr2O4 has a negative effect on CuCr2O4 particle size (Fig. 4b–e). on the other hand, it is noticed that inclusion of different doses of PANI to CuCr2O4, to fabricate various PANI@CuCr2O4 specimens, has no obvious consequence on the morphological structures of the diverse PANI@CuCr2O4 nanocomposites. The tiny dose of PANI explains the absence of the morphology corresponding to PANI in the TEM images of the fabricated PANI@CuCr2O4 nanocomposites.
Figure 5 exhibits HRTEM images for 0.06% PANI@CuCr2O4 specimen. It is concluded from the presence of lattice spacing at 0.476 nm, which characterizes the plane of (101) of CuCr2O4, that the major phases in PANI@CuCr2O4 specimen are CuCr2O4. On the other hand, the very low dose of PANI included in the PANI@CuCr2O4 specimen explains the absence of the lattice spacing corresponding to PANI.
Table 1 depicts the BET values for pure CuCr2O4 as well as the diverse PANI@CuCr2O4 nanocomposites. It is clear from the data tabulated in Table 1 that the specific surface areas of pure CuCr2O4, 0.02% PANI@CuCr2O4, 0.04% PANI@CuCr2O4, 0.06% PANI@CuCr2O4 and 0.08% PANI@CuCr2O4 specimens are in the sequence of 100.00, 98.00, 95.00, 93.00 and 90.00 m2/g. The lower specific surface areas of the specimens accommodating PANI compared to that of the pure CuCr2O4 clarifies that inclusion of PANI to CuCr2O4 has a negative effect on the specific surface areas corresponding to CuCr2O4.
Figure 6 shows the UV–Vis spectra of the pure CuCr2O4 and PANI@CuCr2O4 specimens. It is obvious from the data of Fig. 6 that the absorption zones for the pure CuCr2O4, 0.02% PANI@CuCr2O4, 0.04% PANI@CuCr2O4, 0.06% PANI@CuCr2O4 and 0.08% PANI@CuCr2O4 specimens are corresponding to visible light. Evidently, the bandgap magnitudes of the diverse fabricated specimens could be resolved from their UV–Vis spectral curves (Table 2). The bandgap magnitudes are found to be 2.12, 2.00, 1.92, 1.73 and 1.72 eV corresponding to the pure CuCr2O4, 0.02% PANI@CuCr2O4, 0.04% PANI@CuCr2O4, 0.06% PANI@CuCr2O4 and 0.08% PANI@CuCr2O4 specimens, respectively. Hence, it could be concluded that band gap magnitude of the CuCr2O4 specimen is declined when PANI is included in the CuCr2O4 to form PANI@CuCr2O4 specimens. In general, decline of bandgap energy brings about an enhancement in the photocatalytic reactivity of the fabricated PANI@CuCr2O4 nanocomposites.
The PL spectra of pure CuCr2O4, as well as PANI@CuCr2O4 specimens, are interpreted in Fig. 7. The outcomes of Fig. 7 clarify that the CuCr2O4 specimen possesses high emission peak intensity announcing that the reconsolidation speed of the photo-generated charge transporters of the pure CuCr2O4 specimen is high. Whereas, the intensities of emission peaks for the diverse PANI@CuCr2O4 nanocomposites are small when correlated to that of the pure CuCr2O4 specimen. It is noticed that the intensities of the different peaks are in the order of; CuCr2O4 > 0.02% PANI@CuCr2O4 > 0.04% PANI@CuCr2O4 > 0.06% PANI@CuCr2O4 > 0.08% PANI@CuCr2O4. Accordingly, 0.06% PANI@CuCr2O4 specimen possesses the lowest emission peak intensity compared to those of other specimens. Besides, the reconsolidation speed of the photo-generated transporters is very small for the same specimen (0.06% PANI@CuCr2O4 sample) that brings about an enhancement of its photocatalytic efficacy.
Transient photocurrent responses of the fabricated CuCr2O4 and PANI@CuCr2O4 specimens are displayed in Fig. 8. The attained responses reflect that CuCr2O4 specimen possesses low photocurrent intensity. This finding suggests that the recombination speed of the photo-generated charge carriers for the CuCr2O4 specimen is high. On the contrary, the photocurrent transient responses for the diversity PANI@CuCr2O4 specimens are large when correlated to those of pure CuCr2O4 specimens. Furthermore, the transient photocurrent responses corresponding to the diverse fabricated nanocomposites are arranged in the following regulation; CuCr2O4 < 0.02% PANI@CuCr2O4 < 0.04% PANI@CuCr2O4 < 0.06% PANI@CuCr2O4 < 0.08% PANI@CuCr2O4. Hence, it could be terminated that 0.06% PANI@CuCr2O4 specimen possesses the greatest photocurrent response and in a similar way, the greatest photocatalytic efficacy.
Photocatalytic achievement investigation
Destruction of acridine orange (AO) dye has been inspected on the diversity fabricated PANI@CuCr2O4 nanocomposites so to assess their photocatalytic achievement (Fig. 9). The photocatalytic destruction of acridine orange dye was implemented beneath Vis light irradiation. Figure 9 elucidates that when the photocatalytic destruction of acridine orange dye is conducted over CuCr2O4 specimen, the destruction speed of the dye is found to be about 60% after 1 h under the consequence of Vis light irradiation. Whereas, the inclusion of 0.02%PANI to CuCr2O4 in the fabrication of 0.02% PANI@CuCr2O4 nanocomposite leads to an enhancement in the photocatalytic destruction speed of the dye to 90% after 1 h. Additionally, when the novel nanocomposites (0.04% PANI@CuCr2O4, 0.06% PANI@CuCr2O4, and 0.08% PANI@CuCr2O4) have been endorsed, the destruction speeds of acridine orange dye have been promoted up to 100% after diverse reaction times (60, 50 and 50 min, respectively) beneath Vis light irradiation. The attained data elucidate that 0.06% PANI@CuCr2O4 nanocomposite possesses the greatest photocatalytic efficiencies as compared to those of other fabricated specimens (CuCr2O4, 0.02% PANI@CuCr2O4, 0.04% PANI@CuCr2O4, and 0.08% PANI@CuCr2O4 specimens even if short exposure time is applied (50 min).
Figure 10 elucidates the consequence of photo-catalyst dose on acridine orange dye photocatalytic destruction. It is noticed from the data of Fig. 10 that enlarging nanocomposite (0.06% PANI@CuCr2O4) dose from 0.5 to 1.0 g/L brings about an enhancement in the photocatalytic activity of 0.06% PANI@CuCr2O4 photo-catalyst from 80 to 90% after 60 min. on the other hand, the time needed to complete the photocatalytic destruction of the dye is declined from 50 to 40 min if the catalyst does is boosted from 1.5 to 2.0 g/L. It is obvious that enlarging of the catalyst dose (from 1.5 to 2.0 g/L) leads to a reduction in the time needed to complete the reaction (from 50 to 40 min) clarifying the enhancement in the photocatalytic efficiency, which might be associated with the upgrading of the feasible active centers. On contrary, utilization of higher dose of the fabricated photocatalyst (higher than 2.0 g/L) reduces the photocatalytic performance (down to 95%) at 1 h. This finding could be associated with the hindrance of Vis light dissipation to the surface of the photo-catalyst that retards acridine orange dye destruction.
Figure 11 exhibits the stability of 0.06% PANI@CuCr2O4 nanocomposite towards the photocatalytic destruction of acridine orange dye upon its utilization up to five cycles. The observation of Fig. 11 affirms the greater stability of 0.06% PANI@CuCr2O4 nanocomposite upon its handling up to five times.
Conclusions
The outcomes of this investigation could be summarized as follows:
- 1.
PANI@CuCr2O4 nanocomposite with the homogenous nanospherical design was successfully fabricated.
- 2.
PANI@CuCr2O4 nanocomposite has been identified via FTIR, XPS, BET, XRD, TEM, and UV–Vis and PL analyses.
- 3.
PANI@ CuCr2O4 nanocomposite could be utilized for acridine orange dye photocatalytic destruction beneath Vis light irradiation.
- 4.
The photocatalytic performance of 0.06% PANI@CuCr2O4 non-composite reached 100% towards the destruction of acridine orange dye after 40 min of Vis light exposure via utilization of 2.0 g/L of the photocatalyst.
- 5.
0.06% PANI@CuCr2O4 nanocomposite could be reutilized up to five times preserving its stability.
References
Acharyya SS, Ghosh S, Adak S, Tripathi D, Bal R (2015a) Fabrication of CuCr2O4 spinel nanoparticles: a potential catalyst for the selective oxidation of cycloalkanes via activation of Csp3–H bond. Catal Commun 59:145–150
Acharyya SS, Ghosh S, Siddiqui N, Sivakumar Konathala LN, Bal R (2015b) Cetyl alcohol mediated synthesis of CuCr2O4 spinel nanoparticles: a green catalyst for selective oxidation of aromatic C–H bonds with hydrogen peroxide. RSC Adv 5:4838–4843
Acharyya SS, Ghosh S, Tiwari R, Pendem C, Sasaki T, Bal R (2015c) Synergistic effect between ultrasmall Cu(II) oxide and CuCr2O4 spinel nanoparticles in selective hydroxylation of benzene to phenol with air as oxidant. ACS Catal 5:2850–2858
Aljahdali MS, Amin MS, Mohamed RM (2018) Gd-cobalt selenite as an efficient nanocomposite for aniline synthesis from the photocatalytic reduction of nitrobenzene. Mater Res Bull 99:161–167
An C, Wang S, Sun Y, Zhang Q, Zhang J, Wang C, Fang J (2016) Plasmonic silver incorporated silver halides for efficient photocatalysis. J Mater Chem A 4:4336–4352
Arboleda J, Echavarria A, Amparo Palacio L (2014) Synthesis and characterization of (NH4)1.5Cu2Cr2O8(OH)1.5⋅H2O. Powder Diffr 24:244–246
Baoum AA, Amin MS, Mohamed RM (2018) Decoration of SnO2 nanosheets by AgI nanoparticles driven visible light for norfloxacin degradation. Appl Nanosci 8:2093–2102
Chen S, Cao G (2005) Study on the photocatalytic reduction of dichromate and photocatalytic oxidation of dichlorvos. Chemosphere 60:1308–1315
Delmon B (2007) Preparation of heterogeneous catalysts. J Therm Anal Calorim 90:49–65
Faisal M, Tariq AM, Muneer M (2007) Photocatalysed degradation of two selected dyes in UV-irradiated aqueous suspensions of titania. Dyes Pigments 72:233–239
Faisal M, Harraz FA, Ismail AA, Alsaiari MA, Al-Sayari SA, Al-Assiri MS (2019) Novel synthesis of polyaniline/SrSnO3 nanocomposites with enhanced photocatalytic activity. Ceram Int 45:20484–20492
George K, Sugunan S (2008) Nickel substituted copper chromite spinels: preparation, characterization and catalytic activity in the oxidation reaction of ethylbenzene. Catal Commun 9:2149–2153
Girish S, Kumar KSR, Rao K (2017) Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3, and ZnO). Appl Surf Sci 391:124–148
Haghighat Mamaghani A, Haghighat F, Lee CS (2017) Photocatalytic oxidation technology for indoor environment air purification: the state-of-the-art. Appl Catal B Environ 203:247–269
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res 88:428–448
Liu L, Ding L, Liu Y, An W, Lin S, Liang Y, Cui W (2017) A stable Ag3PO4@PANI core@shell hybrid: enrichment photocatalytic degradation with p–p conjugation. Appl Catal B Environ 201:92–104
Radoicic M, Ciric-Marjanovic G, Spasojevic V, Ahrenkiel P, Mitric M, Novakovic T, Šaponjic Z (2017) Superior photocatalytic properties of carbonized PANI/TiO2 nanocomposites. Appl Catal B Environ 213:155–166
Ma P, Geng Q, Gao X, Yang S, Liu G (2016) CuCr2O4 spinel ceramic pigments synthesized by sol–gel self-combustion method for solar absorber coatings. J Mater Eng Perform 25:2814–2823
Mageshwari K, Sathyamoorthy R, Yong Lee J, Park J (2015) Novel CuCr2O4 embedded CuO nanocomposites for efficient photodegradation of organic dyes. Appl Surf Sci 353:95–102
Meng X, Zhang Z (2016) Bismuth-based photocatalytic semiconductors: introduction, challenges and possible approaches. J Mol Catal A Chem 423:533–549
Paul B, Bhuyan B, Purkayastha DD, Dhar SS, Behera S (2015) Facile synthesis of spinel CuCr2O4 nanoparticles and studies of their photocatalytic activity in degradation of some selected organic dyes. J Alloys Compd 648:629–635
Peng RY, Fan JH (2005) Ozonalytic kinetic order of dye decoloration in aqueous solution. Dyes Pigments 67:153–159
Rani M, Shanker U, Jassal V (2017) Recent strategies for removal and degradation of persistent and toxic organochlorine pesticides using nanoparticles: a review. J Environ Manag 190:208–222
Reddy PA, Reddy PVL, Kwon E, Kim KH, Akter T, Kalagara S (2016) Recent advances in the photocatalytic treatment of pollutants in aqueous media. Environ Int 91:94–103
Sanoop AP, Rajeev R, George BK (2015) Synthesis and characterization of a novel copper chromite catalyst for the thermal decomposition of ammonium perchlorate. Thermochim Acta 606:34–40
Shang M, Wang W, Sun S, Ren J, Zhou L, Zhang L (2009) Efficient visible light induced photocatalytic degradation of contaminant by spindle-like PANI/BiVO4. J Phys Chem C 113:20228–20233
Sobahi TR, Amin MS (2019a) Synthesis of ZnO/ZnFe2O4/Pt nanoparticles heterojunction photocatalysts with superior photocatalytic activity. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.10.073
Sobahi TR, Amin MS (2019b) Upgrading the photocatalytic achievement of g-C3N4 nanosheets along with decoration with Ag@TiO2 nanospheres for the preparation of vitamin B3. Appl Nanosci. https://doi.org/10.1007/s13204-019-00960-3
Sobahi TR, Amin MS, Mohamed RM (2018) Enlargement of photocatalytic efficiency of BaSnO3 by indium doping for thiophene degradation. Appl Nanosci 8:365–557
Sohabi T, Amin MS, Mohamed RM (2017) Photocatalytic degradation of methylene blue dye by F doped Co3O4 nanowires. Desalin Water Treat 74:346–353
Szczepanik B (2017) Photocatalytic degradation of organic contaminants over clay–TiO2 nanocomposites: a review. Appl Clay Sci 141:227–239
Tanwar R, Kaur B, Kumar Mandal U (2017) Highly efficient and visible light driven Ni0.5Zn0.5Fe2O4@PANI modified BiOCl heterocomposite catalyst for water remediation. Appl Catal B Environ 211:305–322
Wang Q, Hui J, Li J, Cai Y, Yin S, Wang F, Su B (2013) Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl Surf Sci 283:577–583
Wang S, Yun JH, Luo B, Butburee T, Peerakiatkhajohn P, Thaweesak S, Xiao M, Wang L (2017) Recent progress on visible light-responsive heterojunctions for photocatalytic applications. J Mater Sci Technol 33:1–22
Xie Y, Chen F, He J, Zhao J, Wang H (2000) Photoassisted degradation of dyes in the presence of Fe3 and H2O2 under visible irradiation. J Photochem Photobiol A Chem 136:235–240
Yan J, Zhang L, Yang H, Tang Y, Lu Z, Guo S, Dai Y, Han Y, Yao M (2009) CuCr2O4/TiO2 heterojunction for photocatalytic H2 evolution under simulated sunlight irradiation. Sol Energy 83:1534–1539
Yuan W, Liu X, Li L (2014) Synthesis, characterization and photocatalytic activity of cubic-like CuCr2O4 for dye degradation under visible light irradiation. Appl Surf Sci 319:350–357
Zhang H, Zhu Y (2010) Significant visible photoactivity and antiphotocorrosion performance of CdS photocatalysts after monolayer polyaniline hybridization. J Phys Chem C 114:5822–5826
Zhang H, Zong R, Zhu Y (2009) Photocorrosion inhibition and photoactivity enhancement for zinc oxide via hybridization with monolayer polyaniline. J Phys Chem C 113:4605–4611
Zhao J, Ji M, Di J, Ge Y, Zhang P, Xia J, Li H (2017) Synthesis of g-C3N4/Bi4O5Br2 via reactable ionic liquid and its cooperation effect for the enhanced photocatalytic behavior towards ciprofloxacin degradation. J Photochem Photobio A Chem 347:168–176
Acknowledgements
This Project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G-98-130-40. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baoum, A., Amin, M.S. & Mohamed, R.M. Development of CuCr2O4 nanocomposite adopting decoration with polyaniline for acridine orange dye degradation. Appl Nanosci 10, 1501–1510 (2020). https://doi.org/10.1007/s13204-019-01218-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01218-8