Abstract
Membrane technologies are a useful solution for water treatment, especially for removing trace amounts of different pollutants. Current research is focused on improving the removal efficiency of polyvinylidene fluoride (PVDF) membranes to heavy metal ions, such as lead(II), cadmium(II), and chromium(III). The surface chemistry and morphology of PVDF membranes were varied applying the sol–gel approach for functionalization. It involved (1) diethylphosphatoethyltriethoxysilane (DPTES) or 3-mercaptopropyltrimethoxysilane (MPTMS) as functionalizing precursors, (2) different concentrations of the initial sols, and (3) NH4OH or HCl as the catalysts for the hydrolysis and co-condensation reactions. According to the SEM analysis of the surface texture, alkaline catalyst and low sol concentration result in the formation of porous membrane active layers. Membrane weight loss after water filtration under pressure indicated the dependence of layer stability on the conditions of functionalization, namely the membranes with phosphonic groups prepared in an acidic medium are more resistant to rinsing. Functionalized PVDF membranes demonstrated a significant increase in sorption efficiency along with high water flux. Overall, such membranes were sufficient for treating water with ion concentrations lower than 100 mg/L, whereas the applied functionalization technique is promising for adjusting the parameters of the final membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Membrane separation is a very important process for chemical technology with multiple applications, including water purification, gas separation, pharmaceutics, electrodialysis, proton conductivity, and others. By materials, the membranes can be classified into organic, inorganic, and composite (Oyama and Stagg-Williams 2011). Among the composite membranes, there are hybrid membranes containing components of various nature (Chen et al. 2013; Shi et al. 2013; Worthley et al. 2013; Choudhury et al. 2018). Composite membranes are usually produced by functionalization of polymeric or ceramic membranes, involving the introduction of certain substances or particles with predetermined properties, e.g., complexation, hydrophobicity, or hydrophilicity. The process of introducing additional components can be carried out either by modification of the membrane support (Worthley et al. 2013; Li et al. 2015; Choudhury et al. 2018) or at the stage of synthesis of the membrane itself (Wang et al. 2011; Galve et al. 2013). Modification of the top layers of the membranes leads to the formation of composite materials with a layered structure (Bauman et al. 2013; Worthley et al. 2013; Li et al. 2015).
For the processes of membrane modification and functionalization, trialkoxysilanes RSi(OX)3 (where –R is a functional group and –OX is a hydrolyzable alkoxy group) are widely used. There are several techniques of membrane functionalization with trialkoxysilanes. One of them assumes direct treatment of the membrane with trialkoxysilanes (Singh et al. 2005; Sah et al. 2004; Abidi et al. 2006; Han et al. 2013; Atallah et al. 2017); another one suggests applying trialkoxysilanes (usually in combination with tetraalkoxysilanes) for depositing polysiloxane layers on the membrane supports (Wang et al. 2011; Bauman et al. 2013; Sadeghi et al. 2013; Worthley et al. 2013; Martin et al. 2015). It is also possible to use trialkoxysilane as a “gluing agent” when attaching additional components to the membrane surface (Hyun et al. 1996; Yang et al. 2014) or introduce during membrane synthesis, as one of the monomers in polymerization (Chakrabarty et al. 2013). Trialkoxysilanes with hydrophobic groups were used to modify ceramic membranes to remove the charge on their surface and thereby improve their resistance to pores clogging (Sah et al. 2004; Atallah et al. 2017). Meanwhile, the functionalization with silanes containing complexing groups can be carried out to increase the adsorption of heavy metals in the filtration of aqueous solutions (Boroujeni et al. 2016; Tomina et al. 2013a, b, 2015, 2017).
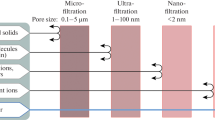
It should be mentioned that by their pore sizes and, accordingly, the size of the retained particles (as well as the working pressure and water flux), the membranes can be classified into micro-, ultra-, nanofiltration, and reverse osmosis (MF, UF, NF and RO, respectively). MF and UF membranes with relatively high water flux and low working pressures work like sieves rejecting only substances exceeding their pore sizes and can be used to clean water from turbidity or microorganisms (AWWA 2008). However, there is a problem of water purification from metal ions coming from water-soluble industrial waste. Such waters contain chrome, nickel, zinc, and other metals widely used in the steel industry. In addition, wastewater may contain lubricants that may pollute the membranes. At high concentrations of metal ions, they are precipitated, while the residual amounts are removed by membrane filtration. UF separates and concentrates insoluble heavy metal salts, reducing the amount of contaminants in wastewater to sufficiently low levels (several mg/L). Then, NF and RO membranes with much smaller pore sizes can be used to purify water from molecules and ions. For example, Qdaisa and Moussa (2004) showed that RO cleans water from ions of copper and cadmium more efficiently, 98–99% compared to 90–97% for NF. Functionalization of the membranes (MF, UF, or NF) with polysiloxane coatings applying the sol–gel technique allows increasing the degree of water purification, even if their pores are larger than the ion sizes due to the complexation with the surface functional groups (Tomina et al. 2013a, b) in addition to the membrane mechanism when bridged silanes are used in the formation of siloxane network (Tomina et al. 2015).
Thus, we aimed to improve the heavy metal ion removal efficiency of polymeric PVDF UF membranes by functionalizing them with porous polysiloxane layers containing thiol [≡Si(CH2)3SH] or phosphonic [≡Si(CH2)2P(O)(OH)2] groups. Thiol groups are known to possess high affinity to the ions of silver, mercury, cadmium, and lead (Samiey et al. 2014; Stolyarchuk et al. 2018). The addition of MPTMS for the synthesis of silica makes it possible to prepare sorbents with very high distribution coefficients and increase the efficiency of water purification by ceramic membranes (Boroujeni et al. 2017; Tomina et al. 2017). Materials with phosphonic groups also possess high sorption properties (Gunathilake et al. 2015; Milyutin et al. 2014; Melnyk et al. 2012). They can be synthesized using DPTES, containing phosphate groups –P(O)(OC2H5)2, but it requires subsequent treatment with concentrated hydrochloric acid to produce the phosphonic groups –P(O)(OH)2 (Mel’nik et al. 2010). However, the majority of polymeric membranes are unstable in the concentrated acids; therefore, such functionalization of PVDF membranes by polysiloxane layers with phosphonic groups pursues several goals: introduction of acidic groups, increasing hydrolytic stability (especially in acidic environment), increasing retention of heavy metal ions. To avoid the action of concentrated hydrochloric acid on initial membrane support, the phosphate groups of DPTES were hydrolyzed into phosphonic prior to the functionalization.
It also should be mentioned that we conducted the synthesis and deposition of functional polysiloxane coatings in one stage. It means that there occurred the co-condensation of structure-forming tetraethoxysilane (TEOS) with functionalizing silane (either DPTES or MPTMS), rather than successive deposition of polysiloxane layer from TEOS followed by modification of the silica coating with trialkoxysilanes. Such technique allows controlling the morphology of the formed layers, depending on the ratio of the reacting components, the type of catalyst, the nature and size of the functional groups. Therefore, we also tried to analyze the impact of the reaction conditions in the systems TEOS/DPTES and TEOS/MPTMS on the morphology and filtration properties of functionalized PVDF membranes.
Experimental section
Materials
The ultrafiltration membrane PVDF 400 from SEPRO Membranes Inc. (Polyvinylidene fluoride membrane on polyester support) was used as initial membrane support.
Tetraethoxysilane (TEOS, 98%, Aldrich); 3-mercaptopropyltrimethoxysilane (MPTMS, ≥ 95%, Merck), diethylphosphatoethyltriethoxysilane (DPTES, 92%, ABCR), ammonium hydroxide solution (NH4OH, 25%, Aldrich), ethanol (95.6%, Sigma-Aldrich), n-isopropanol (99.8%, Sigma-Aldrich), and hydrochloric acid (37%, Sigma-Aldrich) were used to synthesize functional layers.
The following salts were used to study sorption: lead(II) nitrate (Pb(NO3)2, 99.0%, Sigma-Aldrich), cadmium(II) nitrate tetrahydrate (Cd(NO3)2·4H2O, 98.0%, Sigma-Aldrich), and chromium(III) nitrate nonahydrate (Cr(NO3)3·9H2O, 99.9%, Sigma-Aldrich).
Techniques of membrane functionalization
First of all, there were prepared functionalizing sols, which were diluted and deposited on the membrane surfaces to functionalize the membranes.
Preparation of functionalizing sols using DPTES
In the beginning, it was necessary to hydrolyze ethoxy groups near the phosphorus atom of DPTES. 1 mL of DPTES and 10 mL of 37% HCl were boiled (80 °C) on a water bath with reflux condenser for 24 h. Then, the acid was evaporated to a glassy substance, followed by the fourfold addition and evaporation of 10 mL of water. The resulting glassy substance was ground to a white powder, dried at 110 °C, and further labeled as H-DPTES.
The NH4OH-catalyzed sol was prepared by mixing the solutions of TEOS (6 mL of n-isopropanol, 0.63 mL of H2O, 0.2 mL of NH4OH, and 0.27 mL of TEOS) and H-DPTES (0.15 g of H-DPTES in 1 mL of H2O and 2 mL of n-isopropanol) at 3:2 molar ratio of TEOS:H-DPTES.
To prepare the HCl-catalyzed sols, preliminary hydrolysis of TEOS was carried out: 2.5 mL of TEOS, 2.5 mL of 0.0024 M HCl, and 2.5 mL of ethanol were stirred at 70 °C for 30 min. Then, hydrolyzed TEOS solution was mixed with H-DPTES solution (0.2 g of H-DPTES, 4 mL of 0.0024 M HCl, and 4 mL of ethanol) at 3:2 molar ratio of TEOS:H-DPTES.
Preparation of functionalizing sols using MPTMS
The sols with thiol groups were prepared in the same manner as described above, both with NH4OH and HCl as catalysts of hydrolysis, but instead of a batch of H-DPTES, 0.15 and 0.2 mL of MPTMS were used for the syntheses with NH4OH and HCl, accordingly.
Depositing sols on the membranes
Each of the prepared sols was divided into three portions and diluted with corresponding alkohol (n-isopropanol for NH4OH- and ethanol for HCl-catalyzed sols) as indicated in Table 1. First portion remained undiluted, second was diluted 1/4 (v/v), and third 1/10 (v/v), and the final samples were marked, respectively, with 1, 2, and 3. The membranes were seeped by the sols with a ratio of 0.008 mL of sol per 1 cm2 of membrane and dried at 40 °C for 24 h.
Characterization
SEM images were acquired using a scanning electron microscope JSM-6060 LV (JEOL, Japan).
Potentiometric titration was used to determine the molecular weight and phosphonic ionization constants of H-DPTES. The batches of 0.01, 0.02, and 0.04 g of H-DPTES were poured with 20 mL of 0.1 N NaNO3 solution (solid-to-liquid ratios 1:500, 1:1000, and 1:2000, respectively). 0.11 N NaOH solution was used as a titrant; it was added dropwise and under constant stirring in portions of 0.1 mL, and a change in the pH was measured using Ionometric I-500.
The dissociation constants (K) of phosphonic acid groups of H-DPTES were calculated using Eqs. (1)–(5) (Albert and Serjeant 1962):
where pK = − logK; [H+], [A−] and [HA] are equilibrium concentrations of protons, anions and undissociated acid groups, respectively, (mol/L); \( \nu_{{{\text{P(O)}}({\text{OH}})_{2} }} \) and νNaOH are the amounts of functional groups and added titrant (mol), and V is a total volume of the solution (L).
The ability of functionalized membranes to sorb metal cations from the water solution was determined for lead(II), cadmium(II) and chromium(III) ions in static conditions with a solid-to-liquid ratio of 1:450 at a constant temperature of 25 °C. The initial concentrations of metal ions in their salt solutions were 100 mg/L (pH 6). The amount of metal cations in the solution before and after sorption was determined by atomic absorption spectrometer AAS/AES Perkin Elmer 1100 AA Spectra. Sorption capacity (Qe, mg/g) of functionalized membranes was calculated using Eq. (6):
where C0 and Ceq are the initial and the equilibrium concentrations of metal ions, respectively, (mg/L), V is the volume of heavy metal ions solution (L), and m is the weight of membrane with sorption layers (g).
The membranes were tested in the filtration of water and lead(II) ion solutions using the Amicon stirred ultrafiltration cell. Working pressure was 0.1 MPa. The ratio of the volume of the lead(II) ion solution to the membrane area was 10 mL per 5.3 cm2. The removal efficiency (R, %) was calculated using Eq. (7):
where Cp and Cf are the concentrations of permeate and feed, respectively, (mg/L).
The water flux (J0, L/m2 h) of distilled water through the membrane was determined using Eq. (8):
where V is the volume of permeate (L), S is the effective membrane area (m2), and t is the permeation time (h).
Results and discussion
Sol–gel method based on the reaction of hydrolytic co-condensation of alkoxysilanes was used to functionalize polymeric PVDF 400 membranes with polysiloxane layers containing thiol and phosphonic functional groups. However, the peculiarity of such a reaction is the necessity to determine suitable synthetic conditions for each particular system. As it was already mentioned, DPTES is widely applied to incorporate phosphonic groups in the structure of silica sorbents, but the ordinary procedure usually involves post-synthetic acidic hydrolysis of its phosphate groups. Whereas the structure of PVDF 400 membranes can deteriorate when in contact with an aggressive medium of concentrated hydrochloric acid, we altered the conventional approach, and the phosphate groups of DPTES were hydrolyzed to phosphonic prior to the sol–gel synthesis.
Synthesis and properties of phosphonic acid group precursor
Preparation of the phosphonic acid group precursor was carried out by the treatment of DPTES with boiling concentrated HCl, following the procedure described by Barnes and David (1960). The hydrolytic reaction of DPTES is shown in the following scheme.

The resulting substance H-DPTES is hydrolytically unstable and soluble in water and alcohol. To functionalize the membranes with the required content of phosphonic groups, it is necessary to know the equivalent of H-DPTES weight corresponding to one phosphonic group. For this purpose, potentiometric titration of the H-DPTES was performed, which allowed calculating its molecular weight assuming the composition (OH)3SiCH2CH2P(O)(OH)2 × nH2O. The potentiometric titration curves and calculations are given in Fig. 1 and Table 2. The molecular weight of (OH)3SiCH2CH2P(O)(OH)2 is 189 g/mol, but the estimated molecular weight of obtained compound is higher (see Table 2), which may be due to the presence of water. In fact, the molecular weight of the prepared hydrolyzed sample, calculated from potentiometric titration data, varies as shown in the Table 2. Thus, it can be concluded that we have a mixture of (OH)3SiCH2CH2P(O)(OH)2, its dimer (OH)2(O)PCH2CH2Si(OH)2–O–(OH)2SiCH2CH2P(O)(OH)2, and water.
Potentiometric titration curves of H-DPTES with varying solid-to-liquid ratios indicated in Table 2
The presence of two clearly pronounced steps in the potentiometric titration curves (Fig. 1) indicates the dissociation of two protons from the phosphonic group, which indirectly confirms the hydrolysis of ethoxy groups near the phosphorus atom.
Phosphonic ionization constants of H-DPTES were also determined from the potentiometric titration curves. For comparison, the ionization constants for phosphoric acid are pK1 = 2.2, pK2 = 7.1, pK3 = 12.3. The lower the pK value, the more molecules dissociate in solution and, consequently, a stronger acid. According to Table 2, the acidity of the groups in H-DPTES is close to the values for phosphoric acid, while somewhat lower pK2 value may be due to the impact of silanol groups.
Formation, morphology, and stability of polysiloxane layers
The UF PVDF membranes were functionalized by the deposition of the active layers containing phosphonic or thiol groups as schematically depicted in Fig. 2a. We analyzed the peculiarities of the deposited layers, depending on the catalyst of the reaction used in the systems of TEOS/DPTES and TEOS/MPTMS, as well as the impact of the functionalizing sol dilution on the characteristics of the formed layers. The initial PVDF 400 membrane consists of a three-dimensional network of polyvinylidene fluoride on the support of coarse polyester fibers. According to SEM images, the cavities of PVDF network are about 1 μm in size (Fig. 2b), and the diameter of polyester fibers is about 10 μm (Fig. 2c).
The formation of polysiloxane layers on the membrane surface was investigated using SEM. The nature of the catalyst and trialkoxysilane is reflected on the texture of the resulting layers. Specifically, the processes of dissolution and precipitation of silica occur quickly in the alkaline environment while the particles are negatively charged and mutually repelled, so that the particle growth prevails over their aggregation (Iler 1979). Therefore, NH4OH-catalyzed reaction should result in the formation of the layer with distinct particles (Košak et al. 2013), which was observed for the membranes with phosphonic groups (Fig. 3a). The active layer of POH1 consists of polydisperse particles and has multiple cracks. A similar picture is observed for the membrane with thiol groups produced in the NH4OH-catalyzed process (Fig. 3b). However, its particles are not so noticeable and there are no cracks, which can be explained by the interaction of the sol with the membrane, since both the membrane and the thiol groups possess hydrophobic properties.
In the HCl-catalyzed reaction, the processes of polymerization and depolymerization of silica are slowed down, and the particles are practically uncharged, so they aggregate into gel networks (Iler 1979). Therefore, the layers prepared in an acidic medium are fairly homogeneous. Membranes functionalized with thiol groups in an acidic medium have breakages on the surface (Fig. 3c), which can be explained by the shrinkage of the gel during drying. Meanwhile, the membranes coated with phosphonic groups-bearing layers in an acidic medium are distinguished by the homogeneity of the coating and the absence of cracks (Fig. 3d–f). In Fig. 3d, there is a defect in the coverage of PH1 (it should be mentioned that this is a single case), which can be used to estimate that the coating on the membrane features a dense layer. In the case of PH3 (Fig. 3f), the layer is granulous and is about 1.3 μm thick. Obviously, the dilution of the functionalizing sol results in the reduction of particles mutual collisions and they are more likely to grow in size.
The stability of the functional layer on the membranes was analyzed based on the weight difference before and after the water pressure treatment. During the washing of the membranes, their weight decreased by 0.1–0.7% (Fig. 4). Increasing dilution of functionalizing sol tends to decrease the loss of functional layer, except for samples with phosphonic groups synthesized in NH4OH-catalyzed reaction (POH1, POH2, POH3). Since the surface layers of these samples are formed by spherical particles which are more pronounced than for the other samples (Fig. 3a), the denser functional layer (POH1) is held better on the surface of the membrane. It distorts the general tendency of changing the weight loss with the degree of sol dilution. Samples PH2 and PH3 are the most resistant to washing.
Retention of metal ions from aqueous solutions
The PVDF membranes functionalized with thiol or phosphonic groups were tested for the static sorption of lead(II), cadmium(II), and chromium(III) cations (Fig. 5). The maximum capacities for lead(II) ions were above 13 mg/g for PH1, about 10 mg/g for PH2, above 8 mg/g for SH1 (Tomina et al. 2014) and POH1, and about 5 mg/g or lower for other samples. Thus, functionalization with phosphonic groups was preferable to the functionalization with thiol groups in the uptake of lead(II) ions. Comparing the samples with the same groups’ content, the layers prepared via HCl-catalyzed process possess higher sorption capacity. To compare, Košak et al. (2017) synthesized silica nanoparticles with a much higher SH groups content using MPTMS in alkaline medium, but their capacity was 10.8 mg (Pb)/g.
The extraction of cadmium(II) ions by all membranes was higher than 5 mg/g, with the most effective SH1 and SH2 (above 12 mg/g) and slightly less effective POH1, POH2 and PH2 (about 10 mg/g). Regardless of the catalyst, membranes with thiol groups showed somewhat higher removal of cadmium(II) than chromium(III) or lead(II) ions. It also should be mentioned that the thiol-functionalized membranes are characterized by higher sorption capacity than, for example, membranes functionalized with an additional sorption layer containing Schiff bases—5.2 mg (Cd)/g (Gao et al. 2017), or polydopamine—11.9 mg (Cd)/g (Fang et al. 2017). Interestingly, that Irani et al. (2011) prepared membranes using MPTMS, where SH groups were located in the entire volume of the composites, but their capacity of 5–25 mgCd/g does not exceed significantly the results obtained in our research.
The maximum extraction of chromium(III) ions was 10.5 mg/g by sample PH1, 7 mg/g by PH3, about 5 mg/g by PH2 and SH3.
The experiments with non-functionalized PVDF membrane support showed that it is also capable of absorbing a certain amount of chromium(III), lead(II) and cadmium(II) ions Cd2+ > Pb2+ > Cr3+. However, the ions uptake after functionalization is drastically increased due to the complexation with functional groups and additional ion-exchange adsorption on silanol groups of silica (Stolyarchuk et al. 2018).
The adsorption capacity of the active layer may depend on the size of the cation (Melnyk et al. 2012), the distance between the functional groups, as well as the composition of complexes that form on the surface (Graillota et al. 2013). In some cases, when it comes to the sorption of bivalent and trivalent metals, the presence of two or three functional groups together on the surface of the silica particles is necessary for complexation. When such location is impossible due to geometric obstacles or blocking of groups, sorption decreases. Therefore, sometimes there is no direct relationship between the number of functional groups fixed on the surface of the membrane and the sorption capacity. However, the adsorption of the divalent cation can also occur on one phosphonic acid residue, since it is a diprotic acid.
The relatively low adsorption of lead(II) ions on thiol-containing membranes can be explained by the formation of disulfide bridges between closely spaced groups, whereupon they cannot interact with lead(II) ions. Meanwhile, cadmium(II) ions can adsorb on sulfur-containing sulfide and sulfhydryl groups (Liang et al. 2009).
Based on the above-mentioned data on the morphology of the active layer, its resistance to flushing and sorption abilities, PVDF membranes functionalized with phosphonic groups via HCl-catalyzed reaction were selected for testing in the membrane cell. A solution of lead(II) ions with a concentration of 100 mg/L (0.48 mmol/L) was filtrated through the membranes in Amicon stirred ultrafiltration cell. Such a concentration was chosen based on the considerations that the membrane separation was used for purification of water with ion concentrations of 100 mg/L and lower. The water flow through the initial UF membrane support was 400 L/m2 h and it showed no rejection of lead(II) ions. The results of testing functionalized membranes are presented in Table 3. The degree of water purification from lead(II) ions depended on the concentration of functionalizing sol: the higher the sol dilution, the lower the removal efficiency. Portions of permeate were taken for analysis and the maximum removal efficiency was observed at 10% degree of permeate collection, decreasing subsequently. The concentration of lead(II) ions in the concentrate after the first cycle was 5% lower than the initial one, which indicates the processes of ions adsorption on the surface of the functional layers. According to Table 3, after the second cycle, which was carried out without regeneration, there is a slight increase in ion concentration in the concentrate for membranes PH1 (5% higher than the initial) and PH3 (3% higher), which indicates an insignificant manifestation of the membrane separation mechanism.
The flux of water through the functionalized membranes is quite high and increases with increasing sol dilution. The water permeability of the membranes in the second cycle is lower than in the first, indicating a change in the structure of the functional layers at the initial stage. The flux of water through the membranes depends on the parameters of the initial membranes and tends to decrease up to 70% after functionalization with an additional complexing layer, similar to Tomina et al. (2016), Fang et al. (2017), and Choudhury et al. (2018). The flux of water through the resulting composites is quite high, which explains the low degree of purification by the membrane separation mechanism (Yin et al. 2016). The results that were obtained in our research are consistent with the results of the removal of copper(II) ions by ceramic membranes with amino groups (Tomina et al. 2016), which were part of the polysiloxane active layer. Apparently, this testifies to the identity of the water purification mechanisms with similar materials.
Conclusions
The surfaces of UF PVDF membranes were functionalized by phosphonic [≡Si(CH2)2P(O)(OH)2] or thiol [≡Si(CH2)3SH] groups incorporated into the polysiloxane layers using the NH4OH- and HCl-catalyzed reactions. According to SEM images, the most developed porous structure is inherent in the samples with phosphonic groups synthesized using acidic catalyst and the highest sol dilution (sample PH3). The most hydrolytically stable are the active layers of the membranes PH2 and PH3. Membranes with thiol groups are suitable for purification of water from ions of cadmium(II), whereas the membranes functionalized with phosphonic groups are promising for purification of water from chromium(III), cadmium(II) and lead(II) cations. Membranes with phosphonic groups are characterized by high water flux values, and water purification occurs due to the sorption mechanism.
References
Abidi N, Sivade A, Bourret D et al (2006) Surface modification of mesoporous membranes by fluoro-silane coupling reagent for CO2 separation. J Membr Sci 270(1–2):101–107
Albert A, Serjeant EP (1962) Ionization constants of acids & bases. Wiley, New York
Atallah C, Tremblay AY, Mortazavi S (2017) Silane surface modified ceramic membranes for the treatment and recycling of SAGD produced water. J Petrol Sci Eng 157:349–358
Barnes GH, David MP (1960) Synthesis and hydrolytic stability of some organosilicon phosphonate esters. J Org Chem 25(7):1191–1194
Bauman M, Košak A, Lobnik A, Petrinić I, Luxbacherd T (2013) Nanofiltration membranes modified with alkoxysilanes: surface characterization using zeta-potential. Colloids Surf A Physicochem Eng Asp 422:110–117
Boroujeni AR, Karimi M, Javanbakht M (2016) Application of novel surface-modified PES membranes for removal of heavy metals from aqueous solutions. Desalin Water Treat 57(42):19794–19809
Boroujeni AR, Javanbakht M, Karimi M, Akbari-Adergani B (2017) Adsorption properties of thiol-functionalized silica nanoparticles prepared for application in poly(ether sulfone) nanocomposite membranes. J Text Polym 5(1):37–47
Chakrabarty T, Prakash S, Shah VK (2013) End group cross-linked 2-(dimethylamino) ethylmethacrylate based anion exchange membrane for electrodialysis. J Membr Sci 428:86–94
Chen JH, Zheng JZ, Liu QL et al (2013) Pervaporation dehydration of acetic acid using polyelectrolytes complex (PEC)/11-phosphotungstic acid hydrate (PW11) hybrid membrane (PEC/PW11). J Membr Sci 429:206–213
Choudhury PR, Majumdar S, Sahoo GC (2018) High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr(VI) and Pb(II) from contaminated water. Chem Eng J 336:570–578
Fang X, Li J, Li X et al (2017) Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem Eng J 314:38–49
Galve A, Sieffert D, Staudt C et al (2013) Combination of ordered mesoporous silica MCM-41 and layered titanosilicate JDF-L1 fillers for 6FDA-based copolyimide mixed matrix membranes. J Membr Sci 431:163–170
Gao A, Xie K, Song X et al (2017) Removal of the heavy metal ions from aqueous solution using modified natural biomaterial membrane based on silk fibroin. Ecol Eng 99:343–348
Graillota A, Bouyera D, Monge S et al (2013) Sorption properties of a new thermosensitive copolymeric sorbent bearing phosphonic acid moieties in multi-component solution of cationic species. J Hazard Mater 260:425–433
Gunathilake C, Kadanapitiye MS, Dudarko O et al (2015) Adsorption of lead ions from aqueous phase on mesoporous silica with P-containing pendant groups. ACS Appl Mater Interfaces 7(41):23144–23152
Han HH, Ryu SH, Nakao S, Lee YT (2013) Gas permeation properties and preparation of porous ceramic membrane by CVD method using siloxane compounds. J Membr Sci 431:72–78
Hyun SH, JoBeom SY, Kang S (1996) Surface modification of γ-alumina membranes by silane coupling for CO2 separation. J Membr Sci 120(2):197–206
Iler Ralph K (1979) The chemistry of silica: solubility, polymerization, colloid and surface properties and biochemistry of silica. Wiley, New York
Irani M, Keshtkar AR, Mousavian MA (2011) Removal of Cd(II) and Ni(II) from aqueous solution by PVA/TEOS/TMPTMS hybrid membrane. Chem Eng J 175:251–259
Košak A, Lobnik A, Bauman M (2013) Adsorption of mercury(II), lead(II), cadmium(II) and zinc(II) from aqueous solutions using mercapto-modified silica particles. Int J Appl Ceram Technol 12:461–472
Košak A, Bauman M, Padežnik-Gomilšeka J, Lobnik A (2017) Lead (II) complexation with 3-mercaptopropyl-groups in the surface layer of silica nanoparticles: sorption, kinetics and EXAFS/XANES study. J Mol Liq 229:371–379
Li X, Li J, Van der Bruggen B et al (2015) Fouling behavior of polyethersulfone ultrafiltration membranes functionalized with sol–gel formed ZnO nanoparticles. RSC Adv 5:50711–50719
Liang X, Xu Y, Sun G, Wang L, Sun Y, Qin X (2009) Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+. Colloids Surf A Physicochem Eng Asp 349:61–68
Martin A, Arsuaga JM, Roldán N et al (2015) Enhanced ultrafiltration PES membranes doped with mesostructured functionalized silica particles. Desalination 357(2):16–25
Mel’nik IV, Stolyarchuk NV, Dudarko OA et al (2010) Bridged polysilsequioxane adsorption materials containing phosphonic acid residues. Prot Met Phys Chem Surf 46(2):206–214
Melnyk IV, Goncharyk VP, Kozhara LI et al (2012) Sorption properties of porous spray-dried microspheres functionalized by phosphonic acid groups. Microporous Mesoporous Mater 153:171–177
Milyutin VV, Gelis VM, Nekrasova NA et al (2014) Sorption of actinide ions onto mesoporous phosphorus-containing silicas. Radiochemistry 56(3):262–266
Oyama ST, Stagg-Williams SM (2011) Inorganic polymeric and composite membranes: structure, function and other correlations. Elsevier, New York
Qdaisa HA, Moussa H (2004) Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination 164(2):105–110
Sadeghi M, Talakesh MM, Ghalei B, Shafiei M (2013) Preparation, characterization and gas permeation properties of a polycaprolactone based polyurethane-silica nanocomposite membrane. J Membr Sci 427:21–29
Sah A, Castricum HL, Bliek A et al (2004) Hydrophobic modification of γ-alumina membranes with organochlorosilanes. J Membr Sci 243:125–132
Samiey B, Cheng C-H, Wu J (2014) Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions. Rev Mater 7:673–726
Shi F, Ma Y, Ma J et al (2013) Preparation and characterization of PVDF/TiO2 hybrid membranes with ionic liquid modified nano-TiO2 particles. J Membr Sci 427:259–269
Singh RP, Way JD, Dec SF (2005) Silane modified inorganic membranes: effects of silane surface structure. J Membr Sci 259(1–2):34–46
Stolyarchuk NV, Kolev H, Kunachova M et al (2018) Synthesis and sorption properties of bridged polysilsesquioxane microparticles containing 3-mercaptopropyl groups in the surface layer. Colloids Surf A 538:694–702
The AWWA subcommittee on periodical publications of the membrane process committee (2008) Microfiltration and ultrafiltration membranes for drinking water. AWWA 100(12):84–97. www.jstor.org/stable/41313420
Tomina VV, Mel’nik IV, Pogorilyi RP et al (2013a) Functionalization of the surface of ceramic membranes with 3-mercaptopropyl groups using the sol–gel method. Prot Met Phys Chem Surf 49(4):386–391
Tomina VV, Melnyk IV, Zub YuL (2013b) The Impact of the nanostructure of the functional polysiloxane layer in planar ceramic membranes on their sorption properties. In: Proceedings of the international conference “nanomaterials: applications and properties”, vol 2, no 2, p 02FNC17
Tomina VV, Zub YuL, Bauman M, Kosak A, Lobnik A (2014) Mercapto-modified UF/NF membranes in heavy metals retention. In: 21st international congress of chemical and process engineering, CHISA 2014 and 17th conference on process integration, modelling and optimisation for energy saving and pollution reduction, PRES 2014
Tomina VV, Stolyarchuk NV, Melnyk IV et al (2015) Sorptive ceramic membranes functionalized with HS-groups. Microporous Mesoporous Mater 209:66–71
Tomina VV, Stolyarchuk NV, Melnyk IV et al (2016) Surface functionalization of ceramic membranes with 3-aminopropyl groups using the sol–gel method. Prot Met Phys Chem Surf 52(1):55–60
Tomina VV, Stolyarchuk NV, Melnyk IV et al (2017) Composite sorbents based on porous ceramic substrate and hybrid amino- and mercapto-silica materials for Ni(II) and Pb(II) ions removal. Sep Purif Technol 175:391–398
Wang C, Chalkova E, Lee JK et al (2011) Composite membranes with sulfonic and phosphonic functionalized inorganics for reduced relative humidity PEM fuel cells. J Electrochem Soc 158(6):B690–B697
Worthley CH, Constantopoulos KT, Ginic-Markovic M et al (2013) A study into the effect of POSS nanoparticles on cellulose acetate membranes. J Membr Sci 431:62–71
Yang H, Dong X, Wang D, Xu W (2014) Effect of silane coupling agent on physical properties of polypropylene membrane reinforced by native superfine down. Powder Polym Polym Compos 22(6):509–518
Yin N, Wang K, Wang L, Li Z (2016) Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb(II) removal. Chem Eng J 306:619–628
Acknowledgements
This research was carried out in the framework of NATO Science for Peace Program SPS.NUKR.SFP 984398. The authors would like to express their gratitude to Tetiana Tkachenko for her help with translation into English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sliesarenko, V., Tomina, V., Dudarko, O. et al. Functionalization of polymeric membranes with phosphonic and thiol groups for water purification from heavy metal ions. Appl Nanosci 10, 337–346 (2020). https://doi.org/10.1007/s13204-019-01170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-019-01170-7