Abstract
The present study involves the development of nanobiosensor to determine toxicological behavior of Mitoxantrone (MTX). Mitoxantrone intercalates with DNA and produces MTX–DNA adduct, resulting in blockade of protein synthesis and excessive production of free radicals in the myocardium eventually leads to cardiac toxicity. Potentiometry was applied to develop an electroanalytical procedure for the determination of MTX and its interaction with DNA immobilized on the electrode surface modified with Silicon dioxide (SiO2) nanoparticles. The nanobiosensor immersed in MTX solution to monitor MTX–DNA interaction with respect to time and alters the resistance of the nanobiosensor. It was observed that MTX–DNA interaction is fast initially and as time elapses, the change in interaction gets slow due to formation of MTX–DNA adduct. Determination limit of the nanobiosensor is 100–10 ng/ml. This study suggests that the nanobiosensor allows real-time monitoring of the drug–DNA interaction changes by measuring the potential at sensor interface which can prove to be an important tool in drug discovery pipelines and molecular toxicology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanobiosensors and nanobiochips are gaining importance in the field of life science because of its faster, direct, more accurate, more selective detection at very low concentrations. Enormous research has been carried out for the development of nanobiosensor which can be useful in life science fields such as clinical diagnosis, genomics, proteomics and toxicology. But, until now, a very few nanodevices have been developed which can monitor or detect toxicity at nano gram level. Nanoparticles play a key role in adsorption of biomolecules due to their large specific surface area and high surface free energy (Lad and Agrawal 2012c). The combination of nanomaterials and biomolecules is of considerable interest in the field of nanobiotechnology. Recently, many kinds of nanometer materials such as gold (Maxwell et al. 2002; Xiao et al. 1999; Jia et al. 2002), platinum (Ningning et al. 2005) and silicon dioxide (He and Hu 2004; Qhobosheane et al. 2001) nanoparticles are widely applied for electrochemical-based nanobiosensor due to their conducting and semiconducting properties. Also, these nanoparticles have been used to catalyze biochemical reactions, improving coverage and binding ability of the functional components and this capability can be usefully employed in biosensor design (Martin et al. 2007). Recently, we have developed multi-walled carbon nanotube-based DNA nanosensor and platinum nanoparticle-based nanobiosensor for monitoring drug–DNA interaction. Also, we developed optical nanobiosensor for determining drug–DNA interaction. (Lad and Agrawal 2012a, b).
SiO2 nanoparticles have been used to construct biosensor due to its biocompatibility as well as good electron transfer properties. In the work of Luo et al. (2004), SiO2 nanoparticles were introduced in the construction of field-effect transistors (ENFETs) biosensor which could provide a biocompatible environment and improve the enzyme activity. Chen and his team reported the effect of SiO2 nanoparticles on the adsorbability and enzymatic activity of glucose oxidase (Chen et al. 1996). The oligonucleotide-modified silica nanoparticles were prepared by Lisa R. Hilliard and her coworkers which provide an efficient substrate for hybridization and used in the development of DNA biosensors and biochips (Hilliard et al. 2002). Ningning et al. (2005) developed electrochemical DNA nanobiosensor which consists of platinum nanoparticles combined with Nafion-solubilized Multi-walled carbon nanotubes. M. Yousef Elahi and his team developed polypyrrole (PPy) nanofiber modified electrode to monitor DNA–salicylic acid/Aspirin interaction. A Platinum electrode was electrochemically modified by the polymerization of pyrrole to obtain a nanofiber PPy film using a pulse potential method. The reaction rate of Aspirin with DNA was lower than that of Salicylic Acid with DNA, potentially due to the steric hindrance of the acetyl group when binding to the minor groove (Yousef et al. 2011). Many research papers have been reported on silicon dioxide nanowire-based nanosensor to study DNA interaction and DNA hybridization studies (Zhang et al. 2011; Ryu et al. 2010). Overall, these studies suggest that silicon dioxide shows good biocompatibility as well as good electron transfer properties. Hence, it can be useful for construction of biosensor to improve its functionality.
The electromotive force (EMF) is the maximum potential difference or charge between two electrodes. This causes electrons to move so that there is an excess of electrons at one point and a deficiency of electrons at a second point (Robinson et al. 2005). The electrochemical signals are usually generated by redox reactions and changes in ionic composition. Potentiometric sensors measure the potential of an electrode at equilibrium (i.e., in the absence of the appreciable currents) by measuring the electrochemical cell potential versus a reference electrode potential (Wang et al. 2010). MTX allows extensive stabilization of the intercalated adduct by hydrogen-bonding interactions with DNA (Thurston 2008). Thus, this potentiometric nanobiosensor has been designed to monitor interaction of MTX with DNA.
In this report, we developed a real-time potentiometric nanobiosensor by modifying the electrode with SiO2 nanoparticle and DNA. This nanobiosensor was immersed in the solution containing MTX to monitor MTX–DNA interaction (Fig. 1). Mitoxantrone, an anti-cancer agent, has a planar heterocyclic ring structure and the basic side groups are critical for intercalation into DNA. Binding of MTX to DNA inhibits both DNA replication and RNA transcription and leads to excessive production of free radicals in myocardium responsible for producing cardiotoxicity (Hajihassan and Rabbani-Chadegani 2011; Oliveira Brett et al. 1998). Therefore, the change in MTX–DNA interaction was observed by measuring changes in EMF (mV). All experiments were carried out at neutral pH and at room temperature since double—strand of DNA breaks at neutral pH and single—strand breaks at high pH (Smart and Hodgson 2008).
Experimental section
Chemicals
Highly polymerized calf thymus DNA (MP Biomedicals, US) was used in this study. DNA dilutions were prepared in phosphate buffer pH 7. Phosphate buffer was prepared by dissolving 0.1 M disodium hydrogen phosphate in water and adjusting the pH by adding 0.1 M HCl. Tetraethylorthosilicate (TEOS), ammonium hydroxide, and ethanol were used to prepare SiO2 nanoparticles. All chemicals were purchased from E-Merck (India, Mumbai) and were all of analytical reagent grade. Mitoxantrone was obtained from Cipla Ltd (India, Mumbai) and used without purification. All aqueous solutions were prepared in Milli-Q water from a Millipore purification system and all experiments were done at room temperature.
Apparatus
The potential measurements were carried out at 25.0 ± 0.1 °C with a digital pH meter (Model LI120, ELICO, India). A saturated calomel electrode (SCE) was used. Particle size of SiO2 was measured by Malvern Zeta-sizer (Model—The Zetasizer Nano ZS, UK). The morphology of SiO2 was studied using a scanning electron microscope (SEM) EVO-18, special edition, Carl-Zeiss.
Preparation of SiO2 nanoparticles
SiO2 nanoparticles were prepared according to the literature (Stöber et al. 1968; Rossi et al. 2005). To 20 ml of ethanol, 2 ml of TEOS was added followed by 4 ml of concentrated NH4OH. After that it was stirred for 12–14 h at 200–300 rpm. Then the mixture obtained was centrifuged at 3,000 rpm for 30–50 min. Finally a white color powder was formed which was named as silica nanoparticle.
Fabrication of electrode by SiO2 nanoparticles
The surface of calomel electrode was modified with SiO2 nanopaticles. An amount of 2.0 mg of SiO2 were dispersed in a 10 ml of ethanol solution. After about 10 min of sonication, uniformly dispersed SiO2 nanoparticles were formed. Before modifying the electrode with SiO2, the electrode was cleaned by washing it with distilled water and was allowed to dry. Then the dry electrode was immersed in solution containing SiO2 nanoparticles for 30 min with stirring at room temperature. The electrode was removed and was left for drying for about 15 min.
Immobilization of DNA on SiO2 modified electrode
10 ppm DNA solution was prepared in phosphate buffer pH 7. The electrode was immobilized by drop casting technique. A 10 μL (100 ng) drop of DNA was delivered on the modified SiO2 surface of electrode by micropipette and allowed to dry in air. After drying, this nanobiosensor was used for monitoring toxicological behavior of MTX. Potentiometric measurement was performed at working calomel electrode versus reference calomel electrode.
Results and discussion
Characterization of SiO2
The morphology of SiO2 was observed by SEM. Figure 2 illustrates that the particles are predominantly spherical in shape with diameter ranging from 20 to 25 nm. Larger and uneven shaped particles with diameter 35–70 nm were also obtained.
Particle sizes of SiO2 were determined by Malvern zeta-sizer which was found as an average of 20 nm (Fig. 3). Particles were ranging from 46.98 nm (81.3 %), 0.6549 nm (7.3 %), and 2,210 nm (5.8 %).
MTX–DNA interaction in solution
MTX solution of varying concentration (100, 75, 50, 25, and 10 ng/ml) was prepared in distilled water. The MTX–DNA interaction in solution was carried out at room temperature. 1 ml of 100 ng/ml of MTX and 10 μL of 10 μg/ml of DNA was taken to perform interaction study by potentiometry. As shown in Fig. 4, the electrode potential shifted to negative direction steadily. But, at one point, the change in EMF was not increased. This indicates the formation of MTX–DNA adduct. Initially the change in EMF was very fast, but as time elapse, the change in EMF gets slow. In all MTX concentration series, potential shifted steadily but at one stage it gets stopped due to the formation of MTX–DNA adduct.
MTX interacts preferentially with DNA binding with guanine, cytosine base pairs (Oliveira Brett et al. 1998). The sensor measures the two-electron oxidation process of 5,8- hydroxyl substituents on MTX while interacting with DNA. No more hydrogen from guanine and cytosine base can be liberated and oxidized which could lead to the stopped EMF change. The change in EMF with respect to time indicates the interacting behavior of MTX with DNA.
In case of 100 ng/ml of MTX, the interaction of MTX with DNA showed more potential difference (Fig. 4) as compared to 75, 50 and 25 ng/ml of MTX because higher amount of MTX was available to interact with DNA. Thus, this study suggests that concentration of drug is directly proportional to the sensitivity of sensor and shows significant EMF changes. It was also observed that no measurable change was found in EMF at 10 ng/ml of MTX concentration. Thus, this study suggests that concentration of drug is directly proportional to the sensitivity of sensor and at very low concentration no EMF changes are observed.
Nanobiosensor monitoring MTX–DNA interaction
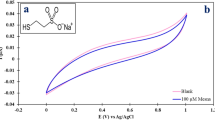
MTX (100, 75, 50, 25 and 10 ng/ml) and DNA interaction was performed by developed nanobiosensor. The results obtained from developed nanobiosensor were significant from without modified sensor (Fig. 5). In all MTX concentration series, the MTX–DNA interaction shows more change in electrode potential. The electrode potential decreases steadily until all the amount of MTX gets interacted with DNA. At one stage, no change in EMF was observed due to formation of MTX–DNA adduct. In case of 100 ng/ml of MTX, more EMF changes were observed by nanobiosensor as compared to without nanobiosensor. This suggests that the sensitivity of sensor improves much better due to SiO2. The biocompatibility of SiO2 nanoparticles provides a suitable environment for DNA to keep its bioactivity and prevent DNA leakage. Moreover, signals from sensor improve much better due to conducting properties of SiO2, which provide a faster pathway for electrons to be transferred between the active sites of the DNA and the surface of the SiO2. Thus, nanobiosensor reveals high sensitivity.
The linearity and reproducibility of the nanobiosensor were investigated by performing three experiments using the same working calomel electrode. It has been observed that in all MTX concentration series, a significant change in EMF was reported with nanobiosensor. Also, the change in EMF was remarkable at lower concentration, i.e., 10 ng/ml of MTX determined by nanobiosensor, while without nanobiosensor did not show any change in EMF. So, sensitivity was also improved at lower concentration. Thus, the developed nanobiosensor allows real-time monitoring of MTX–DNA interaction, which can play a pivotal role in screening of drugs while developing series of new drugs.
Sensitivity and selectivity of nanobiosensor
To evaluate the performance of the nanobiosensor, potential shift ∆E was calculated, i.e., the potential change from equilibrium time to the end of the experiment. From Fig. 6, it is clearly seen that the potential shift is directly proportional to MTX concentration. If the concentration of MTX is higher, more potential shift was observed. Nanobiosensor showed more ∆E than without modification of sensor at all MTX concentration series. At 10 ng/ml of MTX concentration series, nanobiosensor showed remarkable change in potential shift ∆E, while without modified sensor does not show any change in potential shift ∆E. This confirms the improvements of electrical signals and thus, nanobiosensor reveals high sensitivity.
On addition of incremental concentrations of MTX to DNA, potential difference increases in all concentration series. It is evident from the experiment that interacting behavior of DNA with the stock concentrations of MTX are as follows:
100 > 75 > 50 > 25 > 10 ng/ml.
Analytical performance of nanobiosensor
The linearity and reproducibility of the nanobiosensor were investigated by performing three different experiments using the same working electrode. It was observed that the nanobiosensor showed good reproducibility for all three measurements. The working electrode was water-washed to take away the DNA residuals from the surface of the electrode after each measurement. The stability of the nanobiosensor was tested by performing the experiments daily for a period of 15 days while storing in a suitable environment when not in use. Almost 90 % of the initial sensitivity was retained at the end of the period and the biosensor half-life is estimated to almost 1 month.
The comparison of analytical performances for determining MTX–DNA interaction by nanobiosensor and without nanobiosensor is given in Table 1 For nanobiosensor, the values of correlation coefficient (R2), slope, and intercept were found as 0.994, 32.83, and 4.83, respectively. Limit of detection (LOD) and limit of quantification (LOQ) were found as 1.42 and 4.36 μg/mL, respectively. In case of without nanobiosensor, the values of correlation coefficient (R2), slope, and intercept were found as 0.842, 13.067, and 2.267, respectively; limit of detection (LOD) and limit of quantification (LOQ) were found as 2.51 and 7.63 μg/mL, respectively.
Comparative study of MTX–DNA interaction by nanobiosensor
In order to compare the results and hence detect systematic errors between the two methods, a student t test was employed to check whether the standard deviations for the same sample differ significantly (Table 2). Since the experimental value of t test is higher than the critical, it is concluded that the proposed nanobiosensor technique is more precise than without nanobiosensor. Table 2 shows the statistical comparison between two methods at various concentration of MTX. Thus, the results obtained from both the methods were not in agreement, indicating significant difference between two.
Conclusion
This work has shown experimental evidence of interaction of MTX with DNA and may contribute to the understanding of the mechanism of action of this drug with DNA. It was observed that drug–DNA interaction occurring with time which suggests that MTX intercalates with DNA and slowly interacts with it causing some breaking of the hydrogen bonds. It is interesting to note that the nanobiosensor experiments suggest preferential interaction of MTX with DNA at very low concentration. Without surface modified electrode does not seem to be suitable for the monitoring MTX–DNA interaction at low concentrations of MTX. The sensor revealed high sensitivity and selectivity. Overall, we developed nanobiosensor which allows real-time monitoring of the drug–DNA interaction changes by measuring potential at sensor interface which can be crucial biosensor in molecular toxicology and drug discovery pipelines.
References
Chen ZJ, Ou XM, Tang FQ, Jiang L (1996) Effect of nanometer particles on the adsorbability and enzymatic activity of glucose oxidase. Colloids Surf B 7:173–179. doi:10.1016/0927-7765(96)01291-X
Hajihassan H, Rabbani-Chadegani A (2011) Interaction of Mitoxantrone, as an anticancer drug, with chromatin proteins, core histones and H1, in solution. Int J Biol Macromol 48:87–92. doi:10.1016/j.ijbiomac.2010.10.002
He P, Hu N (2004) Electrocatalytic properties of heme proteins in layer-by-layer Films assembled with SiO2 nanoparticles. Electroanalysis 16:1122–1131. doi:10.1002/elan.200403000
Hilliard LR, Zhao X, Tan W (2002) Immobilization of oligonucleotides onto silica nanoparticles for DNA hybridization studies. Anal Chim Acta 470:51–56. doi:10.1016/S0003-2670(02)00538-X
Jia J, Wang B, Wu A, Cheng G, Li Z, Dong S (2002) A method to construct a third-generation horseradish peroxidase biosensor:self-assembling gold nanoparticles to three-dimensional sol–gel network. Anal Chem 74:2217–2223. doi:10.1021/ac011116w
Lad A, Agrawal YK (2012a) DNA labeled gold nanoparticles based optical nanobiosensor monitoring DNA-Mitoxantrone interaction. Bionanoscience 2:9–15. doi:10.1007/s12668-011-0030-5
Lad A, Agrawal YK (2012b) Optical nanobiosensor monitoring carboplatin-DNA interaction in vitro-study. Talanta 97:218–221. doi:10.1016/j.talanta.2012.04.020
Lad A, Agrawal YK (2012c) Nanodevices determining toxicological behaviour of therapeutic agent. Rev Nano Nanotech 3(1):217–227. doi:10.1166/rnn.2012.1016
Luo X, Xu J, Zhao W, Chen H (2004) Glucose biosensor based on ENFET doped with SiO2 nanoparticles. Sens Actuators B 97:249–255. doi:10.1016/j.snb.2003.08.024
Martin P, Samuel S, Izumi I, Jie T (2007) Electrochemical nanobiosensors. Sens Actuators B 123:1195–1205. doi:10.1016/j.snb.2006.11.016
Maxwell D, Taylor MJ, Nie S (2002) Self-assembled nanoparticle probes for recognition and detection of biomolecule. J Am Chem Soc 124:9606–9612. doi:10.1021/ja025814p
Ningning Z, Zhu C, Pingang H, Yuzhi F (2005) Electrochemical DNA biosensors based on platinum nanoparticles combined carbon nanotubes. Anal Chim Acta 545:21–26. doi:10.1016/j.aca.2005.04.015
Oliveira Brett AM, Macedo TRA, Raimundo D, Marques MH, Serrano SHP (1998) Voltammetric behaviour of Mitoxantrone at a DNA-biosensor. Biosens Bioelectron 13:861–867. doi:10.1016/S0956-5663(98)00053-0
Qhobosheane M, Santra S, Zhang P, Tan W (2001) Biochemically functionalized silica nanoparticles. Analyst 126:1274–1278. doi:10.1039/B101489G
Robinson JW, Skelly Frame EM, Frame GM (2005) Undergraduate instrumental analysis. Marcel Dekker, New York
Rossi LM, Shi L, Quina FH, Rosenzweig Z (2005) Stöber synthesis of monodispersed luminescent silica nanoparticles for bioanalytical assays. Langmuir 21:4277–4280. doi:10.1021/la0504098
Ryu SW, Kim CH, Han JW, Kim CJ, Jung C, Park HJ, Choia YK (2010) Gold nanoparticle embedded silicon nanowire biosensor for applications of label-free DNA detection. Biosens Bioelectron 25:2182–2185. doi:10.1016/j.bios.2010.02.010
Smart RC, Hodgson E (2008) Molecular and biochemical toxicology. John Wiley and Sons, USA
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse Silica spheres in the Micron size range. J Colloid Interface Sci 26:62–69. doi:10.1016/0021-9797(68)90272-5
Thurston D (2008) Chemistry and pharmacology of anti-cancer drugs. CRC Press, Boca Raton
Wang Y, Chen Q, Zeng X (2010) Potentiometric biosensor for studying hydroquinone cytotoxicity in vitro. Biosens Bioelectron 25:1356–1362. doi:10.1016/j.bios.2009.10.027
Xiao Y, Ju H, Chen H (1999) Hydrogen peroxide sensor based on horseradish peroxidase-labeled Au colloids immobilized on gold electrode surface by cysteamine monolayer. Anal Chim Acta 391:73–82. doi:10.1016/S0003-2670(99)00196-8
Yousef EM, Bathaie SZ, Kazemi SH, Mousavi MF (2011) DNA immobilization on a polypyrrole nanofiber modified electrode and its interaction with salicylic acid/aspirin. Anal Biochem 411:176–184. doi:10.1016/j.ab.2011.01.006
Zhang GJ, Luo ZHH, Huang MJ, Tay GK, Lim EK (2011) Morpholino-functionalized silicon nanowire biosensor for sequence-specific label-free detection of DNA. Biosens Bioelectron 25:2447–2453. doi:10.1016/j.bios.2010.04.001
Acknowledgments
The authors would like to acknowledge Cipla Ltd (Mumbai, India) for providing Mitoxantrone for monitoring toxicological studies by Nanodevice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lad, A.N., Agrawal, Y.K. SiO2-based nanobiosensor monitoring toxicological behavior of Mitoxantrone in vitro. Appl Nanosci 4, 523–529 (2014). https://doi.org/10.1007/s13204-013-0239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0239-4