Abstract
Kinetics and mechanism of heterogeneous transesterification reaction of African pear seed oil (APO) catalyzed by phosphoric acid-activated kaolin clay to produce biodiesel were investigated. Heterogeneous catalyst synthesized by activating clay with phosphoric acid was used to examine the effect of time, temperature, methanol/oil molar ratio, catalyst concentration and agitation speed on the production of biodiesel. The kinetics was studied using two elementary reaction mechanisms: Eley–Rideal (ER) and Langmuir–Hinshelwood–Hougen–Watson (LHHW). The results obtained showed that the clay belongs to kaolinite group and acid-activated clay catalyst, AAC was able to convert APO to standard biodiesel with the variation of catalyst concentration, temperature methanol, speed and reaction time having significant effect in the production. About 78–80% biodiesel production was obtained with 10:1 methanol/oil molar ratio, 3 wt% AAC catalyst concentration, time 3 h, speed 300 rpm and at 60 °C temperature. The kinetics result revealed that the LHHW is the most reliable representation of the experimental data using acid-activated clay catalyst with surface reaction between adsorbed triglyceride and adsorbed methanol as rate determining step (RDS). The activation energy for the forward reaction was determined to be 10.08 kJ/mol. Hence, the production of biodiesel from non edible oil APO with cheap and available heterogeneous catalyst (AAC) is achievable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is a rapid decline in petroleum diesel reserve in recent years due to high demand of this energy source. The usage of this energy source poses serious environmental problems to the society. These challenges have stimulated the search for renewable fuels such as biodiesel, etc. Biodiesel is the most promising alternative diesel fuel which has attracted attention worldwide [11]. It is environmentally friendly because it is produced from natural resources such as vegetable oils, animal fats and waste oils through a process known as transesterification to produce glycerol and fatty acid alkyl esters [8, 10, 32, 33, 35]. In addition to its environmental benign, biodiesel production is cheaper than production of petroleum-based diesel because the cost of its raw materials is cheap and typically accounts for about 70–80% of the total cost of the production. This has stimulated research for production of biodiesel from non-edible resources using cheap and natural catalysts [13, 14, 21].
Most cheap and natural catalysts used for transesterification are heterogeneous catalysts. Comparing homogenous catalysts with heterogeneous catalysts, the later have the advantages of easy and cheap separation and regeneration [4, 13]. Among the heterogeneous catalysts, the basic heterogeneous catalysts have attracted the most attention because of its high catalytic activity, regenerability/reusability, and that there are plenty of relatively inexpensive resources for its production (waste shells, egg shells, clay, etc.). Moreover, they are not sensitive to small amounts of FFA and moisture, and they are, therefore, suitable for crude vegetable oil [5, 35].
Clay is one of natural and cheap heterogeneous catalysts discovered to have high selectivity and product yield. Clays are solid acidic (heterogeneous) catalysts which can function as both Bronsted and Lewis acids in their natural and ion-exchanged form [7]. Modified smectite clays can be very selective catalysts for a wide range of organic transformations. Clay minerals are made up of layered silicates [36]. They are crystalline materials of very fine particle size ranging from 150 to less than 1 micron. There are two basic building blocks—tetrahedral and octahedral layers, which are common to clay minerals [36]. A variety of organic reactions that are catalysed by Brønsted acids such as H2SO4, HCl, and other protonic acids or Lewis acids such as AlCl3, FeCl3, have been shown to take place in clays, more efficiently under milder conditions, with greater selectivity, better yields, shorter reaction times, etc.
Clay is a type of soil which is naturally available in most of the states in Nigeria. Nigerian clay is essentially alumina silicates which have resulted from weathering of rocks and aluminum silicates [15]. The range of reactions that have been successfully performed on clay catalysts includes addition, dehydration, elimination, oxidation, rearrangement reactions, substitution and esterification [36]. They can be used as catalysts for transesterification reaction. Some researchers worldwide have investigated clay catalysts for esterification and transesterification but very few for biodiesel production [22]. Prakash et al. [27] reported transesterification of dicarboxylic acid with various alcohols by Mn+-montmorillonite clay catalysts. Also Vijayakumar et al. [37] used Indian bentonite as esterification catalyst for ester synthesis. Dubios et al. [9] had prepared biodegradable polyester by transesterification catalysts to improve clay exfoliation. Liu et al. [19] produced ethyl/methyl β-ketoester by montmorillonite K-10 as an efficient reusable catalyst. Manuit and Statit [22] studied biodiesel synthesis from transesterification by clay-based catalyst. They discovered that biodiesels from clay–based catalysts have some encouraging properties to supersede low-speed diesel fuel and to lower the cost of production in some extent. Calgaroto et al. [6] studied production of biodiesel from soybean and Jatropha Curcas oils with KSF and amberlyst 15 catalysts in the presence of co-solvents. Moreover, they obtained a higher yield in the presence of ultrasound as compared to the conventional approach under similar conditions [28].
Most of the reviewed studies involving the use of clay catalysts were focused on the catalyst synthesis, characterization and catalytic activity for fatty acid transesterification with short chain alcohols without measuring and properly exploring the kinetics of these mineral clay catalyzed reactions. The present study, therefore, focused on kinetics of biodiesel from African pear seed oil using acid-activated clay catalyst.
Materials and methods
Materials
African pear was bought from Odegba in New Market Enugu, Enugu State, Nigeria. The seeds were dried in sunlight, deshelled and crushed using a grinder prior to oil extraction. N-hexane was used for oil extraction while methanol was used for transesterification and both are of analytical grade. All other solvents and chemicals used were of analytical grade and were procured from commercial sources. The clay was collected from Abakaliki, Ebonyi State, Nigeria.
Biodiesel production from APO was carried out in the research laboratory of the Projects Development Institute (PRODA), Enugu, Nigeria. The fuel properties of biodiesel and blends were determined according to American Society for Testing Materials (ASTM) standard methods in PRODA, Enugu, Nigeria.
Methods
Oil extraction
The method employed by Sanjay et al. [31] was used in extraction of oil from the seed. Extractability of oil was evaluated by solvent extraction of the 100 g of crushed kernel. Crushed kernel in n-hexane with solvent/solute ratio of 1.5 ml/g and constant particle size of 900 µm was magnetically stirred at a constant speed of 200 rpm at temperature range of 50 °C for 45 min. The yield of the crude oil extracted was calculated using Eq. (1).
where Y is the oil yield (%), \(W_{\text{o}}\) is the weight of pure oil extracted (g) and W is the weight of the sample of seed used in the experiment
Synthesis of catalyst
The catalyst employed in this study is clay catalyst. The clay was immersed in hydrogen peroxide solution (30%) in the ratio of 1:2 wt/wt at 30 °C for 24 h to remove organic impurities. The mixture was gently heated in a boiling water bath to remove excess H2O2 and subsequently separated from the clay. The purified clay was then suspended in distilled water in the ratio of 1:4 wt/wt and allowed to settle. The water was removed and the purified clay was dried in an oven at a temperature of 110 °C until its moisture content reached 10%. The clay was then crushed and sieved with 80/100 mesh. The dried clay catalyst was modified by phosphoric acid activation as described below to remove excess salt and improve its activity.
The dried clay sample was mixed with a solution of phosphoric acid, H3PO4 0.5 M in a ratio of 1:1 (g/ml). The reaction was carried out in a round bottom flask at the condition under vigorous stirring and at the temperature of 100 °C for 2 h. The system was heated in glycerine bath connected to a reflux condenser. The acid-activated clay was then washed with distilled water until the pH was close to 7 and dried at 110 °C for 6 h. It was finally grounded into a fine powder.
Characterization of the clay sample
The raw and activated clay samples were characterized by X-ray fluorescence (ARL 9400XP + Wavelength-dispersed XRF spectrometer) to determine metallic compositions, Fourier-transform infra-red spectrometer (BUCK model 500 M) to determine the functional group; scanning electron microscope (Carl Zeiss Sigma Field Emission) to determine the morphology of the clay samples and X-ray Diffractometer (model XRD-7000 with Cu Ka X-ray) to determine the group of the clay. The Brunauer–Emmett–Teller (BET) method was used to calculate the surface area, average pore diameter and total pore volume of the clay. Surface area, pore volume and average pore diameter were determined from N2 adsorption isotherms using a micrometrics ASAP 2020 surface analyzer.
Transesterification reaction
The extracted oil from African pear seed reacted with methanol in the presence of acid-activated clay to produce methyl esters of fatty acids (biodiesel) and glycerol. The oil sample was precisely and quantitatively transferred into a flat bottom flask placed on a hot magnetic stirrer. Specific amount of catalyst (by weight of oil sample) mixed with the required amount of methanol was then added. The reaction flask was kept on a hot magnetic stirrer under constant temperature with defined agitation throughout the reaction. At the defined time, the sample was taken out, cooled, and the biodiesel (i.e., the methyl ester in the upper layer) was separated from the by-product (i.e., the glycerol in the lower layer) by settling the mixture overnight under ambient condition in a separating funnel. The percentage of the biodiesel yield was determined by comparing the volume of biodiesel layer with the volume of oil used using Eq. (2).
Reaction mechanism for transesterification of African pear seed oil
The mechanism proposed by Langmuir–Hinshelwood–Hougen–Watson (LHHW) in transesterification of triglyceride was employed for the kinetic model. Basically, the Langmuir–Hinshelwood–Hougen–Watson (LHHW) mechanism is a mechanism that involves adsorption, surface reaction and desorption of atoms and molecules on the surfaces. From Eq. 3, it is proposed that both reactant molecule A (methanol) and T (triglyceride) are adsorbed at different free sites on the catalyst surface. Then, the reaction took place between chemisorbed (chemical bond between the surface and an atom or a molecule) molecules to give the products B (biodiesel) and G (glycerol). Finally, the adsorbed products B and G were desorbed.
Assumptions
The following assumptions were made using LHHW mechanism to model transesterification reaction catalyzed by heterogeneous reaction:
-
1.
The pellet size of the catalyst is small such that the reaction is not diffusion limited.
-
2.
ii. The activity of the surface toward adsorption, desorption or surface reaction is independent of coverage such that the surface is essentially uniform as far as the various steps in the reaction are concerned.
The elementary steps of LHHW were derived in nine-step sequence as presented in Eqs. 4–12 below.
The ‘S’ represents the active site of catalyst surface and D represents diglyceride, M represents monoglyceride molecules.
I. Assuming alcohol adsorption as rate determining step (Eq. 4)
The site balance is given by:
From the concept of the rate determining step, all other steps are in quasi-equilibrium and the known surface coverage [S] and [AS] in Eq. (13) are expressed in terms of fluid species concentrations. Therefore, Eq. (13) then becomes:
where \(K_{2}\) is the \(\frac{{k_{3} }}{{k_{4} }}\), \(K_{3}\) is the \(\frac{{k_{5} }}{{k_{6} }}\), \(K_{4}\) is the \(\frac{{k_{7} }}{{k_{8} }}\), \(K_{5}\) is the \(\frac{{k_{9} }}{{k_{10} }}\), \(K_{6}\) is the \(\frac{{k_{11} }}{{k_{12} }}\), \(K_{7}\) is the \(\frac{{k_{13} }}{{k_{14} }}\), \(K_{8}\) is the \(\frac{{k_{15} }}{{k_{16} }}\), \(K_{9}\) is the \(\frac{{k_{17} }}{{k_{18} }}\)
And for completeness;
A summary of the nine (9) derived rate equations for the LHHW reaction mechanism are shown below.
II. Assuming triglyceride adsorption as rate determining step (Eq. 5)
III. Assuming surface reaction between adsorbed triglyceride and adsorbed alcohol as rate determining step (Eq. 6)
IV. Assuming surface reaction between adsorbed diglyceride and adsorbed alcohol as determining step (Eq. 7)
V. Assuming surface reaction between adsorbed monoglyceride and adsorbed alcohol as rate determining step (Eq. 8)
VI. Desorption of biodiesel as rate determining step (Eq. 9)
VII. Desorption of diglyceride as rate determine step (Eq. 10)
VIII. Desorption of monoglyceride as rate determining step (Eq. 11)
IX. Desorption of glycerol as rate determining step (Eq. 12)
Concentration of T, D, M, A, B and G were determined from GC analysis using equation adopted by Olutoye and Hameed [25].
where \(C_{i}\) is the concentration of triglyceride or diglyceride or monoglyceride or glycerol or alcohol or biodiesel, A is the peak area of the triglyceride/diglyceride/monoglyceride/glycerol/alcohol/biodiesel component as will be determined by GC.
The derived rate equations (Eqs. 15–23) were used as models for the conversion of African seed oil triglyceride on phosphoric acid modified clay. The rate and equilibrium constants were determined using nonlinear regression analysis of POLYMATH 5.1 to search for those parameter values that minimize the sum of the squares of difference between the measured rates and the calculated rates for all the data points as shown in Eq. (25) with initial guess of 0.01 and 10 for rate constant and equilibrium constant, respectively. Each reaction rate was determined using POLYMATH 5.1 by developing polynomial equation with concentration of various species in the reaction obtained by GC–MS analysis. The models were compared using their individual variances calculated using Eq. (26) at 95% confidence level, positiveness of rate and equilibrium constants and goodness of fit according to Onukwuli et al. [26].
where \(S^{2}\) is the sum of squares, N is the no of runs, K is the no of parameters to be determine, \(r_{im}\) is the measured reaction rate for run i, \(r_{ic}\) is the calculated reaction rate of run i, \(\sigma^{2}\) is the variance
The mechanism proposed by Eley–Rideal was also used to model the kinetics of the transesterification reaction catalyzed by acid-activated clay catalyst. The mechanism involves the reaction between an adsorbed molecule and non-adsorbed molecule. In this study, methanol was assumed to be adsorbed while T, D, and M are non-adsorbed.
The elementary steps of Eley–Rideal were derived in seven-step sequence as presented in Eqs. 27–33 below.
The ‘S’ represents the active site of catalyst surface and D represents diglyceride, M represents monoglyceride molecules. Similarly, the rate equations were derived as in LHHW mechanism and summary of the equations are presented below:
I. Assuming alcohol adsorption as rate determining step (Eq. 27)
II. Assuming surface reaction between triglyceride and adsorbed alcohol as rate determining step (Eq. 28)
III. Assuming surface reaction between diglyceride and adsorbed alcohol as determining step (Eq. 29)
IV. Assuming surface reaction between monoglyceride and adsorbed alcohol as rate determining step (Eq. 30)
V. Desorption of glycerol as rate determining step (Eq. 31)
VI. Desorption of diglyceride as rate determine step (Eq. 32)
VII. Desorption of monoglyceride as rate determining step (Eq. 33)
Result and discussion
Extraction of oil
African pear (D. edulis) oil extracted by solvent extraction using n-hexane was liquid at room temperature. This implies that it could be classified as oil. The percentage oil yield of the African pear oil, APO was 53.1% and it falls within the range reported by Umoti and Okyi [34]. Umoti and Okyi [34] in Isaac et al. [17] gave the range of oil yield of African pear oil extracted by solvent extraction as 40–65% depending on the maturity of the fruits, while the range of yield obtained by press extraction was given as 25–49%. It has been established that the oil content of African pear (D. edulis) varies from species to species [16]. The yield was also relatively higher than the yields reported for other non edible seed oil like Mangifera indica; 30.7% [23] almond seed oil; 47% [24]. The observed oil content of edulis was also found comparable to the yields of some edible oil such as soybeans 65% and cottonseed 60% [29]. The relatively high oil content of Dacryodes will encourage less dependence on edible oils as feedstock for biodiesel production. It will promote food security and food availability. Besides, the cost of producing biodiesel will be minimized, since the major feedstock is cheaply available, hence making biodiesel economically pleasant.
Table 1 shows the physiochemical properties of the raw oil of Dacryodes edulis (African pear seed) seed. The oil has moderate acid number and free fatty acid values of 5.49mgKOH/g and 2.75%, respectively. These values suggest the pretreatment step on the raw-oil before the transesterification step using homogeneous catalyst but the process was circumvented using heterogeneous catalysts. The physicochemical properties of the raw oil compared favourably with those of some other non–edible oils such as Pongamia pinnata [1], Jatropha Curcas [2], Madhuca Indica [3].
The densities and high viscosities of both oil will make their atomization difficult in internal combustion engine, hence they cannot be used directly as bio-fuel. The low pour point shows that the oil will hardly solidify at room temperature hence can be stored for a long time. The oxidation stability of the oil was high and is good for the production of biodiesel. The high oxidation stability of the oils could be as a result of method used in extracting the oil. Solvent refining results in the production of base oil, which retain some sulphur compounds that are natural antioxidants. These base oils retained natural ability to prevent oxidation, while hydro-treated base oils must be further fortified with antioxidants to maintain thermal and oxidation stability.
Characterization of clay catalyst
Physicochemical properties of the synthesized catalyst
The physical properties of the raw clay catalyst and modified clay catalysts are presented in Table 2. From the table, it could be observed that the properties of the clay catalyst improved after acid activation. The pore size of the raw clay decreased after the activation and this may be attributed to increase in surface area after activation. The raw clay has lower iodine value which later increased after activation with phosphoric acid. This may be attributed to the high degree of activation by the acid which contributed to improvement of the activity of the clay.
X-ray fluorescence analysis of the clay catalysts
The chemical composition of the raw clay and acid-activated clay used in this study is summarized in Table 3. The main compositions of the clay are Si, Al, Ti and Fe. It contains metallic oxides such as MgO, Al2O3, SiO2, K2O, CaO, TiO2, Cr2O3, Mn2O3, Fe2O3, ZnO and SrO which are constituents of heterogeneous catalysts [30]. The clay has high amounts of SiO2, Al2O3 and Fe2O3. The modification of the raw clay by acid increased the quantity of SiO2, Al2O3 and reduced the quantity of Fe2O3 classifying the modified clay as Brønsted and Lewis acids whose acidity and catalytic properties are largely dependent on the electronegativity of interlamellar spacing of exchangeable cations attached to the negatively charged aluminosilicate sheets. Consequently, the activation enhanced the amount and strength of Brønsted and Lewis acid sites [30].
Fourier-transform infra red (FTIR) analysis of the catalysts
Fourier-transform infra red (FTIR) spectroscope of the raw clay catalyst and the acid modified clay catalysts was done to ascertain the functional groups present in them and depicted in Figs. 1 and 2, respectively. Comparing the raw clay to the acid-activated clay it could be observed that there was no significant decrease in intensity of bands 998.9 and 685 cm−1, suggesting that the structural changes attributed to the modifications are small. According to Zatta et al. [38], the FTIR bands at 998.9, 909.5, and 685 cm−1 are attributed to Al–OH–Al, Al–OH–Mg and Al–OH–O–Si vibrations, respectively. From Figs. 1 and 2, it can be concluded that the catalysts have common functional groups such as Si–O–Si, C–Cl of aliphatic chloro compounds, C–H stretch of aromatic bend, C–H stretch of vinyl, Organic silicone Si–O–C, Si–O, double bond C=C, –OH stretch of primary alcohol, Al–OH–Al, Al–OH–Mg and Al–OH–O–Si vibrations. The raw clay catalyst contains cyanide ion (band 2009 cm−1) which can hinder catalytic activity of the clay but the modification of the clay with acid removed the cyanide content. Moreover, the raw clay and acid-activated clay show broad peak in a region of 3652.8–3693.8 cm−1 that can be assigned to Bronsted acidity and 450–690 cm−1 can be assigned to Lewis acidity. The appearance of peak 1640 cm−1 and 685 cm−1 in activated clay indicates the increase in the strength of both the Bronsted and Lewis active sites. These peaks assignments were done based on the previous work by Jacques [18]. These suggest that the clay catalyst used performed as both Bronsted and Lewis acids.
Scanning electron microscopes (SEM) of the clay catalysts
The morphologies of the raw clay catalyst and acid-activated clay catalyst were performed by SEM as shown in Figs. 3 and 4 respectively. Micrographs of the clay catalyst samples synthesized by acid activation showed increase in number of pores and pore size on the clay. For the acid-activated clay, the formation of more pores on the clay particles was observed and this supports the fact that it has more surface area with higher pore size as reported in “Physicochemical properties of the synthesized catalyst”.
X-ray diffraction pattern of the clay catalysts
Figures 5 and 6 depicts XRD of the raw clay and acid-activated clay catalyst, respectively. It is observed that clay contains kaolinite, quartz and muscovite confirming the presence of alumina and silica. This suggests that the clay belongs to kaolinite group.
Effects of process parameters on biodiesel yield
Effect of time on biodiesel yield
The conversion rate increased with reaction time. In this work, the effect of reaction time from 1 to 5 h on the reaction yield using acid-activated clay catalyst is presented in Fig. 7. It was found that higher yield occurred at reaction time of 3 h and beyond it the yield decreased. The reaction was very slow due to diffusion of methanol and triglyceride into the active site of the catalysts was slow and the decreased in the yield after 3 h reported above may be due to reversible reaction of transesterification resulting in loss of esters.
Effect of catalyst concentration on biodiesel yield
Acid-activated clay catalyst was used as heterogeneous catalyst for the transesterification reaction in this study. The effect of catalyst concentration expressed as weight percentage of the African pear seed oil on the production yield is presented in Fig. 8. From the figure, it could be observed that the yield of methyl ester increased with increase in catalyst weight up to 3 wt% and then began to decrease. Initially the amount of catalyst helped to accelerate the reaction by increasing the reaction rate. The higher yield of ester with increase in catalyst weight is due to more availability of catalyst in the reaction medium. Increasing the catalyst weight beyond the catalyst weight of 3 wt% led to the decrease in ester yield. This may be due to excess catalyst causing dispersion and mixing problems, thereby inhibiting the formation of end product [39].
Effect of methanol/oil molar ratio on biodiesel yield
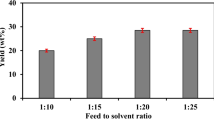
The alcohol–oil molar ratio is one of the most important factors that can affect the yield of esters. The stoichiometry of the transesterification reaction requires 3:1 molar ratio to yield 3 mol of ester and 1 mol of glycerol, but most researchers have found that excess alcohol was required to drive the reaction close to completion [39]. In this research, the effect of methanol/oil molar ratio of 6:1–14:1 was investigated. The yield of methyl esters at the different molar ratio of methanol/oil is shown in Fig. 9. The result indicated that methanol–oil molar ratio has significant impact on biodiesel yield. The maximum ester yield was obtained at a methanolysis of oil molar ratio of 10:1. The higher molar ratio results in higher rate of ester formation. The yield reduced when the molar ratio was beyond 10:1. This may be due to decrease in the catalyst activity with increase in methanol content and difficulty in glycerol separation. The results obtained are in agreement with the reports of earlier works of Zhang et al. [39].
Effect of temperature on biodiesel yield
The influence of temperature on reaction metrics during African pear seed oil transesterification using activated clay was investigated over the temperature range 45–70 °C. As shown in Fig. 10, the reaction rate was slow at low temperatures, but biodiesel yield first increased and then decreased with the increase of the reaction temperature beyond 60 °C. The increase in yield with temperature is consistent with the Arrhenius behaviour of the reaction rate. However, beyond 60 °C, the product yield dropped probably due to significant loss of methanol from the biphasic liquid reaction medium since it has a boiling point of 64.7 °C. Similar results have been reported by Liu et al. [20].
Effect of speed of agitation on biodiesel yield
The mixing appears to be of particular importance for the transesterification process: it ensures homogeneity within the reaction mixture. It increased the contact area between oil/methanol mixture and catalyst [20]. Mixing also facilitates the reaction. In this study, methanolysis was conducted with different rate of stirring such as 100, 200, 300, 400 and 500 revolutions per minutes (rpm). The yield of methyl esters produced from African pear seed oil with acid-activated clay catalyst at different rate of mixing is shown in Fig. 11. It was observed from the figure that the reaction of methanolysis was low at 100 rpm and only exhibited a yield which was difficult to separate. The yield was observed to decrease as the stirring rate went above 300 rpm and this may be that oily phase was stratified close to the rotating shaft leading to the downturn in biodiesel yield at higher speeds.
Fuel properties produced with optimal conditions
Table 4 gives a summary of all the fuel properties analyzed and the limits that they were compared with (ASTM standards). It was observed that the properties of biodiesel produced are within the acceptable limits and can be used in an internal combustion engine without modification.
Product distribution
The formation of products and disappearance of reactants at temperatures of 45, 50, 55 °C are depicted in Figs. 12, 13, and 14, respectively. From the graphs, it could be observed that the products (biodiesel and glycerol) concentration increase as time increases and the reactants (triglyceride, diglyceride, monoglyceride and methanol) concentration decrease as time increases. The changes in concentration of products and reactants became constant after 3 h for all the temperatures. This could be that the reaction reached its end point at 3 h. The figures also show that biodiesel is more predominate in the product with a higher concentration at a temperature of 55 °C. This shows that higher yield is obtained at temperature of 55 °C.
Kinetics study of heterogeneous catalyzed transesterification
The kinetics study of the methanolysis of African pear seed oil (APO) using phosphoric acid activated clay (AAC) was carried out using two elementary reaction mechanisms: Langmuir–Hinshelwood–Hougen–Watson (LHHW) and Eley–Rideal (ER). Nine kinetic models with assumptions of adsorption of species, surface reaction and desorption of species were investigated for LHHW and seven models for Eley–Rideal (ER). It was observed that for all the temperatures considered, the rate constants and variances for surface reaction between adsorbed triglyceride and adsorbed methanol were lowest for LHHW model and the rate equation has best fit of the experimental data in terms of conversion as shown in Fig. 13 while rate constant and variances between adsorbed methanol and non-adsorbed triglyceride for ER model were lowest with the equation having best fit of the experimental data in terms of conversion as depicted in Fig. 14. These can be considered as rate determining steps (RDS) and it is in agreement with assumption that approximately 75% of all heterogeneous reaction mechanisms are surface-reaction-limited [12]. The rate parameters of the rate determining step are presented in Tables 5 (LHHW model) and 6 (ER model). It was observed that the rate constant increased as temperature increases showing that the reaction is endothermic and proceeds at higher temperature below the boiling point of methanol. The activation energies for the forward and backward reactions of the rate determining step were 10.08 kJ/mol and 0.0172 kJ/mol, respectively, for LHHW model while activation energies for the forward and backward reactions of the rate determining step for ER model were 0.081 kJ/mol and 0.029 kJ/mol, respectively. It is evident in the value of activation energy that the reaction is mass transfer resistance and that the reaction was slow because triglyceride is a heavy molecule with higher potential energy than the reactant methanol. From Tables 5 and 6, it is observed that the adsorption energies of steps 1 and 2 for LHHW model were endothermic while desorption energies for steps 6, 7 and 8 for LHHW were exothermic.
The temperature sensitivity of step 9 for LHHW model was inconsistent with others. The reaction energies for steps 2 and 3 for ER model were positive as shown in Table 6 indicating that the reactions are endothermic. The desorption energy for step 6 is positive while that of step 7 is negative. The temperature sensitivity of step 7 for 4 ER model was inconsistent with step 6. Comparing LHHW model and ER model (Table 7), it can be observed that the variances of the RDS (rate Eq. 3) at different temperature for LHHW were smaller than that of RDS (rate Eq. 2) for ER with better fitting. These show that the LHHW model best describes the kinetic data at all temperature most especially at 55 °C (Figs. 15, 16).
Conclusion
The application of economical and environment-friendly waste materials in production of biodiesel will play an important role in economizing the process and consequently expand the use of biodiesel all around the world. In this study, acid-activated clay (AAC) catalyst was used for producing biodiesel from African Pear Seed oil (APO) with methanol and compared with standard quality of biodiesel. The reaction was promoted by the large surface area and acidity of the catalyst and the biodiesel produced has qualities within the acceptable limits for biodiesel. The kinetics study showed that the transesterification of APO with AAC catalyst suits Langmuir–Hinshelwood–Hougen–Watson (LHHW) with surface reaction between adsorbed triglyceride and adsorbed methanol as rate determining step (RDS). The rate constant increased as temperature increase show that the reaction was endothermic and preceded at higher temperature below the boiling point of methanol. Therefore, the production of quality biodiesel from APO with synthesized clay catalyst will make biodiesel production economical and affordable because the raw materials are cheap and easily available. The kinetic parameters will assist in design of reactors for biodiesel production.
References
Agarwal M, Garima C, Chaurasia SP, Singh K (2012) Study of catalytic behaviour of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J Taiwan Inst Chem Eng 43:89–94
Adebayo GB, Ameen OM, Abass LT (2011) Physico-chemical properties of biodiesel produced from Jatropha Curcas oil and fossil diesel. J Microbiol Biotechnol Res 1(1):12–16
Azam MM, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 29:293–302
Birla A, Singh B, Upadhyay SN, Sharma YC (2012) Kinetics studies of synthesis of biodiesel from waste frying oil using a heterogeneous catalyst derived from snail shell. Biores Technol 106:95–100
Boey PL, Maniam GP, Hamid SA (2011) Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: a review. Chem Eng J 168:15–22
Calgaroto C, Calgaroto S, Mazutti MA, de Oliveira D, Sibele Pergher SJ, Vladimir de Oliveira JV (2013 Production of biodiesel from soybean and jatropha curcas oils with ksf and amberlyst 15 catalysts in the presence of co-solvents. Sustain Chem Process 1:17–22
Clark JH (1994) Catalysis of organic reaction. Supp. Inorg. Reagt. VCH, New York
Di Serio M, Tesser R, Pengmei L, Santacesaria E (2008) Heterogeneous catalysts for biodiesel production. Energy Fuels 22:207–217
Dubios P, Pollet E, Delcourt C, Alexandre M (2006) Transesterification catalysts to improve clay exfoliation in synthetic biodegradable polyester nanocomposite. Eur Polymer J 42:1330–1341
Endalew AK, Kiros Y, Zanzi R (2011) Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 35:3787–3809
Fan X, Wang X, Chen F (2011) Biodiesel production from crude cottonseed oil; an optimization process using response surface methology. Open Feuls Energy Sci J 4:1–8
Fogler HS (2011) Element of chemical reaction engineering, 4th edn. Pearson Education Inc, Upper Saddle River, pp 655–703
Gaikwad ND, Gogate PR (2015) Synthesis and application of carbon based heterogeneous catalysts for ultrasound assisted biodiesel production. Green Process Synth 4:17–30
Gole VL, Gogate PR (2012) A review on intensification of synthesis of biodiesel from sustainable feed stock using sonochemical reactors. Chem Eng Process 53:1–9
Igbokwe P, Ogbuagu J (2003) Effects of process parameters on the extraction of alumina from indigenous kaolinitic clay deposit. Niger J Eng Res Dev 2(2):23–26
Isaac IO, Ekpa OD (2009) Minerals and anti-nutrients in two varieties of African pear (Dacryodes edulis). J Food Technol 7(4):106–110
Isaac IO, Ekpa OD, Ekpe UJ (2014) Extraction, characterization of African pear (dacryodes edulis) oil and its application in synthesis and evaluation of surface coating driers. Int J Adv Res Chem Sci 1(4):14–22
Jacques CV (2015) Acid-base characterization of heterogeneous catalysts: an up-to-date overview. Res Chem Intermed 41:9387–9423
Liu DH, Wei D, Xu YY, Jing Z (2004) Novozyrn 435-catalysed transesterification of crude soy bean oils for biodiesel production in a solvent-free medium. Biotechnol Appl Biochem 40:187–190
Liu X, He H, Wang Y, Zhu S, Piao X (2008) Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87:216–221
Maddikeri GL, Pandit AB, Gogate PR (2012) Intensification approaches for biodiesel synthesis from waste cooking oil: a review. Indus Eng Chem Res 51:14610–14628
Manuit J, Statit P (2007) Biodiesel synthesis from transesterification by clay-based catalyst. Chiang Mai J Sci 34(2):201–207
Ogunsuyi HO (2012) Acid and Base Catalysed Transesterification of Mango (Mangifera Indica) Seed Oil to Biodiesel. IOSR J Appl Chem (IOSRJAC) 2(2):18–22
Ogunsuyi HO, Daramola BM (2013) Evaluation of almond seed oil as viable feedstock for biodiesel fuel. Int J Biotechnol Resour 1(8):120–127
Olutoye MA, Hameed BH (2016) Kinetics and deactivation of dual-site heterogeneous oxide catalyst during the transesterification of crude jatropha oil with methanol. J Taibah Univ Sci 10(5):685–699
Onukwuli OD, Ngomo HM, Susu AA (1999) Reforming of n-octane on a Pt/Al2O3 catalyst. 1. Product distribution and kinetics analysis. Pet Sci Technol 17(7–8):711–735
Prakash BSJ, Reddy CR, Iyengar P, Nagendrappa G (2005) Esterification of dicarboxylic acids to diesters over Mn+-montmorillonite clay catalysts. Catalysis Lett 101:87–91
Pukale DD, Maddikeri GL, Gogate PR, Pandit AB, Pratap AP (2015) Ultrasound assisted transesterification of waste cooking oil using heterogeneous solid catalyst. Ultrasonic Sonochem 22:278–286
Rashid U, Farooq A, Gerhard K (2009) Evaluation of biodiesel obtained from cottonseed oil. Fuel Process Technol 90(9):1157–1163
Sani YM, Daud WMAW, AbdulAziz AR (2014) Activity of solid acid catalysts for biodiesel production: a critical review. Appl Catal A 470:140–161
Sanjay B, Dinesh CD, Dibakar CD (2012) Composition of biodiesel from Gmelina arborea seed oil. Adv Appl Sci Res 3(5):2745–2753
Sharma YC, Singh B, Korstad J (2011) Latest developments on application of heterogenous basic catalysts for an efficient and eco friendly synthesis of biodiesel: a review. Fuel 90:1309–1324
Taufiq-Yap YH, Lee HV, Hussein MZ, Yunus R (2011) Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass Bioenergy 35:827–834
Umoti U, Okyi DA (1987) Characteristics and composition of oil and cake of African pear. J Sci Food Agric 38:67–72
Veljkovic VB, Stamenkovic OS, Todorovic ZB, Lazic ML, Skala DU (2009) Kinetics of sunflower oil methanolysis catalyzed by calcium oxide. Fuel 88:1554–1562
Vijayakumar B (2004) Clay catalyst in organic synthesis. SYNLETT 2:388–389
Vijayakumar B, Iyengar P, Nagendrappa G, Prakash BSJ (2005) An eco-friendly method for the synthesis of aryl and alkyl esters of carboxylic acids using acid activated Indian bentonite. J Indian Chem Soc 82:922–925
Zatta L, Ramos LP, Wypych F (2013) Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric acid acid with methanol. Appl Clay Sci 80–81:236–244
Zhang Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Biores Technol 90:229–240
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Onukwuli, O.D., Ude, C.N. Kinetics of African pear seed oil (APO) methanolysis catalyzed by phosphoric acid-activated kaolin clay. Appl Petrochem Res 8, 299–313 (2018). https://doi.org/10.1007/s13203-018-0210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-018-0210-0