Abstract
Lima bean (Phaseolus lunatus L.) is an important legume species that establishes symbiosis with rhizobia, mainly of the Bradyrhizobium genus. The aim of this study was to evaluate the efficiency of rhizobia of the genus Bradyrhizobium in symbiosis with lima bean, in both Leonard jars and in pots with a Latossolo Amarelo distrófico (Oxisol). In the experiment in Leonard jars, 17 strains isolated from nodules of the three legume subfamilies, Papilionoideae (Vigna unguiculata, Pterocarpus sp., Macroptilium atropurpureum, Swartzia sp., and Glycine max), Mimosoideae (Inga sp.), and Caesalpinioideae (Campsiandra surinamensis) and two uninoculated controls, one with a low concentration (5.25 mg L−1) and another with a high concentration (52.5 mg L−1) of mineral nitrogen (N) were evaluated. The six strains that exhibited the highest efficiency in Leonard jars, isolated from nodules of Vigna unguiculata (UFLA 03–144, UFLA 03–84, and UFLA 03–150), Campsiandra surinamensis (INPA 104A), Inga sp. (INPA 54B), and Swartzia sp. (INPA 86A), were compared to two uninoculated controls, one without and another with 300 mg N dm−3 (NH4NO3) applied to pots with samples of an Oxisol in the presence and absence of liming. In this experiment, liming did not affect nodulation and plant growth; the INPA 54B and INPA 86A strains stood out in terms of shoot dry matter production and provided increases of approximately 48% in shoot N accumulation compared to the native rhizobia populations. Our study is the first to indicate Bradyrhizobium strains isolated from the three legume subfamilies are able to promote lima bean growth via biological nitrogen fixation in soil conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lima bean (Phaseolus lunatus L.) is the second most economically important crop among the four commercially exploited species of the Phaseolus genus worldwide (Fofana et al. 1999). Although the use of lima bean is less than that of common bean (P. vulgaris), it is one of the main legume species cultivated in tropical regions and represents an important alternative protein source for human consumption (Maquet et al. 1999). In Brazil, the Northeastern region accounts for approximately 95% of lima bean production, with a planted area of 41,318 ha, production of 17,078 tons of beans, and grain yield of approximately 420 kg ha−1 for the year 2009 (available at http://www.sidra.ibge.gov.br). This low grain yield may be attributed to the fact that most of the production is from small-scale farmers with limited access to agricultural technology.
Like other species of the Phaseolus genus, lima bean is able to establish symbiosis with rhizobia (Thies et al. 1991; Ormeno-Orrillo et al. 2006; Antunes et al. 2011; Matsubara and Zúñiga-Dávila 2015). P. lunatus is a very diverse species with many genotypes which vary in seed morphology, color, and size. The few papers reporting N2 fixation in this species have usually restricted study to one cultivar. The symbiont Bradyrhizobium genus has the main focus for research (Ormeno-Orrillo et al. 2006; López-López et al. 2013; Matsubara and Zúñiga-Dávila 2015; Costa Neto et al. 2017). However, information on the agronomic implications of this symbiosis is scarce, especially in Brazil, where only two studies evaluating the symbiotic efficiency of rhizobia strains in lima bean, have been performed under axenic conditions (Antunes et al. 2011; Costa Neto et al. 2017). Thus, there is considerable need to expand studies, select, and recommend strains to increase grain yield in this crop.
Among the rhizobia genera, Bradyrhizobium has attracted attention in Brazil because it has broad geographic distribution and establishes efficient symbiosis with many legume species of importance for agriculture, in pasture areas, and in forestry. Furthermore, natural and altered ecosystems within the Amazon region accommodate a large diversity of Bradyrhizobium strains (Moreira et al. 1993; Moreira et al. 1998; Lima et al. 2009; Guimarães et al. 2012; Jaramillo et al. 2013) that may be considered important sources of genetic resources for biotechnological application.
In Brazil, rhizobia strains have been selected and authorized by the Ministry of Agriculture (MAPA) for production of inoculants for 83 legume species, Among these, 62% have inoculant strains from the genus Bradyrhizobium (details available at http://www.agricultura.gov.br). A main advantage of this genus is that most of its nodulation and N2 fixation genes are located on the chromosome, so that they tend to be genetically stable. This characteristic is extremely important for the selected strain not to lose its symbiotic efficiency over the years. In addition to the symbiotic efficiency and genetic stability of a strain, the ability to nodulate and fix nitrogen in symbiosis with different legume species and its competitiveness with native rhizobia populations should also be considered when selecting and recommending new rhizobia strains for a certain legume species. Competitiveness of a strain with native rhizobia populations is one of the main factors determining the response of a legume species to inoculation (Singleton and Tavares 1986; Thies et al. 1991). Other factors, such as the genetic traits of both the macro- and microsymbiont, soil acidity, and nutrient availability, can also affect nodulation and the BNF process (Hartwig 1998; Bonilla and Bolaños 2009; Moreira et al. 2010; Rufini et al. 2011).
The aim of this study was to evaluate the efficiency of nitrogen-fixing bacteria of the Bradyrhizobium genus isolated from diverse legume species in symbiosis with lima bean under axenic conditions and in soil.
2 Materials and methods
2.1 Strains evaluated
In this study, 17 Bradyrhizobium strains from the collection of the Sector of Biology, Microbiology, and Biological Processes of the Soil (Setor de Biologia, Microbiologia e Processos Biológicos do Solo - SBMPBS) of the Federal University of Lavras (Universidade Federal de Lavras - UFLA) were used. These strains were recently characterized by housekeeping gene sequencing (Guimarães et al. 2015; Ribeiro et al. 2015), which indicated that they belong to different phylogenetic groups (Table 1). The origin and efficiency of these strains in symbiosis with other legume species - cowpea (Vigna unguiculata), siratro (Macroptilium atropurpureum), or soybean (Glycine max) - is shown in Table 1. The ability of the rhizobia strains to fix nitrogen in symbiosis with different legume species is a desirable characteristic since it facilitates commercialization by companies producing inoculants.
2.2 Strain efficiency under axenic conditions
The experiment was conducted at the SBMPBS (UFLA) from April to May 2014. Mean values of maximum and minimum temperatures in the greenhouse were 38 and 14 °C, respectively, during the experiment period. Treatments consisted of individual inoculations of 17 Bradyrhizobium strains and two uninoculated negative controls, one with a low concentration (5.25 mg L−1) and the other with a high concentration (52.5 mg L−1) of mineral nitrogen (N). The experiment followed a completely randomized design, with three replicates.
A 1:2 mixture of sand (150 cm3) and vermiculite (300 cm3) was placed at the top of Leonard jars. Hoagland nutrient solution (Hoagland and Arnon 1950), modified as described by Guimarães et al. (2012), was placed at the bottom of the jars. The same N concentration used in the nutrient solution for the control with low N concentration was used for the inoculated treatments. After preparation, the jars and nutrient solution were autoclaved for one hour at 1.5 kg cm−2 at 121 °C.
An accession of “criolo” lima bean (white seed), one of the accessions often grown by farmers in Northeastern Brazil, was used. Before sowing, the seeds were surface sterilized using 98% ethyl alcohol (30 s) and 2% sodium hypochlorite (2 min). Seeds were successively washed in sterile distilled water, pre-germinated in sterile petri dishes containing filter paper and wet cotton, and then kept for 48 h in a growth chamber at 28 °C. Four seeds were sown in each Leonard jar and thinning was performed seven days after emergence, leaving one plant per jar.
The bacterial strains were cultured in liquid culture medium 79 (Fred and Waksman 1928) under stirring at 110 rpm at 28 °C for five days. In each inoculated treatment, 1 mL of inoculant at a concentration of 1 × 108 bacterial cells mL−1 was added to each seedling. After inoculation, a layer of paraffin sand (10 kg of sand, 1 L of chloroform, and 10 g of paraffin) was added to each pot to avoid possible contamination. The nutrient solution was prepared, autoclaved, and reapplied to the pots periodically throughout the experiment.
At 45 days after sowing (onset of flowering) the SPAD (Soil Plant Analysis Development) index, which represents an indirect measurement of leaf chlorophyll content, was determined. A total of 15 readings were taken from the last fully developed trifoliate leaf for each plant using a Minolta SPAD-502 chlorophyll meter. After the readings, the plants were collected to evaluate the following variables: number of nodules (NN), nodule dry matter (NDM), shoot dry matter (SDM), root dry matter (RDM), total dry matter (TDM), and shoot nitrogen accumulation (SNA). The nodules, shoots, and roots were placed in paper bags and dried in a forced air oven at 60 °C to constant weight to determine the NDM, SDM, and RDM. Shoot N content was determined using the semi-micro Kjedahl method. The SNA was calculated by multiplying the SDM (mg) by the N content (%) / 100.
According to the Shapiro-Wilk test, the experimental data exhibited normal distribution and were subjected to analysis of variance using the SISVAR 5.3 statistical analysis program (Ferreira 2011). Mean values were grouped by the Scott-Knott test at 5% probability. The NN and NDM data were transformed into the square root of (Y + 0.5). Pearson correlation coefficients were estimated at 1% and 5% probability levels.
2.3 Strain efficiency in pots with soil
The experiment was conducted from October to November 2014 in a greenhouse at the SBMPBS (UFLA). Soil characterized as a Latossolo Amarelo distrófico (Oxisol) collected in the municipality of São Luís, MA, in a site at the Federal Institute of Education, Science, and Technology of Maranhão (Instituto Federal de Educação, Ciência e Tecnologia do Maranhão) (altitude 27 m, 2°36′37″S and 4°16′18″W) was used. The state of Maranhão is one of the main lima bean producers in Northeastern Brazil. Additionally, this soil class is predominant within the state and throughout Brazil. The site where the samples were collected has a history of annual corn (Zea mays L.) cultivation under a conventional tillage system. Every three years, liming and N-P-K fertilization (100–80-90) is performed and there is no history of application of any type of inoculant. Liming was last performed at this site two years before this study.
Soil samples were collected at a depth of 0–20 cm and then crushed, homogenized, passed through a 4-mm sieve, and placed in pots (3.5 dm3 capacity). Before implementing the experiment, the soil had the following characteristics: pH in H2O, 6.0; P (Mehlich 1), 2.3 mg dm−3; K+, 20 mg dm−3; Ca2+ , 1.60 cmolc dm−3; Mg2+, 0.60 cmolc dm−3; Al3+, 0.00 cmolc dm−3; H + Al, 2.32 cmolc dm−3; sum of exchangeable bases, 2.25 cmolc dm−3; cation exchange capacity, 2.25 cmolc dm−3; cation exchange capacity at pH 7.0, 4.57 cmolc dm−3; aluminum saturation, 0.00%; base saturation, 49.26%; organic matter, 1.87 dag kg−1; clay, 13 dag kg−1; silt, 5 dag kg−1; and sand, 82 dag kg−1.
The experiment followed a randomized block design with four replicates in an 8 × 2 factorial arrangement [8 N sources and 2 liming levels (with and without liming)]. The following N sources were used: individual inoculation of the 6 Bradyrhizobium strains that exhibited highest efficiency in the experiment in Leonard jars (UFLA 03–84, UFLA 03–144, INPA 104A, INPA 54B, INPA 86A, and UFLA 03–150) and 2 uninoculated controls, one without and another with mineral N fertilizer (300 mg dm−3). The lime application rate in the liming treatments was calculated according to the base saturation method to raise the saturation to 60% using calcium carbonate (CaCO3) and magnesium carbonate (MgCO3) at a ratio of 4:1, respectively. The soil was kept moist and incubated for 30 days before planting. In all plots, the following fertilization was carried out: 300, 300, 40, 5.0, 1.5, 3.6, 0.8, and 0.15 mg dm−3 of K, P, S, Zn, Cu, Mn, B, and Mo, respectively. For the control with mineral N, NH4NO3 (300 mg N dm−3) was provided, divided into three applications.
The lima bean seeds and the method for disinfecting them were the same as used in the previous experiment. Six seeds were sown per pot, and each inoculated treatment received 1 mL of the inoculant at 1 × 108 bacterial cells mL−1 on each seed. Eight days after emergence, the plants were thinned to two seedlings per pot.
The plants were collected at 45 days after sowing (during flowering) to evaluate the following variables: number of nodules (NN), nodule dry matter (NDM), shoot dry matter (SDM), root dry matter (RDM), total dry matter (TDM), and shoot nitrogen accumulation (SNA). These variables were obtained by the procedure described in the previous experiment. According to the Shapiro-Wilk test, the experimental data exhibited normal distribution and were subjected to analysis of variance using the SISVAR 5.3 statistical analysis program (Ferreira 2011). Mean values were grouped by the Scott-Knott test at 5% probability. The NN and NDM data were transformed into the square root of (Y + 0.5). Pearson correlation coefficients were estimated at 1% and 5% probability levels.
3 Results
3.1 Strain efficiency under axenic conditions
The treatments had effects (p < 0.05) on all the variables evaluated (Table 2). There was no nodulation in the uninoculated controls, indicating that there was no contamination in the experiments. Only four strains did not nodulate lima bean (UFLA 03–268, UFLA 03–290, UFLA 06–24, and UFLA 06–13). Of the strains that nodulated, the mean NN values per plant ranged from 15 to 506 for the treatments inoculated with the UFLA 03–153 and UFLA 03–144 strains, respectively. The UFLA 03–84, UFLA 03–144, INPA 104A, INPA 54B, UFLA 03–150, UFLA 03–197, and UFLA 03–164 strains promoted higher NN (p < 0.05) than the other strains. The highest (p < 0.05) NDM values were obtained in the treatments inoculated with the UFLA 03–144 and INPA 104A strains. The UFLA 03–84, INPA 54B, INPA 86A, UFLA 03–150, and UFLA 03–197 strains formed a second group that also stood out (p < 0.05) in terms of NDM production.
Of the 17 strains evaluated, the highest (p < 0.05) SDM, TDM, SPAD index, and SNA values were obtained from the seven strains that also stood out in terms of NDM production (UFLA 03–84, UFLA 03–144, INPA 104A, INPA 54B, INPA 86A, UFLA 03–150, and UFLA 03–197) (Table 2). Positive correlations (p < 0.01) were detected between NDM and the other variables, except for RDM. There was also high correlation (p < 0.01) between the SPAD index and SNA (Table 3).
The INPA 54B strain promoted higher (p < 0.05) SDM (7.29 g plant−1), TDM (8.77 g plant−1), and SNA (0.26 g plant−1) values than the other strains and the control with high N concentration (Table 2). The UFLA 03–144 strain was the second most efficient for these variables, promoting higher (p < 0.05) values than the control with high N concentration and than the other strains. The treatments inoculated with the UFLA 03–84, INPA 104A, and INPA 86A strains formed a third group with higher (p < 0.05) SDM, TDM, and SNA values than the control with high N concentration. The treatments inoculated with the UFLA 03–150 and UFLA 03–197 strains exhibited similar and lower (p < 0.05) values of these variables, respectively, compared to the control with high N concentration. The other strains were inefficient in BNF, achieving SDM and SNA values similar (p < 0.05) to the control with low N concentration.
For the RDM, 82% of the strains evaluated exhibited higher (p < 0.05) values than the control with low N concentration (Table 2). The INPA 54B strain stood out (p < 0.05) compared to the other strains and led to a response similar (p < 0.05) to the control with high N concentration. The UFLA 03–144, INPA 237B, INPA 104A, and UFLA 03–153 nodulating strains, together with three strains that did not nodulate lima bean (UFLA 03–290, UFLA 06–24, and UFLA 06–13), formed a second group with higher (p < 0.05) RDM values than the other strains and the control with low N concentration, though these values were lower (p < 0.05) than the value of the control with high N concentration.
3.2 Strain efficiency in pots with soil
There were effects (p < 0.05) only of nitrogen sources on the NN, NDM, SDM, TDM, and SNA variables (Table 4). The highest (p < 0.05) NN values were observed in the treatments inoculated with the UFLA 03–144 and INPA 86A strains; however, for NDM, there was no difference between the inoculated treatments and the uninoculated control without mineral N. The plants fertilized with mineral N did not nodulate.
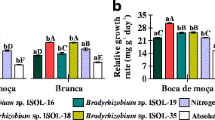
All strains, except UFLA 03–150, promoted higher SDM and TDM production (p < 0.05) than the uninoculated control without mineral N (Table 4). However, no strain was similar or superior (p < 0.05) to the uninoculated control with mineral N. The INPA 54B and INPA 86A strains stood out (p < 0.05) from the others in SDM production and promoted 34 and 36% increases, respectively, compared with the production obtained from the uninoculated control without mineral N. The treatments inoculated with the UFLA 03–84, UFLA 03–144, and INPA 104A strains promoted similar (p < 0.05) SDM production.
The highest (p < 0.05) SNA value was obtained in the uninoculated control with mineral N (Table 4). The INPA 54B and INPA 86A strains were most efficient in N2 fixation, promoting higher (p < 0.05) SNA than the other strains and the uninoculated control without mineral N. The UFLA 03–84 and UFLA 03–144 strains also promoted higher (p < 0.05) SNA than the uninoculated control without mineral N. The treatments inoculated with the INPA 104A and UFLA 03–150 strains exhibited similar and lower (p < 0.05) SNA, respectively, than the uninoculated control without N. Positive correlations (p < 0.05) were observed between the NN, NDM, and SNA (Table 3).
Regarding RDM, there was an interaction (p < 0.05) between the liming and the N sources (Table 5). The UFLA 03–144 and UFLA 03–150 strains induced higher (p < 0.05) RDM production in the presence of liming, whereas the INPA 104A strain promoted higher (p < 0.05) RDM production in the absence of liming. Among the N sources, in the absence of liming, the treatments inoculated with the UFLA 03–84 and INPA 104A strains exhibited higher (p < 0.05) RDM production than the other inoculated treatments and the controls with and without mineral N application. In the presence of liming, the inoculated treatments, except for the one inoculated with the INPA 104A strain, promoted higher (p < 0.05) RDM production than the controls with and without mineral N application.
4 Discussion
Selecting rhizobia strains efficient in biological nitrogen fixation (BNF) in symbiosis with lima bean is an important strategy to economically and sustainably increase the grain yield of this crop. The six strains that exhibited the highest efficiency in N2 fixation in symbiosis with lima bean in Leonard jars were isolated from nodules of Vigna unguiculata (UFLA 03–84, UFLA 03–144, and UFLA 03–150), Campsiandra surinamensis (INPA 104A), Inga sp. (INPA 54B), and Swartzia sp. (INPA 86A) in Amazon soils. These strains were also efficient in symbiosis with cowpea (UFLA 03–144, UFLA 03–84, and UFLA 03–150) and siratro (INPA 104A, INPA 54B, and INPA 86A) in previous studies (Table 1), indicating the potential of Amazon soils as a source of genetic resources for biotechnological applications. Although, seed N may have contributed to plant N, this contribution was similar in all treatments. Thus, the differences observed can be considered as being due to the treatments, i.e., the strains inoculated.
The UFLA 03–84 strain is currently authorized by the MAPA as a cowpea inoculant (information available at http://www.agricultura.gov.br) and its symbiotic efficiency was also observed in pigeon pea (Cajanus cajan L.) in Leonard jars (Rufini et al. 2014). In contrast, the UFLA 03–153, UFLA 03–164, UFLA 03–320, and UFLA 03–321 strains, which are efficient in symbiosis with cowpea (Rufini et al. 2013; Soares et al. 2014), and the INPA 237B and UFLA 04–0212 strains, which form efficient symbiosis with siratro (Table 1), were inefficient in symbiosis with lima bean in this study, indicating that BNF is affected by the intrinsic characteristics of the symbionts, as reported by Hartwig (1998).
High symbiotic efficiency of isolates (genetically unidentified) of lima bean nodules inoculated in this crop was reported by Antunes et al. (2011) in an experiment conducted in Leonard jars. These authors also detected positive correlations (p < 0.05) between NDM and SDM production and SNA, corroborating the results obtained in the present study. Similar correlation results were reported by Ferreira et al. (2012) when working with common bean (Phaseolus vulgaris L.) inoculated with rhizobia strains.
The SPAD index, which represents an indirect measure of leaf chlorophyll content, has been positively correlated with shoot N accumulation, grain yield, and/or shoot dry matter in some legume species, such as common bean, soybean, and cowpea (Fritschi and Ray 2007; Remans et al. 2008; Jaramillo et al. 2013). However, for lima bean, our study is the first to report this index and its excellent correlation with both SNA and SDM production. Thus, we suggest evaluating the SPAD index during the process of selecting rhizobia strains for this crop, especially in the first steps because when large numbers of strains are tested, the typical procedure, the cost for N analyses becomes very expensive.
The INPA 237B, UFLA 03–153, and UFLA 03–320 strains that were inefficient in BNF and the four strains that did not nodulate lima bean (UFLA 03–268, UFLA 03–290, UFLA 06–24, and UFLA 06–13) in Leonard jars most likely increased (p < 0.05) the RDM production by acting in biological processes other than BNF. Although the main function of the Bradyrhizobium genus has already been reported as BNF, this genus is quite versatile and may also act in other plant growth-promoting processes, such as inorganic phosphate solubilization and phytohormone production (Boiero et al. 2007; Marra et al. 2011; Oliveira-Longatti et al. 2014).
In the experiment in pots with soil, the absence of a liming effect on lima bean nodulation and shoot growth differs from the results obtained by Rufini et al. (2011). They worked with common bean and found positive responses (p < 0.05) to liming on nodulation, plant growth, and N2 fixation. However, these results were observed in a Oxisol with an initial pH (H2O) of 5.1 and a base saturation of 21%. Liming is an important practice for two reasons: it raises soil pH, reducing acidity, and increases base saturation, supplying calcium and magnesium, which are important nutrients for plant and diazotrophic bacterial development and, consequently, for establishment of the symbiosis (Norris 1958; Lodeiro et al. 1995). In the present study, the relatively high pH (6.0) and Ca+2 (1.60 cmolc dm−3) and Mg2+ (0.60 cmolc dm−3) levels present in the soil probably adequately sustained the plants during the experimental period and could explain why the plant did not respond to the addition of lime. However, two strains (UFLA 03–144 and UFLA 03–150) increased (p < 0.05) the RDM in response to liming.
The lack of correlation (p > 0.05) between NDM and SDM and the lower correlation (p < 0.05) between NDM and SNA in the experiment in pots with soil differs from the results obtained in Leonard jars. This may be due mainly to the effects of edaphic factors. The absence of nodulation in the uninoculated control with mineral N in pots with soil indicates a mitigating role of nitrogen in lima bean nodulation, which has also been observed in other legume species, such as cowpea (Costa et al. 2014), common bean (Rufini et al. 2011), and pigeon pea (Rufini et al. 2014). The similarity in NDM between the uninoculated control without mineral N and the inoculated treatments is evidence for the effective nodulating capacity of the native rhizobia populations present in the soil under study. Despite this similarity, four strains (UFLA 03–144, UFLA 03–84, INPA 54B, and INPA 86A) exhibited a competitive ability and promoted increased (p < 0.05) SDM and TDM production and SNA. This confirms the potential of some strains for use as lima bean inoculants, especially INPA 54B and INPA 86A, which were the most efficient in pot trials.
The rapid plant response to mineral N shows that no strain is equal to the control fertilized with high NH4NO3 concentration (300 mg N dm−3), as the establishment and functioning of a symbiosis takes time. However, once the symbiosis is established, the performance of the inoculated treatments can equal that of the mineral N treatment. This stage is reached during the grain production phase in crops such as cowpea (Soares et al. 2006; Ferreira et al. 2013). There is no data yet regarding this in lima bean.
Our study is the first to report the symbiotic efficiency of rhizobia strains with lima bean in an experiment conducted in soil. It constitutes the first step in selecting strains to recommend strains for lima bean inoculation. In future experiments we plan to evaluate INPA 54B and INPA 86A as lima bean inoculants under field conditions taking into account other edaphoclimatic conditions to confirm the symbiotic efficiency of these strains.
5 Conclusions
Strains isolated from nodules of hosts - Vigna unguiculata (UFLA 03–144 and UFLA 03–84), Campsiandra surinamensis (INPA 104A), Inga sp. (INPA 54B), and Swartzia sp. (INPA 86A) - belonging to the three legume subfamilies are efficient in symbiosis with lima bean under axenic conditions and in pots with soil. Liming does not affect lima bean nodulation and shoot growth in the soil evaluated during the experimental period. The INPA 54B and INPA 86A strains, both belonging to phylogenetic group V, promote higher shoot dry matter production and shoot nitrogen accumulation compared to the other strains and the native rhizobial populations, exhibiting potential for use as lima bean inoculants.
References
Antunes JEL, Gomes RLF, Lopes ÂCA, Araújo ASF, Carmo M, Lyra CP, Figueiredo MVB (2011) Symbiotic efficiency of rhizobia isolated from nodules of lima bean (Phaseolus lunatus L.). R Bras Ci Solo 35:751–757. doi:10.1590/S0100-06832011000300011
Boiero L, Perrig D, Masciarelli O, Penna C, Cassán F, Luna V (2007) Phytohormone production by three strains of Bradyrhizobium japonicum and possible physiological and technological implications. Appl Microbiol Biotechnol 74:874–880. doi:10.1007/s00253-006-0731-9
Bonilla I, Bolaños L (2009) Mineral nutrition for legume-rhizobia symbiosis: B, Ca, N, P, S, K, Fe, Mo, Co, and Ni: a review. Sustain Agric Rev 1:253–274. doi:10.1007/978-1-4020-9654-9_13
Costa Neto VP, Mendes JBS, Araújo ASF, Alcântara Neto F, Bonifacio A, Rodrigues AC (2017) Symbiotic performance, nitrogen flux and growth of lima bean (Phaseolus lunatus L.) varieties inoculated with different indigenous strains of rhizobia. Symbiosis (in press)
Costa EM, Nóbrega RSA, Ferreira LVM, Amaral FHC, Nóbrega JCA, Silva AFT, Moreira FMS (2014) Growth and yield of the cowpea cultivar BRS Guariba inoculated with rhizobia strains in Southwest Piauí. Semin Cienc Agrar 35:3073–3084. doi:10.5433/1679-0359.2014v35n6p3073
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc Agrotec 35:1039–1042. doi:10.1590/S1413-70542011000600001
Ferreira PAA, Bomfeti CA, Soares BL, Moreira FMS (2012) Efficient nitrogen-fixing Rhizobium strains isolated from amazonian soils are highly tolerant to acidity and aluminium. World J Microbiol Biotechnol 28:1947–1959. doi:10.1007/s11274-011-0997-7
Ferreira LVM, Nóbrega RSA, Nóbrega JCA, Aguiar FL, Moreira FMS, Pacheco LP (2013) Biological nitrogen fixation in production of Vigna unguiculata (L.) Walp, family farming in Piauí, Brazil. J Agr Sci 5:153–160. doi:10.5539/jas.v5n4p153
Florentino LA, Guimarães AP, Rufini M, Silva K, Moreira FMS (2009) Sesbania virgata Stimulates the occurrence of its microsymbiont in soils but does not inhibit microsymbionts of other species. Sci Agric 66:667–676. doi:10.1590/S0103-90162009000500012
Fofana B, Baudoin JP, Vekemans X, Debouck DG, Du Jardin P (1999) Molecular evidence for an Andean origin and a secondary gene pool for the lima bean (Phaseolus lunatus L.) using chloroplast DNA. Theor Appl Gen 98:202–212. doi:10.1007/s001220051059
Fred EB, Waksman SA (1928) Laboratory manual of general microbiology: with special reference to the microorganisms of the soil. McGraw-Hill, New York
Fritschi FB, Ray JD (2007) Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 45:92–98. doi:10.1007/s11099-007-0014-4
Guimarães AA, Jaramillo PMD, Nóbrega RSA, Florentino LA, Silva KB, Moreira FMS (2012) Genetic and symbiotic diversity of nitrogen-fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Appl Environ Microb 78:6726–6733. doi:10.1128/AEM.01303-12
Guimarães AA, Florentino LA, Almeida KA, Lebbe L, Silva KB, Willems A, Moreira FMS (2015) High diversity of Bradyrhizobium strains isolated from several legume species and land uses in Brazilian tropical ecosystems. Syst Appl Microbiol 38:433–441. doi:10.1016/j.syapm.2015.06.006
Hartwig UA (1998) The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Perspect Plant Ecol 1:92–120. doi:10.1078/1433-8319-00054
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Agric Exp Stn (Circular, n.347). Univ. of California, Berkeley, California
Jaramillo PMD, Guimarães AA, Florentino LA, Silva KB, Nóbrega RSA, Moreira FMS (2013) Symbiotic nitrogen-fixing bacterial populations trapped from soils under agroforestry systems. Sci Agric 70:397–404. doi:10.1590/S0103-90162013000600004
Lima AS, Nóbrega RSA, Barberi A, Silva K, Ferreira DF, Moreira FMS (2009) Nitrogen-fixing bacteria communities occurring in soils under different uses in the western Amazon region as indicated by nodulation of siratro (Macroptilium atropurpureum). Plant Soil 319:127–145. doi:10.1007/s11104-008-9855-2
Lodeiro AR, Lagares A, Martínez EN, Favelukes G (1995) Early interactions of Rhizobium leguminosarum bv. phaseoli and bean roots: specificity in the process of adsorption and its requirement of Ca2+ and Mg2+ ions. Appl Environ Microbiol 61:1571–1579
López-López A, Negrete-Yankelevich S, Rogel MA, Ormeño-Orrillo E, Martínez J, Martínez-Romero E (2013) Native bradyrhizobia from Los Tuxtlas in Mexico are symbionts of Phaseolus lunatus (lima bean). Syst Appl Microbiol 36:33–38. doi:10.1016/j.syapm.2012.10.006
Maquet A, Vekemans XZ, Baudoin JP (1999) Phylogenetic study on wild allies of lima bean, Phaseolus lunatus L. (Fabaceae) and implications on its origin. Plant Syst Evol 218:43–54. doi:10.1007/BF01087033
Marra LM, Oliveira SM, Soares CRF, Moreira FMS (2011) Solubilisation of inorganic phosphates by inoculant strains from tropical legumes. Sci Agric 68:603–609. doi:10.1590/S0103-90162011000500015
Matsubara M, Zúñiga-Dávila D (2015) Phenotypic and molecular differences among rhizobia that nodulate Phaseolus lunatus in the Supe valley in Peru. Ann Microbiol 65:1803–1808. doi:10.1007/s13213-015-1054-9
Moreira FMS, Gillis M, Pot B, Kersters K, Franco AA (1993) Characterization of rhizobia isolated from different divergence groups of tropical Leguminosae by comparative polyacrylamide gel electrophoresis of their total proteins. Syst Appl Microbiol 16:135–146. doi:10.1016/S0723-2020(11)80258-4
Moreira FMS, Haukka K, Young JPW (1998) Biodiverity of rhizobia isolated from a wide range of forest legumes in Brazil. Mol Ecol 7:889–895. doi:10.1046/j.1365-294x.1998.00411.x
Moreira FMS, Carvalho TS, Siqueira JO (2010) Effect of fertilizers, lime and inoculation with rhizobia and mycorrhizal fungi on the growth of four leguminous tree species in a low-fertility soil. Biol Fertil Soils 46:771–779. doi:10.1007/s00374-010-0477-5
Norris DO (1958) Rhizobium needs magnesium, not calcium. Nature 182:734–735. doi:10.1038/182734a0
Oliveira-Longatti SM, Marra LM, Moreira FMS (2014) Bacteria isolated from soils of the western Amazon and from rehabilitated bauxite-mining areas have potential as plant growth promoters. World J Microbiol Biotechnol 30:1239–1250. doi:10.1007/s11274-013-1547-2
Ormeno-Orrillo E, Vinuesa P, Zúñiga-Davila D, Martinez-Romero E (2006) Molecular diversity of native bradyrhizobia isolated from (Phaseolus lunatus L.) in Peru. Syst Appl Microbiol 29:253–262. doi:10.1016/j.syapm.2005.09.002
Remans R, Ramaekers L, Schelkens S, Hernandez G, Garcia A, Reyes JL, Mendez N, Toscano V, Mulling M, Galvez L, Vanderleyden J (2008) Effect of Rhizobium-Azospirillum coinoculation on nitrogen fixation and yield of two contrasting Phaseolus vulgaris L. genotypes cultivated across different environments in Cuba. Plant Soil 312:25–37. doi:10.1007/s11104-008-9606-4
Ribeiro PRA, Santos JV, Costa EM, Lebbe L, Assis ES, Louzada MO, Guimarães AA, Willems A, Moreira FMS (2015) Symbiotic efficiency and genetic diversity of soybean bradyrhizobia in Brazilian soils. Agric Ecosyst Environ 212:85–93. doi:10.1016/j.agee.2015.06.017
Rufini M, Ferreira PAA, Soares BL, Oliveira DP, Andrade MJB, Moreira FMS (2011) Symbiosis of nitrogen fixing bacteria with common bean in different pH values. Pesq Agrop Brasileira 46:81–88. doi:10.1590/S0100-204X2011000100011
Rufini M, Silva MAP, Ferreira PAA, Cassetari AS, Soares BL, Andrade MJB, Moreira FMS (2013) Symbiotic efficiency and identification of rhizobia that nodulate cowpea in a Rhodic Eutrudox. Biol Fertil Soils 50:115–122. doi:10.1007/s00374-013-0832-4
Rufini M, Oliveira DP, Trochmann A, Soares BL, Andrade MJB, Moreira FMS (2014) Bradyrhizobium strains in symbiosis with dwarf pigeon pea under greenhouse and field conditions. Pesq Agrop Brasileira 49:197–206. doi:10.1590/S0100-204X2014000300006
Singleton PW, Tavares JW (1986) Inoculation response of legumes in relation to the number and effectiveness of indigenous rhizobium populations. Appl Environ Microbiol 51:1013–1018
Soares ALL, Ferreira PAA, Pereira JPAR, Vale HMM, Lima AS, Andrade MJB, Moreira FMS (2006) Agronomic efficiency of selected rhizobia strains and diversity of native nodulating populations in Perdões (MG - Brazil). II – beans. Rev Bras Ciênc Solo 30:795–802. doi:10.1590/S0100-06832006000500006
Soares BL, Ferreira PAA, Oliveira-Longatti SM, Marra LM, Rufini M, Andrade MJB, Moreira FMS (2014) Cowpea symbiotic efficiency, pH and aluminum tolerance in nitrogen-fixing bacteria. Sci Agric 71:17–180. doi:10.1590/S0103-90162014000300001
Thies JE, Bohlool BB, Singleton PW (1991) Subgroups of the cowpea miscellany: symbiotic specificity within Bradyrhizobium spp. for Vigna unguiculata, Phaseolus lunatus, Arachis hypogaea, and Macroptilium atropurpureum. Appl Environ Microbiol 57:1540–1545
Acknowledgements
We thank the Coordination for the Improvement of Higher Education Personnel [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)] and the National Council for Scientific and Technological Development [Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)] for financial support and for granting scholarships. We also thank the Federal Institute of Education, Science and Technology of Maranhão [Instituto Federal de Educação, Ciência e Tecnologia do Maranhão (IFMA)] for granting soil for the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martins da Costa, E., de Almeida Ribeiro, P.R., de Lima, W. et al. Lima bean nodulates efficiently with Bradyrhizobium strains isolated from diverse legume species. Symbiosis 73, 125–133 (2017). https://doi.org/10.1007/s13199-017-0473-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-017-0473-8