Abstract
There is increasing interest in the use of plant growth-promoting rhizobacteria (PGPR) as environmental-friendly and healthy biofertilizers. Strawberries (Fragraria x ananassa) are mainly consumed fresh and hence any PGPRs used for biofertilization must be safe for humans, which is the case for members of the genus Rhizobium. In this study, the effects of inoculation of strawberry plants with Rhizobium sp. strain PEPV16, which belongs to the phylogenetic group of R. leguminosarum, and whose plant growth promotion ability has been reported previously for lettuce (Lactuca sativa) and carrots (Daucus carota), was examined. The results demonstrated that PEPV16 promotes strawberry growth through significant increases in the number of stolons, flowers and fruits as compared with uninoculated controls. Compared to uninoculated controls, the fruits of the inoculated plants had higher concentrations of Fe, Zn, Mn and Mo, and they also had higher concentrations of organic acids, such as citric and malic acid, and lower amounts of ascorbic acid than fruits. Although decreases in ascorbic acid have previously been described after the inoculation of strawberry with strains from different PGPR genera, this is the first study to report increases in organic acids after PGPR inoculation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biofertilizers or plant probiotics (Berlec 2012) include rhizospheric and endophytic microorganisms with the ability to promote and directly and/or indirectly regulate plant growth (Compant et al. 2010 and Glick 2012). Direct plant growth mechanisms involve those that facilitate the acquisition of nutrients through biological nitrogen fixation (BNF), phosphate solubilization, iron sequestration and the modulation of phytohormone levels. Indirect mechanisms involve the induction of systemic resistance and the production of antibiotics, enzymes (lytic enzymes and ACC deaminase) and siderophores (Glick 2012). There are many bacteria that possess one or more of these beneficial mechanisms. Many of them are human pathogens and, therefore, cannot be used as biofertilizers (García-Fraile et al. 2012). Strains from different species of Rhizobium are now well known as plant growth-promoters capable of fixing atmospheric nitrogen within legume root nodules (Peix et al. 2015), and they have also been reported to act as root colonizers and plant growth-promoters in some vegetables, such as pepper (Capsicum annuum), tomato (Solanum lycopersicum), lettuce (Lactuca sativa) and carrot (Daucus carota) (García-Fraile et al. 2012; Flores-Félix et al. 2013). Recently, we have shown that Phyllobacterium, related phylogenetically to the Rhizobiaceae family, promotes the growth of strawberries (Flores-Félix et al. 2015b), which are one of the most important soft fruits consumed in European countries, including Spain (López-Aranda et al. 2011). They contain nutritive compounds comprising sugars, vitamins and minerals (Giampieri et al. 2015), together with bioactive compounds such as vitamin C, flavonoids, anthocyanins and phenolic acids (Basu et al. 2014; Giampieri et al. 2015). Their high antioxidant potential is mainly due to their vitamin C content (Giampieri et al. 2015) and several studies have focused on the effect of bacterial inoculation on the vitamin C content and the contents of various minerals in the fruits (Pirlak and Köse 2009; Erturk et al. 2012; Bona et al. 2015; Ipek et al. 2014; Flores-Félix et al. 2015b). Interestingly, however, strawberry is peculiar regarding its ability to propagate both sexually, via seeds and vegetatively via stolons (Heide et al. 2013). Accordingly, stolon production is as important as that of fruits, and the production of both can be enhanced by biofertilization with some PGPRs, as recently shown for Phyllobacterium strains (Flores-Félix et al. 2015b).
Although there are several reports addressing the ability of Rhizobium strains to enhance the growth of the aerial parts of non-legumes (García-Fraile et al. 2012; Glick 2012), there are no studies about the effect of Rhizobium strains on strawberry plants. We have recently reported a strain of Rhizobium, PEPV16, which nodulates Phaseolus vulgaris and has several in vitro plant promotion mechanisms, that was able to colonize the roots of lettuce, an important step required for obtaining beneficial effects on plant growth (Lugtenberg et al. 2001; Compant et al. 2010); the strain had a positive effect on the growth of lettuce leaves (Flores-Félix et al. 2013). This strain was identified in that previous study as R. leguminosarum on the basis of its 16S rRNA gene sequence, but since 2013 several species of the genus Rhizobium with identical 16S rRNA genes have been published, such as R. laguerreae (Saïdi et al. 2014), R. sophorae (Jiao et al. 2015), R. anhuiense (Zhang et al. 2015), and Rhizobium acidisoli (Román-Ponce et al. 2015). Moreover, there are two accession numbers in Genbank for the 16S rRNA gene of the R. indigoferae CCBAU71042T type strain, one of them identical to that of the above sequences. Nevertheless, these species are distinguishable on the basis of the results of different housekeeping gene analyses, such as recA and atpD, which are available for all type strains of these species (Peix et al. 2015).
The aims of this study were to analyse the recA and atpD genes of strain PEPV16 in order to establish more exactly its relationship with species in the R. leguminosarum group, and to explore the effect of inoculation with this strain on the growth of strawberry plants under greenhouse conditions, as well as on the content of minerals and organic acids, including ascorbic acid (vitamin C), of the fruits.
2 Material and methods
2.1 Housekeeping gene analysis
The atpD and recA genes of strain PEPV16 were amplified and sequenced as described by Gaunt et al. (2001). The sequences obtained were compared with those held in GenBank using the BLASTN program (Altschul et al. 1990). They were aligned using Clustal W software (Thompson et al. 1997). Distances calculated according to Kimura’s two-parameter model (Kimura 1980) were used to infer phylogenetic trees with the neighbour-joining method (Saitou and Nei 1987), using MEGA5 software (Tamura et al. 2011). Confidence values for nodes in the trees were generated by bootstrap analysis using 1000 permutations of the data sets.
2.2 Biofilm production and plant colonization assays
The ability of strain PEPV16 to form biofilms on abiotic surfaces at the macro and micro-scale and to produce cellulose was analysed as described by Flores-Félix et al. (2015b). The microstructure of the biofilm was detected using 25 ml of TY medium placed in a 50 ml glass tube containing a sterile microscope slide. The medium was inoculated with a 2 day old culture of strain PEPV16 (measured at 600 nm) using 100 μl of a 0.5 OD suspension. The inoculated slides were examined after 7 days of incubation. The slides were then removed and placed in water for 5 s three times to remove the cells that had not adhered to the slide surface, after which they were placed in a solution of 40 mg l−1 acridine orange in phosphate buffer (pH 7.2) for 30 s. Microscopic examination was carried out using an appropriate filter with a NIKON Eclipse 80i fluorescence microscope. Cellulose production was checked on plates containing YMA medium supplemented with 0.25 % Congo Red, inoculated with strain PEPV16 and incubated at 28 °C for 2 days (Robledo et al. 2012). In order to confirm cellulose production, the strain was inoculated in 30 ml of YMB medium, which was shaken at 180 rpm and 28 °C for 5 days, followed by static growth for 2 days. Five ml were taken from the bottom of the flask and centrifuged at 1500 × g for 5 min. The flocs were then washed with 5 ml of 100 mM phosphate citrate buffer (PCA) pH 5, recovered by centrifugation, and resuspended in 5 ml of the same buffer. This suspension was placed in 5 cm diameter Petri plates and treated with 10 U ml−1 cellulase produced by Trichoderma viride (Sigma Co., USA) for 2 h at 37 °C in an orbital shaker at 180 rpm. Controls without cellulase were incubated under the same conditions.
For plant colonization assays, 30 achenes of strawberry (Fragraria x ananassa) var. Camarosa were surface-sterilized by immersion in 70 % ethanol for 30 s, followed by soaking in an aqueous 5 % sodium hypochlorite solution for 15 min. The achenes were then washed six times with sterile water and germinated in 1 % water-agar plates overlaid with sterile Whatman number 1 filter paper wetted with sterile water. Five day old strawberry seedlings were inoculated with 1 ml of a suspension (108 CFU ml−1) of the GFP-tagged PEPV16 strain obtained in a previous study (Flores-Félix et al. 2013). The plates were placed in the darkness in a growth chamber at 24 °C with mixed incandescent and fluorescent illumination (400 microeinsteins m−2 s−1; 400–700 nm) programmed for a 16 h photoperiod and 50–60 % relative humidity (Robledo et al. 2008) until the seedling roots were 1–2 cm in length. In order to remove unbound bacteria, the roots were gently washed three times with sterile distilled water before microscopic examination. Uninoculated strawberry roots were included in the experiment as negative controls. Fluorescence microscopy was carried out using a Nikon Eclipse 80i, and excitation of the green fluorescent protein (GFP) was accomplished using a mercury lamp. The seedlings were examined at 3, 5, 8 and 12 days after inoculation.
To increase the resolution of the biofilm observations, calcofluor white staining was used as described by Flores-Félix et al. (2015a). Seedlings were placed on slides and stained with 50 μl of 50 mg l−1 Calcofluor White solution (Calcofluor White stain, Sigma®) and 50 μl of 10 % potassium hydroxide solution to improve resolution, as recommended by the manufacturer. Preparations were covered and incubated for 1 min before examination under an epifluorescence microscope.
2.3 Growth promotion assays in planta
The ability of strain PEPV16 to promote the growth of strawberry var. Camarosa plants was evaluated. Fifteen plants were included in each treatment: a control without inoculation and inoculation with strain PEPV16. The seedlings (conserved at −4 °C) were obtained from a commercial distributor of strawberry plants in Canada and were planted on “SEED PRO 6040”/vermiculite (3:1 V/V) (PROJAR, Spain) non-sterile commercial peat, using black plastic trays containing 6 Kg each. Six days after planting, each tray was inoculated with 100 ml of a suspension of strain PEPV16 containing 106 CFU ml−1. To obtain this suspension, the cells of the strain were cultivated on YMA plates for 48 h at 28 °C and then suspended in sterile water.
The plants were irrigated with water from a bottom reservoir every 48 h for 3 months in a lab greenhouse under day/night temperatures of 25–35/15–20 °C, humidity being set at 70 %. During the experiment, stolons, flowers and fruits were counted, the fully ripened fruits from first to third categories were harvested. The fresh and dry weights of 25 fully ripened fruits were measured and their mineral content was analysed. Determination of N, P, K, Ca and Mg was performed at the Ionomic Service of the Centro de Edafología y Biología Aplicada del Segura (CEBAS)-CSIC (Spain). Statistical analyses using One-way Analysis of Variance were carried out using the StatView 4.1 program for Macintosh computers (Abacus Concepts, USA) and mean values were compared with Fisher’s Protected LSD test (Least Significant Differences (LSD) at a confidence level of 95 %, P ≤ 0.05).
2.4 Analysis of organic acids
Fifteen fully ripened fruits from each treatment were frozen at −20 °C, lyophilized (Labconco Freezone 4.5 apparatus, USA), and ground to a mean particle size of less than 910 μm. The material obtained was divided into three aliquots, which were analyzed separately. Organic acids were analyzed according to the procedure described by Dopico-García et al. (2007). Each lyophilized powdered sample (ca 0.3 g) was extracted using 0.01 N H2SO4 (ca. 50 ml) for 30 min whilst stirring at 300 rpm. The aqueous solution was then passed through a Chromabond C18 NEC column previously conditioned with 30 ml of methanol and 70 mL of water acidified to pH 2 using HCl. The aqueous extract containing the organic acids was evaporated to dryness under reduced pressure at 30 °C and redissolved in 1 ml 0.001 N H2SO4 for HPLC-UV analysis, using 20 μl of the suspension.
The separation and quantification of organic acids was carried out in a system consisting of an analytic HPLC–UV unit (Gilson Inc., Middleton, WI) with a Nucleogel® Ion 300 O ion-exclusion column (300 × 7.7 mm; Macherey–Nagel, Düren, Germany), as previously reported by Dopico-García et al. (2007). Elution was performed in isocratic mode with 0.01 N H2SO4 at a flow rate of 0.2 ml min−1. Detection of organic acids was achieved using a UV detector set at 214 nm. Organic acid quantification was achieved by measuring the absorbance recorded in the chromatograms relative to external standards: oxalic, cis- and trans-aconitic, citric, ascorbic, malic, shikimic and fumaric acids (Sigma–Aldrich, St. Louis, MO). The data thus acquired were analyzed using One-way Analysis of Variance, as reported above (García-Fraile et al. 2013).
3 Results and discussion
3.1 Housekeeping gene analysis
Strain PEPV16 was classified within the phylogenetic group of R. leguminosarum because its 16S rRNA gene exhibited 100 % similarity with the type strain of this species (Flores-Félix et al. 2013). Nevertheless, since 2013 several new species of Rhizobium with identical 16S rRNA genes have been described, such as R. laguerreae (Saïdi et al. 2014), R. sophorae (Jiao et al. 2015), R. anhuiense (Zhang et al. 2015) and R. acidisoli (Román-Ponce et al. 2015). These species are distinguishable by their housekeeping genes, which are phylogenetically divergent from one another. This does not occur in the case of R. indigoferae, an old species (Wei et al. 2002), whose housekeeping genes suggest that it would be synonymous with R. leguminosarum and that its taxonomic status should be revised according to the current rules of bacterial taxonomy (Ferreira et al. 2011).
The results for the recA and atpD genes of strain PEPV16 and the closely related species on the basis of the 16S rRNA gene revealed that this strain is closely related to different species, depending on the gene analysed (Fig. 1). The high relatedness of the atpD gene to the type strain of R. laguerreae (98.9 % similarity) suggested that strain PEPV16 belonged to this species (Fig. 1a). Nevertheless, the recA gene was more closely related to R. leguminosarum, with 98.3 % similarity (Fig. 1b). Therefore, strain PEPV16 could be an example of genome recombination, as has been shown previously for R. leguminosarum strains (Kumar et al. 2015), and it could be a good candidate for further characterization through complete genome sequencing.
Neighbour-joining phylogenetic trees of the atpD (a) and recA (b) genes showing the position of strain PEPV16 within the phylogenetic group of Rhizobium leguminosarum. Bootstrap values (percentages) calculated for 1000 replications are indicated. The Genbank accesion numbers of atpD and recA genes of strain PEPV16 are KU196776 and KU196777, respectively. Bar, 0.5 nucleotide substitution per 100 nt
3.2 Biofilm production and colonization of strawberry roots
Strain PEPV16 was able to produce biofilms on abiotic surfaces (Fig. 2a) and cellulose (Fig. 2a and b), as confirmed by treatment with cellulase (data not shown). Cellulose microfibrils constitute part of the biofilm polysaccharides and the production of cellulose is an important mechanism for legume root colonization by Rhizobium and Phyllobacterium (Robledo et al. 2012; Flores-Félix et al. 2015b). The examination by fluorescence microscopy of GFP-tagged Rhizobium sp. PEPV16 revealed the attachment of its cells to the strawberry seedling roots, forming typical microcolonies (Fig. 2c and d). Moreover, contrast staining with Calcofluor White revealed the presence of biofilms around the seedling roots (Fig. 2e) and the presence of globular masses on root hairs (Fig. 2f). These results are in agreement with those previously found in strawberry roots inoculated with the Phyllobacterium strain PEPV15, which was also able to promote the growth of strawberries (Flores-Félix et al. 2015b). Therefore, the effect of strain PEPV16, which is also able to colonize strawberry roots and has demonstrated in vitro mechanisms of plant growth promotion (Flores-Félix et al. 2013), was analysed.
Biofilm formation by Rhizobium strain PEPV16 on abiotic surfaces observed in glass slides with acridine orange (a, bar 500 μm). Cellulose formed in plates containing Congo Red (b, left). Fluorescence optical micrographs of roots of strawberry seedlings colonized by GFP-tagged cells of PEPV16 (c, bar 100 μm, and d, bar 10 μm). The micrographs show the ability of strain PEPV16 to colonize the root surfaces of strawberry 3 days post-inoculation (c) and the initiation of microcolonies 5 days post-inoculation (d). Fluorescence optical micrographs of roots of strawberry seedlings colonized with GFP-tagged cells of PEPV16 contrast stained with calcofluor white revealing the presence of biofilms on the strawberry roots 8 days post-inoculation (e, bar 500 μm) and a globular mass of bacteria on root hairs 12 days post-inoculation (f, bar 100 μm)
3.3 Effect on plant growth
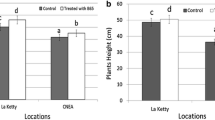
Inoculation with the test strain resulted in an increase in the number and length of stolons as compared to the uninoculated control (p-value < 0.05), together with a significantly higher number of flowers and fruits (Table 1). When the fruits produced were classified using the commercial categories determined by the EU (Commission Regulation (EEC) No 899/87), depending on their calibre (first, second and third category), all of them were found to correspond to fresh fruits weighing more than 7 g. Although no significant differences were found in the fresh weight of the fruits from the second and third categories (data not shown), a significant increase was found in the fresh and dry weights of fruits from the first category after inoculation with the test strain (Table 1). This is in accordance with the results of other authors, who found significant increases in fruit weight after inoculation with strains of Pseudomonas (Esitken et al. 2010; Bona et al. 2015), Bacillus megaterium and Bacillus sp. (Erturk et al. 2012), and Alcaligenes faecalis and Agrobacterium rubi (Ipek et al. 2014). Other authors found no differences after the inoculation of two combined strains of Bacillus and Pseudomonas (Pırlak and Köse 2009). Variable results, depending on the inoculated strain, have been reported in other studies after the inoculation of strawberris with several strains of Bacillus megaterium, B. simplex, Bacillus sp. and Paenibacillus polymyxa (Erturk et al. 2012) or after the inoculation with strains of Alcaligenes faecalis, Staphylococcus arlettae, S. simulans, Pantoea agglomerans, Agrobacterium rubi and Bacillus megaterium (Ipek et al. 2014).
In this study the content of N, P and K increased slightly while the Ca content decreased slightly after inoculation with the test strain, but the differences with respect to the uninoculated plants were not statistically significant (Table 1). Inoculation significantly increased the Na content. However, other researchers have found increases in the contents of several macronutrients when non-rhizobial strains were inoculated onto strawberry plants (Ipek et al. 2014; Flores-Félix et al. 2015b).
With regard to micronutrients, inoculation with strain PEPV16 increased the iron content of the strawberry fruits (Table 1), as also occurred after inoculation with the Phyllobacterium strain PEPV15 (Flores-Félix et al. 2015b) and with several strains from different genera and species, such as Alcaligenes faecalis, Staphylococcus arlettae, S. simulans, Pantoea agglomerans, Agrobacterium rubi and Bacillus megaterium (Ipek et al. 2014). Inoculation with strain PEPV16 increased the Mn content in strawberry fruits, in agreement with the results of Ipek et al. (2014). Nevertheless, an increase in the Zn content and a decrease in the B content were also found after inoculation with strain PEPV16, in contrast to the results of Ipek et al. (2014). The Mo content also increased after inoculation with strain PEPV16; this element is not usually analyzed in strawberries despite its importance for fruit development (Kaiser et al. 2005) and also in human health (Mendel and Schwarz 2011).
Taken together with previous studies on the effects of strain PEPV16 on lettuce and carrots, it can be concluded that it is a good plant probiotic that is able to increase the yield and mineral contents of the edible parts of various horticultural plants, such as strawberries, carrots and lettuce.
3.4 Effect on fruit organic acid content
Strawberries are particularly appealing for human consumption because of their antioxidant potential, which is connected to the preservation of cardiovascular health (Giampieri et al. 2012) and the enhancement of bodily defences against oxidative challenges (Tulipani et al. 2014). Giampieri et al. (2012) reported that ascorbic acid (vitamin C) was involved in these antioxidant activities. In other studies on strawberries, increases in this vitamin have been found after inoculation with several strains of Bacillus megaterium, B. simplex, Bacillus sp. and Paenibacillus polymyxa (Erturk et al. 2012), Pseudomonas fluorescens and Pseudomonas sp. (Bona et al. 2015) and Phyllobacterium endophyticum (Flores-Félix et al. 2015b). However, as occurred after inoculation with strain PEPV16, decreases in ascorbic acid have been reported by other authors after inoculation with Bacillus sp. and Pseudomonas sp. (Pırlak and Köse 2009), Bacillus sp. and Pseudomonas sp. (Esitken et al. 2010) and with Alcaligenes faecalis, Staphylococcus arlettae, S. simulans, Pantoea agglomerans, Agrobacterium rubi and Bacillus megaterium (Ipek et al. 2014).
Contrary to what was observed for ascorbic acid, significant increases were noted in oxalic, aconitic, citric, malic, shikimic and fumaric acid contents after inoculation with strain PEPV16 (Table 2), although in all cases they were within the range of levels commonly found in strawberry fruits. To date, no studies have addressed the changes in the content of these organic acids in strawberries after bacterial inoculation, and hence further work is necessary to determine whether this would be a general effect of this practice.
3.5 Concluding remarks
Strain PEPV16 of the genus Rhizobium colonized the roots of strawberry, and exerted a positive effect on strawberry yield in both its production modes (seeds and stolons). Inoculation can increase the number of stolons and fruits as well as the weight of first-category fruits, which are those most appreciated by consumers, and also the content of some minerals and organic acids. Our results show that biofertilization with Rhizobium can increase both the yield and quality of strawberries, contributing to preserving both human health and the environment.
References
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Basu A, Nguyen A, Betts NM, Lyons TJ (2014) Strawberry as a functional food: an evidence-based review. Crit Rev Food Sci Nutr 54:790–806
Berlec A (2012) Novel techniques and findings in the study of plant microbiota: search for plant probiotics. Plant Sci 193–194:96–102
Bona E, Lingua G, Manassero P et al (2015) AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 25:181–193
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Dopico-García MS, Valentão P, Guerra L et al (2007) Experimental design for extraction and quantification of phenolic compounds and organic acids in white “Vinho Verde” grapes. Anal Chim Acta 583:15–22
Erturk Y, Ercisli S, Cakmakci R (2012) Yield and growth response of strawberry to plant growth-promoting rhizobacteria inoculation. J Plant Nutr 35:817–826
Esitken A, Yildiz HE, Ercisli S et al (2010) Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci Hortic (Amsterdam) 124:62–66
Ferreira L, Sánchez-Juanes F, García-Fraile P et al (2011) MALDI-TOF mass spectrometry is a fast and reliable platform for identification and ecological studies of species from family Rhizobiaceae. PLoS One 6, e20223
Flores-Félix JD, Menéndez E, Rivera LP et al (2013) Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J Plant Nutr Soil Sci 176:876–882
Flores-Félix JD, Menéndez E, Marcos-García M et al (2015a) Calcofluor white, an alternative to propidium iodide for plant tissues staining in studies of root colonization by fluorescent-tagged rhizobia. J Adv Biol Biotechonol 2:65–70
Flores-Félix JD, Silva LR, Rivera LP et al (2015b) Plants probiotics as a tool to produce highly functional fruits: the case of Phyllobacterium and vitamin C in strawberries. PLoS One 10, e0122281
García-Fraile P, Carro L, Robledo M et al (2012) Rhizobium promotes non-legumes growth and quality in several production steps: towards a biofertilization of edible raw vegetables healthy for humans. PLoS One 7, e38122
García-Fraile P, Silva LR, Sánchez-Márquez S et al (2013) Plums (Prunus domestica L.) are a good source of yeasts producing organic acids of industrial interest from glycerol. Food Chem 139:31–34
Gaunt MW, Turner SL, Rigottier-Gois L et al (2001) Phylogenies of atpD and recA support the small sub- unit rRNA-based classification of rhizobia. Int J Syst Evol Microbiol 51:2037–2048.
Giampieri F, Tulipani S, Alvarez-Suarez JM et al (2012) The strawberry: composition, nutritional quality, and impact on human health. Nutrition 28:9–19
Giampieri F, Forbes-Hernandez TY, Gasparrini M et al (2015) Strawberry as a health promoter: an evidence based review. Food Funct 6:1386–1398
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo) 2012:15
Heide OM, Stavang JA, Sønsteby A (2013) Physiology and genetics of flowering in cultivated and wild strawberries - A review. J Hortic Sci Biotechnol 88:1–8
Ipek M, Pirlak L, Esitken A et al (2014) Plant Growth-Promoting Rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high-calcareous soil conditions. J Plant Nutr 37:990–1001
Jiao YS, Yan H, Ji ZJ et al (2015) Rhizobium sophorae sp. nov. and Rhizobium sophoriradicis sp. nov., nitrogen-fixing rhizobial symbionts of the medicinal legume Sophora flavescens. Int J Syst Evol Microbiol 65:497–503
Kaiser BN, Gridley KL, Ngaire Brady J et al (2005) The role of molybdenum in agricultural plant production. Ann Bot 96:745–754
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kumar N, Lad G, Giuntini E et al (2015) Bacterial genospecies that are not ecologically coherent: population genomics of Rhizobium leguminosarum. Open Biol 5:140133
López-Aranda JM, Soria C, Santos BM et al (2011) Strawberry production in mild climates of the world: a review of current cultivar use. Int J Fruit Sci 11:232–244
Lugtenberg BJJ, Dekkers LC, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490
Mendel RR, Schwarz G (2011) Molybdenum cofactor biosynthesis in plants and humans. Coord Chem Rev 255:1145–1158
Peix A, Ramírez-Bahena MH, Velázquez E, Bedmar EJ (2015) Bacterial associations with legumes. Crit Rev Plant Sci 34:17–42
Pirlak L, Köse M (2009) Effects of plant growth promoting rhizobacteria on yield and some fruit properties of strawberry. J Plant Nutr 32:1173–1184
Robledo M, Jiménez-Zurdo JI, Velázquez E et al (2008) Rhizobium cellulase CelC2 is essential for primary symbiotic infection of legume host roots. PNAS 105:7064–7069. doi:10.1073/pnas.0802547105
Robledo M, Rivera L, Jiménez-Zurdo JI et al (2012) Role of Rhizobium endoglucanase CelC2 in cellulose biosynthesis and biofilm formation on plant roots and abiotic surfaces. Microb Cell Fact 11:125
Román-Ponce B, Zhang YJ, Vásquez-Murrieta MS et al (2015) Rhizobium acidisoli sp. nov., isolated from root nodules of Phaseolus vulgaris in acid soils in Mexico. Int J Syst Evol Microbiol. doi:10.1099/ijsem.0.000732
Saïdi S, Ramírez-Bahena MH, Santillana N et al (2014) Rhizobium laguerreae sp. nov. nodulates Vicia faba on several continents. Int J Syst Evol Microbiol 64:242–247
Saitou N, Nei M (1987) A neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 44:406–425
Tamura K, Peterson D, Peterson N et al (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F et al (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tulipani S, Armeni T, Giampieri F et al (2014) Strawberry intake increases blood fluid, erythrocyte and mononuclear cell defenses against oxidative challenge. Food Chem 156:87–93
Wei GH, Wang ET, Tan ZY et al (2002) Rhizobium indigoferae sp. nov. and Sinorhizobium kummerowiae sp. nov., respectively isolated from Indigofera spp. and Kummerowia stipulacea. Int J Syst Evol Microbiol 52:2231–2239
Zhang YJ, Zheng WT, Everall I et al (2015) Rhizobium anhuiense sp. nov., isolated from effective nodules of Vicia faba and Pisum sativum grown in Southern China. Int J Syst Evol Microbiol 65:2960–2967
Acknowledgments
This work was sponsored by the “Junta de Castilla y León” (Grant SA183A11-2), MINECO (Grant AGL2011-29227) and by the “Fundação para a Ciência e a Tecnologia”–FCT (PEst-C/SAU/UI0709/2014). Co-funded by the Fundo Europeu de Desenvolvimento Regional - FEDER via the Programa Operacional Factores de Competitividade-COMPETE/QREN. PGF is the recipient of a postdoctoral researcher contract from the Academy of Sciences of the Czech Republic. JDFF, MMG and EM were supported by PhD fellowships from the University of Salamanca, the “Miguel Casado San José” foundation, and MICINN, respectively. LRS acknowledges FCT for a Post-doc Grant (SFRH/BPD/105263/2014). The authors thank Nicholas Skinner for English corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flores-Félix, JD., Marcos-García, M., Silva, L.R. et al. Rhizobium as plant probiotic for strawberry production under microcosm conditions. Symbiosis 67, 25–32 (2015). https://doi.org/10.1007/s13199-015-0373-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0373-8