Abstract
Issues, like emerging insecticide resistance in Anopheles mosquitoes, have led to a breakdown in many vector control programs. In this study, a recombinant Escherichia coli with plasmid expressing a green fluorescent protein (E.coli-GFP) was used as a paratransgenesis model to determine: the possibility of E. coli-GFP trans-stadial transmission. The effect of the water microflora, of bacteria-impregnated sugar solutions, and of blood-feeding on E. coli-GFP colonization and localization within An. stephensi tissues, were studied. The results demonstrated the persistence of E. coli-GFP during molting and metamorphosis events and its trans-stadial transmission. Also the efficacy of bacteria-impregnated sugar solutions for transferring the bacteria to the adult mosquito’s midgut was shown. A blood meal dramatically increased the number of bacteria within 24–48 h post feeding. In addition to fluorescence microscope evaluation, GFP gene PCR amplification showed the presence of the bacteria in the midgut of larvae, pupae, and adults up to 13 days after eclosion. Massive colonization of bacteria was observed in the larvae and in the adult mosquito’s malpighian tubules which may play a role in retaining bacteria in adult mosquitos. The results of this study showed that E. coli could be used as a laboratory model in paratransgenesis studies for the evaluation of various effector molecules as anti-parasite agents for malaria and filariasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vector-borne diseases (VBD) affect the human population in many parts of the world. Malaria is the most important VBD causing more than one million deaths annually (Breman et al. 2001; WHO 2010). Different control approaches have been designed and employed to reduce the burden of malaria. Until recently, pyrethroid insecticides and particularly pyrethroid-impregnated bed nets have been considered as one of the most effective parts of vector control programs. However, the emergence and expansion of pyrethroid-resistant mosquitoes has shed doubt on the further use pyrethroids and treated bed nets (Magesa et al. 1994; Chandre et al. 1999a, b; Enayati et al. 2003; Brengues et al. 2003; Vatandoost et al. 2005; Munhenga et al. 2008; Howard et al. 2010; Hunt et al. 2010; Ranson et al. 2011). In addition, to emerging drug resistant Plasmodium parasites and environmental issues, there are other concerns which have resulted in a breakdown of control programs and that necessitate the invention of new strategies for controlling malaria (Hill et al. 2005; Coutinho-Abreu et al. 2010).
The genetic modification of vectors (transgenic mosquitoes) or their symbionts (paratransgenesis) in order to reduce a mosquito’s vectorial competence are among the new strategies that have received a great deal of consideration by researchers (Riehle and Jacobs-Lorena 2005; Riehle et al. 2007; Aksoy et al. 2008; Ren et al. 2008; Coutinho-Abreu et al. 2010). Paratransgenesis has been defined as “Using symbiotic microorganisms to deliver anti-parasite effector molecules to wild vector populations” (Riehle and Jacobs-Lorena 2005). Moreover, other goals are envisaged and these include (1) expressing a substance with pathogenic effect on the vector, or its reproduction, in order to shorten its life span, (2) blocking the pathogen transmission cycle in the vector body or (3) reducing the vectorial capacity in order to limit the chain of disease transmission (Favia et al. 2007; Aksoy et al. 2008; Coutinho-Abreu et al. 2010).
Paratransgenesis, in contrast to transgenic mosquitoes, is low-tech and simpler. Therefore its implementation as a new control strategy in the relatively near future is more feasable (Wang et al. 2011a). Microorganisms such as viruses (Ren et al. 2008) and fungi (Fang et al. 2011) have been tested as paratransgenesis candidates in malaria vectors, although until recently bacterial symbionts have received more attention (Straif et al. 1998; Gonzalez-Ceron et al. 2003; Lindh et al. 2005; Riehle and Jacobs-Lorena 2005; Favia et al. 2007; Riehle et al. 2007; Lindh et al. 2008; Terenius et al. 2008; Rani et al. 2009; Chavshin et al. 2012). The important criteria for a paratransgenesis candidate include the ability to be cultured in common and inexpensive media, genetic malleability, dominance in the vector’s body and safety with respect to humans and non-target animals. In the case of malaria vectors, other crucial characteristics like trans-stadial transmission of candidates have to be considered. The discovery and insertion of a suitable promoter to help with the expression and secretion of the effector molecule/s at the right time and at right place in mosquito’s body is of key importance (Beard et al. 2002). Recently several pioneer studies have been carried out in the field of finding suitable paratransgenesis candidates (Bisi and Lampe 2011; Wang et al. 2012).
In the present study a recombinant Escherichia coli with a plasmid expressing a green fluorescent protein (E. coli-GFP) was used as a paratransgenesis model to determine 1) the possibility of trans-stadial transmission of E. coli-GFP (larvae to adult), 2) the effect of any water microflora, of bacteria-impregnated sugar solutions, and of blood-feeding on the bacteria colonization, and 3) the localization of E. coli-GFP in different tissues of An. stephensi, the main malaria vector in Asia (Oshaghi et al. 2006; Vatandoost et al. 2006).
2 Methods
2.1 Mosquito’s specimens
A laboratory strain of An. stephensi (Department of Medical Entomology, School of Public Health, Tehran University of Medical Sciences, SPH-TUMS) was used for experiments. The mosquitoes were reared at the temperature 25 ± 2 and humidity 70 % conditions (Benedict 2007).
2.2 Bacteria and its transformation
Electro-competent cell of E. coli k12DH5α was prepared and successfully transformed with (pGEM52f) plasmids by electroporation system (Sambrook and Russell 2001). The engineered bacterium was cultured on LB-amp and the resultant colonies were examined using a fluorescence microscope as well as by amplification of GFP gene using GFP-specific primers. Colonies with successful GFP expression were selected and used for further studies.
2.3 Larvae experiments using E. coli-GFP
Two experiments were done using different water sources supplemented by bacteria and ampicillin. For the first set the first instar larvae of An. stephensi was reared in trays that included the 106 CFU E. coli-GFP plus ampicillin 100 μg/ml. After 24 h, the larvae were washed twice in ddH2O for removing the possible surface bacteria and transferred to clean ddH2O plus ampicillin. The larvae were reared for rest of their life in the bacteria free ddH2O. For the second set of experiments, first instar larvae of An. stephensi were reared in trays that included E. coli-GFP and ordinary (tap) water without ampicillin. After 24 h exposure with E. coli-GFP, the larvae were transferred to ordinary water without E. coli-GFP after washing twice with double-distilled water. In addition, An. stephensi larvae were reared in trays using ordinary (tap) water with no E. coli-GFP or ampicillin and provided the negative controls. Feeding and rearing of larvae was carried out according to the previously described standard methods (Benedict 2007).
2.4 Pupae dissection
A subset of ten old pupae, close to eclosion, from the two experiment sets were dissected separately and the persistence of GFP-producer bacteria in their tissues was examined.
2.5 Adult experiments using E. coli-GFP
In order to test trans-stadial transmission and the effect of blood meal on colonization and localization of E. coli-GFP in the mosquito’s tissues, two parallel experiments were performed using adult mosquitoes. In the first experiment, a portion of the adults which were exposed to E. coli-GFP bacteria during laraval stage were kept in sterile containers without any sugar solution or blood meal and dissected 2 days after eclosion. The remaining females were blood fed on day three and were subjected to dissection or kept alive for a subsequent blood meal on day ten. The blood-fed mosquitoes were dissected and examined 24, 48 and 72 h post feeding using a fluorescence microscope (BX61 microscope, with U-LH100HGAPO lamphouses and DP-70 imaging system, Olympus). In the second experiment, ordinary adult specimens, which were not exposed to E. coli-GFP in larval stage, were fed sugar-containing E. coli-GFP bacteria. As with the first experiment, the adults were fed one or two blood meals on days three or ten after eclosion, respectively, and were then tested for traces of E. coli-GFP bacteria 24, 48 and 72 h post first and second blood meal.
In all the fluorescence microscope surveys, larvae or adult of mosquitoes with no exposure to the GFP-expressing bacteria were examined as negative controls to ensure the correct detection of the green fluorescence due to GFP and assess any auto-fluorescence by the inner parts of the mosquitoes.
2.6 PCR amplification of GFP gene in mosquito specimens
To confirm the persistence of E.coli-GFP in the specimens and diminish the risk of contamination of the mosquitoes with GFP-DNA, mosquito midguts were plated on the Amp-selective LB and the bacteria which grew were checked under the fluorescence microscope prior to DNA extraction. The frequency of colonies on the plates was compared to test of the effect of a blood meal on the propagation of the E.coli-GFP in the mosquitoes’ midgut 24, 48 and 72 h post blood feeding. For DNA extraction, before dissection of the specimens, the surface of the specimens were briefly sterilized with 70 % ethanol (Pidiyar et al. 2004) in a sterile hood. Then under sterile conditions, specimens were dissected individually and the midgut was mashed and suspended in 500 μL of Brain Heart Infusion (BHI) and incubated for 24–48 h at 37 °C. The DNA of the bacteria that grew was extracted using QIAGEN DNeasy Kit (Qiagen, Germany) according to the manufacturer supplied manual. The specific GFP gene amplifying primers, GFP-F 5′-caagagtgccatgcccgaagg-3′ and GFP-R 5′-gacagggccatcgccaattgg-3′, were used to obtain a 280 bp band (Li et al. 2006; Sarrazin et al. 2009). The PCR conditions were set as an initial denaturation at 94 °C for 10 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 40 s, and extension at 72 °C for 30 s, followed by a final extension at 72 °C for 8 min.
3 Results
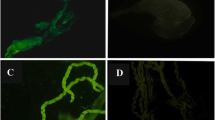
The persistence of the E. coli-GFP bacteria in the midgut of An. stephensi specimens was monitored following molting, metamorphosis, and blood feeding to investigate the dynamics and maintenance of these bacteria during the transition from larvae to pupae, pupae to adult, and from unfed to fed status respectively. These observations revealed that the bacteria were associated in the midguts of all tested (10–20 specimens for each stage) larvae, pupae, emerging imagoes and adult mosquitoes. Our observations showed that the E. coli-GFP bacteria have trans-stadial transmission in An. stephensi mosquitoes indicating that several molting or ecdysis events during larval stages, as well as hydrolytic processes during metamorphosis cannot remove or delete the E. coli-GFP bacteria from the midgut or malpighian tubules organs (Fig. 1a, b, c and d). The results also showed that a short contact time (24 h) between the bacteria and the first instar larvae was sufficient for the larvae to acquire the bacteria from the larval habitat.
E. coli-GFP colonization and trans-stadial transmission in different life stage of Anopheles stephensi which is demonstrated by the expression of GFP gene in the colonized bacteria resulted in a green light under the microscope: a midgut of larva, b live pupae c, midgut of pupae, d midgut (MG) of adult and e negative control
The study on the effect of a blood meal on colonization by the bacteria in the adult midguts showed that a blood meal can dramatically increase the number of bacteria during 24–48 h after first or second blood meal (Fig. 3a, b). The results were revealed by an enormous increase in fluorescent light throughout of the mosquito abdomen (Fig. 3c, d). The fluorescence microscope analysis of ordinary adults which were fed with sugar containing E. coli-GFP bacteria (bacteria-impregnated sugar solution) revealed the successful acquisition and colonization of the bacteria in the mosquito’s midgut, 24, 48 and 72 h after the first or the second blood meal (Fig. 2a, b).
There was no significant difference between the successful colonization and persistence of E. coli-GFP in the lines of larvae maintained in tap water versus those in sterilized water, although a stronger fluorescence was observed in larvae kept in non-sterile conditions. The reason for this is unclear but it seems likely that the larval surroundings may influence the establishment of the E.coli-GFP in some way.
The PCR amplification of specimens with the GFP specific primers showed the presence of bacteria in the midgut of larvae, pupae, newly emerged flies, and blood fed mosquitoes up to three days post blood meal (Fig. 5). There was a positive correlation between the sharpness of PCR band and the frequency of E.coli-GFP colonies in the plates. However, there was no positive PCR amplification against the midgut bacterial DNA of the 14 days old specimen. Furthermore, no green fluorescence was observed in the midgut of 14 days old specimens or in the midgut (after 24–48 h) of adults given a third blood meal. We assume that the bacteria had disappeared from the midgut of the 14 days old specimens but the cause is obscure.
Testing localization of the E. coli-GFP bacteria in the mosquito’s tissues showed that, in addition to midgut, there was a massive and surprising colonization by bacteria in the malpighian tubules of both larvae and adult mosquitoes (Fig. 4a, b). The bacteria remained there in excessively high numbers throughout of the mosquito life stages regardless of blood meal. In contrast to the midgut, there was a very sharp PCR amplification band or a bright green fluorescent light in the malpighian tubules of 14 day old, or even older, adults as well as in those examined 48 h after a third blood meal.
Examination by the fluorescence microscope of larvae or adult mosquitoes reared without GFP-expressing bacteria (Figs. 1e, 2c, 3e and 4c) or PCR assays (Fig. 5), revealed the lack of E. coli-GFP in the negative controls, although some minor green fluorescence was observed that was due to auto-fluorescence of the inner parts of the larval or adult’s body (Figs. 1e, 2c, 3e and 4c).
Colonization of E. coli-GFP in An.stephensi midgut post blood meal (pbm) resulted in increased propagation of the bacteria within 24–48 h after second blood meal (pbm) (a) the distance between pritorphic matrix (PM) and epithelial cell line (EP), (b) disrupted midgut and pritrophic matrix showing blood clots and green light 48 h pbm, (c and d) An.stephensi abdomen 24 and 48 h pbm respectively, e negative control
Amplification of partial GFP gene (286 bp) against E. coli-GFP genome in a fourth instar larva, b pupae, c newly emerged, and d 10 days old adult of An.stephensi with two round blood feeding, M: A 100 base pair molecular weight marker (Cinnagen, Iran). (NA) E. coli-GFP free adult as negative control and (NL) E. coli-GFP free larvae as negative control
4 Discussion
Finding suitable candidate for paratransgenesis is a complex process and appropriate studies needs to be carried out in order to understand aspects of the symbiotic relationship between bacteria and its mosquito host. In the present study we found that the E. coli-GFP exhibited trans-stadial transmission, localized in midgut, that increased dramatically following a blood meal, and remained in the lumen of adult mosquitoes for at least 13 days after eclosion. These characteristics make these bacteria a suitable candidate for paratransgenesis. However, the 13 day durability of E. coli-GFP in the current study does not agree with the observations of Riehle et al. (2007) who found that their E.coli strain was completely eliminated after 96 h. This difference may be due to intrinsic factors including the biological form or strain of An. stephensi, or that of the E.coli. Other biotic (symbionts) or abiotic factors in the mosquito’s midgut may also have differed in the two studies. Further investigations are clearly required.
The results of the present study demonstrated the ability of mosquitoes to acquire E. coli-GFP bacteria from both bacteria-impregnated sugar solutions and the water of breeding sites. This finding is in agreement with the study of (Lindh et al. 2006) indicating the possibility of using both sugar-baits and oviposition sites to introduce manipulated bacteria into a mosquitoes’ population in the field. However, introducing the engineered bacteria into wild adult mosquitoes could be complicated and challenging, so it may be easier to introduce manipulated bacteria at the larval stage. Some studies suggest that the majority of midgut bacteria in mosquito larvae are not transferred to the adult stage, being removed during metamorphosis (Moll et al. 2001). The egestion of the meconium (a food bolus plus bacteria surrounded by the peritrophic matrix) or ingestion of molting fluid containing antimicrobial substances, are among the suggested reasons for the enormous decrease of bacteria in the mosquito midgut observed during metamorphosis and adult emergence (Riehle and Jacobs-Lorena 2005). The number of bacteria before and after metamorphosis was not tested in this study but the results showed that at least some bacteria remained in the midgut and so bacteria were not completely egested with the meconium.
Trans-stadial transmission of microorganisms from larvae to adult stage has been reported previously in different groups of arthropods including bacteria in Anopheles mosquitoes (Favia et al. 2007; Favia et al. 2008; Lindh et al. 2008; Damiani et al. 2010; Boissière et al. 2012); Serratia odorifera in Aedes aegypti (Apte-Deshpande et al. 2012); LaCrosse Encephalitis virus in Ochlerotatus triseriatus (McJunkin et al. 2001); Rift Valley fever virus in Culex pipiens, Aedes circumluteolus and Abydosaurus mcintoshi (Turell et al. 1990), and Francisella tularensis in ticks(Reese et al. 2011) and mosquitoes (Lundström et al. 2011) . However, the mechanisms leading to trans-stadial transmission of microorganisms have not been well described. It is believed that despite of “gut sterilization” during metamorphosis from pupae to adult, microbiota clearance is not complete and a part of the larval midgut is retained to adulthood during the metamorphosis (Rani et al. 2009; Wang et al. 2011b). Apte-Deshpande et al. (2012) speculated that elevated environmental temperatures and strong hemolytic and proteolytic properties of Serratia odorifera can potentially favor selective preservation and trans-stadial transmission of S. odorifera in the Ae. aegypti midgut (Apte-Deshpande et al. 2012). Andreadis (2005) suggested that strategies such as delayed virulence, low pathogenicity and high tissue specificity, and polymorphic development, well synchronized with host mosquito physiology, could lead to trans-stadial transmission of microsporidium in mosquitoes of Ochlerotatus cantator (Andreadis 2005). The presence of E. coli-GFP in the malpighian tubules of the larval and adult stages, in the present study, may explain how trans-stadial transmission of E.coli-G7FP occurs and is retained in the adult. It is known that under the influence of ecdysone, most of the larval tissues degenerate but the larval malpighian tubules of Anopheles mosquitoes remain intact (Singh and Hou 2009; Gautam and Tapadia 2010). Malpighian tubules are attached to the insect’s alimentary canal at a point where the midgut and hindgut are joined. The proximal region of the tubule is open to lumen and this may facilitate draining of tubular fluid including bacteria from the malpighian tubules to the midgut.
The results of this study showed trans-stadial transmission of E. coli in An. stephensi. This phenomena had already been reported in An.gambiae, An.stephensi and An.albimanus (Pumpuni et al. 1996). It seems that trans-stadial transmission of bacteria is controlled by species specific intrinsic factors. Studies of Moll et al. (2001) showed the trans-stadial transmission of a wider range of midgut bacteria in some Culex species that in An. punctipennis and Aedes aegypti (Moll et al. 2001). Furthermore, bacterial infection of newly emerged mosquitoes varies widely: An. albimanus (17 %), An. gambiae (19 %) and An. stephensi (73 %) (Pumpuni et al. 1996).
The findings of this study demonstrate the value of E. coli-GFP bacteria as a laboratory model to evaluate the efficacy of effector gene/s against malaria and filarial parasites. E. coli has been found to be the commonest bacterium isolated from the mosquito midgut (Straif et al. 1998) and thus a good candidate for paratransgenesis. It has already been reported that E. coli bacteria expressing SM1 and PLA2, anti-Plasmodium peptides, can partially inhibit the parasite’s development within the mosquito vector (Riehle and Jacobs-Lorena 2005; Riehle et al. 2007; Bisi and Lampe 2011). However, further studies are needed to understand the symbiotic relationship between the bacteria and their environment including the physical and biological quality of mosquito’s larval breeding places and how this affects the bacteria life cycle. In the present study, it was shown that ordinary tap water favored bacterial colonization of the larvae over those reared in ddH2O and previous have documented that waters vary with respect to their bacterial load and function (Chapelle 2001). The results of the present study also confirmed the significant positive relationship between blood feeding and bacterial colonization in the midgut of An. stephensi. This is in agreement with previous studies indicating that a blood meal has positive effect on bacterial colonization in the mosquito’s midgut (Pumpuni et al. 1996; Riehle et al. 2007). Female Anopheles mosquitos acquire the malaria parasites during blood-feeding from an infected human. After blood-feeding, the symbiotic bacteria grow, colonize and quickly occupy the midgut. Connecting the expression of the desired effector gene/s to blood-feeding in mosquito’s tissues should lead to the production of an interfering protein against Plasmodium at the right time. Thus, researchers should look for a suitable promoter that links the expression of effector gene/s in bacteria with blood feeding.
It was also shown that E. coli-GFP successfully established and colonized in malpighian tubules of An.stephensi. This finding provides further support for using this bacterium as a candidate for a paratransgenesis control strategy for other vector-borne agents in which the mosquito’s malpighian tubules are involved. For instance, this approach could be applied against Dirofilaria immitis, one of the causing agents of zoonotic dirofilariasis, which is endemic in several parts of southern Europe (Muro et al. 1999) the United States of America (Theis 2005), some parts of Asia (Kan et al. 1977), and the middle east, e.g. Iran (Azari-Hamidian et al. 2009).
5 Conclusion
Paratransgenesis is a new and multi-dimensional approach in which a step by step development is possible. Finding suitable bacteria for paratransgenesis in order to control malaria is the first step. This should be followed by testing effector molecules and by studying the symbiotic relationship between midgut bacteria and their host (Anopheles mosquitoes). E. coli is a good laboratory model for evaluating the efficacy of the various effector proteins against Plasmodium and Dirofilaria during the development of new strategies to control these human diseases and their insect vectors.
References
Aksoy S, Weiss B, Attardo G (2008) Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv Exp Med Biol 627:35–48. doi:10.1007/978-0-387-78225-6_3
Andreadis TG (2005) Evolutionary strategies and adaptations for survival between mosquito-parasitic microsporidia and their intermediate copepod hosts: a comparative examination of Amblyospora connecticus and Hyalinocysta chapmani (Microsporidia: Amblyosporidae). Folia parasitologica 52(1–2):23–35
Apte-Deshpande A, Paingankar M, Gokhale MD, Deobagkar DN (2012) Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS One 7(7):e40401
Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol 23(2):111–121. doi:10.1111/j.1365-2915.2009.00802.x
Beard CB, Cordon-Rosales C, Durvasula RV (2002) Bacterial symbionts of the triatominae and their potential use in control of Chagas disease transmission. Annu Rev Entomol 47:123–141. doi:10.1146/annurev.ento.47.091201.145144
Benedict M (2007) Methods in Anopheles research. Malaria Research and Reference Reagent Resource Center, Atlanta
Bisi DC, Lampe DJ (2011) Secretion of anti-Plasmodium effector proteins from a natural Pantoea agglomerans isolate by using PelB and HlyA secretion signals. Appl Environ Microbiol 77(13):4669–4675
Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R (2012) Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathogens 8(5):e1002742
Breman JG, Egan A, Keusch GT (2001) The intolerable burden of malaria: a new look at the numbers. AmJTrop Med Hyg 64(1–2 Suppl):iv–vii
Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, Manguin S, Morgan JC, Hemingway J (2003) Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol 17(1):87–94
Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, Guillet P (1999a) Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull World Health Organ 77(3):230–234
Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, Carnevale P, Guillet P (1999b) Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia 41(1–3):319–322
Chapelle F (2001) Ground-Water Microbiology and Geochemistry, John Wily & Sons
Chavshin AR, Oshaghi MA, Vatandoost H, Pourmand MR, Raeisi A, Enayati AA, Mardani N, Ghoorchian S (2012) Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: a step toward finding suitable paratransgenesis candidates. Acta Trop 121(2):129–134
Coutinho-Abreu IV, Zhu KY, Ramalho-Ortigao M (2010) Transgenesis and paratransgenesis to control insect-borne diseases: current status and future challenges. Parasitol Int 59(1):1–8. doi:10.1016/j.parint.2009.10.002
Damiani C, Ricci I, Crotti E, Rossi P, Rizzi A, Scuppa P, Capone A, Ulissi U, Epis S, Genchi M, Sagnon N, Faye I, Kang A, Chouaia B, Whitehorn C, Moussa GW, Mandrioli M, Esposito F, Sacchi L, Bandi C, Daffonchio D, Favia G (2010) Mosquito-bacteria symbiosis: the case of Anopheles gambiae and Asaia. Microb Ecol 60(3):644–654. doi:10.1007/s00248-010-9704-8
Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J (2003) Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol 17(2):138–144
Fang W, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St. Leger RJ (2011) Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331(6020):1074–1077. doi:10.1126/science.1199115
Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S (2007) Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci 104(21):9047–9051
Favia G, Ricci I, Marzorati M, Negri I, Alma A, Sacchi L, Bandi C, Daffonchio D (2008) Bacteria of the genus Asaia: a potential paratransgenic weapon against malaria. Adv Exp Med Biol 627:49–59. doi:10.1007/978-0-387-78225-6_4
Gautam NK, Tapadia MG (2010) Ecdysone signaling is required for proper organization and fluid secretion of stellate cells in the Malpighian tubules of Drosophila melanogaster. Int J Dev Biol 54(4):635–642. doi:10.1387/ijdb.092910ng
Gonzalez-Ceron L, Santillan F, Rodriguez MH, Mendez D, Hernandez-Avila JE (2003) Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol 40(3):371–374
Hill CA, Kafatos FC, Stansfield SK, Collins FH (2005) Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol 3(3):262–268. doi:10.1038/nrmicro1101
Howard AF, Koenraadt CJ, Farenhorst M, Knols BG, Takken W (2010) Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar J 9:168. doi:10.1186/1475-2875-9-168
Hunt R, Edwardes M, Coetzee M (2010) Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasit Vectors 3:122. doi:10.1186/1756-3305-3-122
Kan SP, Rajah KV, Dissanaike AS (1977) Survey of dirofilariasis among dogs in seremban, Malaysia. Vet Parasitol 3(2):177–181
Li J, McLellan S, Ogawa S (2006) Accumulation and fate of green fluorescent labeled Escherichia coli in laboratory-scale drinking water biofilters. Water Res 40(16):3023–3028
Lindh J, Borg-Karlson A, Faye I (2008) Transstadial and horizontal transfer of bacteria within a colony of Anopheles gambiae (Diptera: Culicidae) and oviposition response to bacteria-containing water. Acta Trop 107:242–250
Lindh JM, Terenius O, Eriksson-Gonzales K, Knols BG, Faye I (2006) Re-introducing bacteria in mosquitoes–a method for determination of mosquito feeding preferences based on coloured sugar solutions. Acta Trop 99(2–3):173–183. doi:10.1016/j.actatropica.2006.07.008
Lindh JM, Terenius O, Faye I (2005) 16S rRNA gene-based identification of midgut bacteria from field-caught Anopheles gambiae sensu lato and A. funestus mosquitoes reveals new species related to known insect symbionts. Appl Environ Microbiol 71(11):7217–7223. doi:10.1128/AEM.71.11.7217-7223.2005
Lundström JO, Andersson AC, Bäckman S, Schäfer ML, Forsman M, Thelaus J (2011) Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis 17(5):794
Magesa SM, Aina O, Curtis CF (1994) Detection of pyrethroid resistance in Anopheles mosquitos. Bull World Health Organ 72(5):737–740
McJunkin JE, de los Reyes EC, Irazuzta JE, Caceres MJ, Khan RR, Minnich LL, Fu KD, Lovett GD, Tsai T, Thompson A (2001) La Crosse encephalitis in children. New England. Journal of Medicine 344(11):801–807
Moll RM, Romoser WS, Modrzakowski MC, Moncayo AC, Lerdthusnee K (2001) Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol 38(1):29–32
Munhenga G, Masendu HT, Brooke BD, Hunt RH, Koekemoer LK (2008) Pyrethroid resistance in the major malaria vector Anopheles arabiensis from Gwave, a malaria-endemic area in Zimbabwe. Malar J 7:247. doi:10.1186/1475-2875-7-247
Muro A, Genchi C, Cordero M, Simón F (1999) Human dirofilariasis in the European union. Parasitology Today 15(9):386–389
Oshaghi MA, Yaaghoobi F, Abaie MR (2006) Pattern of mitochondrial DNA variation between and within Anopheles stephensi (Diptera: Culicidae) biological forms suggests extensive gene flow. Acta Trop 99(2–3):226–233
Pidiyar VJ, Jangid K, Patole MS, Shouche YS (2004) Studies on cultured and uncultured microbiota of wild culex quinquefasciatus mosquito midgut based on 16 s ribosomal RNA gene analysis. AmJTrop Med Hyg 70(6):597–603
Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC (1996) Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. AmJTrop Med Hyg 54(2):214–218
Rani A, Sharma A, Rajagopal R, Adak T, Bhatnagar R (2009) Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol 9(1):96
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27(2):91–98. doi:10.1016/j.pt.2010.08.004
Reese SM, Petersen JM, Sheldon SW, Dolan MC, Dietrich G, Piesman J, Eisen RJ (2011) Transmission efficiency of Francisella tularensis by adult american dog ticks (Acari: Ixodidae). Journal of medical entomology 48(4):884–890
Ren X, Hoiczyk E, Rasgon JL (2008) Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog 4(8):e1000135. doi:10.1371/journal.ppat.1000135
Riehle MA, Jacobs-Lorena M (2005) Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol 35(7):699–707. doi:10.1016/j.ibmb.2005.02.008
Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M (2007) Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol 37(6):595–603. doi:10.1016/j.ijpara.2006.12.002
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, vol 1. CSHL press
Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, Otto C, Sieweke MH (2009) MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell 138(2):300–313
Singh SR, Hou SX (2009) Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. J Exp Biol 212(Pt 3):413–423. doi:10.1242/jeb.024216
Straif SC, Mbogo CN, Toure AM, Walker ED, Kaufman M, Toure YT, Beier JC (1998) Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J Med Entomol 35(3):222–226
Terenius O, de Oliveira CD, Pinheiro WD, Tadei WP, James AA, Marinotti O (2008) 16S rRNA gene sequences from bacteria associated with adult Anopheles darlingi (Diptera: Culicidae) mosquitoes. J Med Entomol 45(1):172–175
Theis JH (2005) Public health aspects of dirofilariasis in the United States. Vet Parasitol 133:157–180
Turell MJ, Saluzzo JF, Tammariello RF, Smith JF (1990) Generation and transmission of Rift Valley fever viral reassortants by the mosquito Culex pipiens. J Gen Virol 71(10):2307–2312
Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I (2005) Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Kerman province, southeastern Iran. J Vector Borne Dis 42(3):100–108
Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H (2006) Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran, 2002. Acta Trop 97(2):196–203. doi:10.1016/j.actatropica.2005.11.002
Wang S, Fang W, Vega-Rodriguez J, Ghosh A, Leger RS, Jacobs-Lorena M (2011a) Fighting Malaria with engineered fungi and bacteria. In: Molecular and population biology of disease vectors, Orthodox Academy of Crete Kolymbari, Greece
Wang Y, Gilbreath TM 3rd, Kukutla P, Yan G, Xu J (2011b) Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6(9):e24767. doi:10.1371/journal.pone.0024767
Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M (2012) Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proceedings of the National Academy of Sciences
WHO (2010) World malaria report 2010. World Health Organization.
Acknowledgment
Authors would like to thank Eng. Abaei, MR. and Eng. Rafie, F. for their assistance in An. stephensi rearing. This study was financially supported by the Tehran University of Medical Sciences (TUMS). The authors also thank the anonymous reviewers for their helpful comments and Professor David Richardson for assistance with editing and improving the English.
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
ARC did the experiments, analyzed the results and wrote the draft of manuscript, MAO and HV designed the study and coordinated the team works, BY and FZ assist the bacterial experiments and AR reviewed the manuscript and finalized the study design.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chavshin, A.R., Oshaghi, M.A., Vatandoost, H. et al. Escherichia coli expressing a green fluorescent protein (GFP) in Anopheles stephensi: a preliminary model for paratransgenesis. Symbiosis 60, 17–24 (2013). https://doi.org/10.1007/s13199-013-0231-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-013-0231-5