Abstract
The main goal of this study was to compare the changes that happen during the frying process with the refined oleic sunflower oil (ROSO), refined olive oil (ROO) and refined linoleic sunflower oil (RLSO) in 5-day testing period as well to evaluate the efficacy of fatty acid composition on frying stability. Each day, oil samples were withdrawn after 7 h of frying and were further subjected to analyzes for determination of different chemical properties. As a result, in the current studies were observed significant changes in all oil types in terms of chemical properties. ROSO, which contained lower linoleic acid content, had higher thermal stability than both RLSO and ROO. This result indicated that the frying efficiency of ROSO depended mainly on the linoleic acid content and the iodine value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been global increase in the consumption of fried foods despite that some negative results about degraded frying fats and fried foods have been known for many years. Deep frying is defined as the process of frying and cooking of food through contact with oil at a temperature of 120–190 °C (Velasco et al. 2009; Aydınkaptan and Mazı 2017). The fried products are recognized for their crispy texture, roasted, fried aroma and their pleasant golden to brown color (Brühl 2014). However, during deep frying, the oil is in contact with the air and food at a high temperature and this gives rise to the emergence of oxidative, hydrolytic and thermal alterations of the oil. These co-occurring complex reactions cause the formation of many components in the oil that greatly reduce its nutritional value (Velasco et al. 2009; Aydınkaptan and Mazı 2017).
Fatty acid degradation reaction caused by thermo oxidative factors occurs in the later stages of the frying which creates off-aroma (Brühl 2014). Resulting from these degradation reactions are formed dimers and polymers borned of triacylglycerols containing unsaturated acyl groups. Sometimes, the newly formed compounds have more polar properties than the original triacylglycerol molecule. Many of these structurally new compounds affect negatively the oil quality (Velasco et al. 2009). There are many factors affecting the oil degradation. The most important ones that affect the frying oil quality are the conditions of the process. Besides, there are also some other factors such as oil composition, oil interaction with food, frying period and etc. (Rossell 2001; Aladedunye et al. 2014). The physical and chemical changes that happen in different types of oils during deep frying have been generally studied by different researchers. Besides, in lots of researches there were compared traditional methods of frying with some new ones, such as microwave drying, osmotic pretreatments etc. (Velasco et al. 2009; Zribi et al. 2014; Crosa et al. 2014; Zhang et al. 2016). In the before mentioned researches there were studied different types of oil except for oleic type sunflower oil. There are some studies focusing on frying with oleic type sunflower oil (Kaya et al. 2008; Ali et al. 2013; Smith et al. 2017). Sunflower oil, which contains a high (around 71%) polyunsaturated fatty acid (PUFA) content, is a commonly used vegetable oil in Turkey. Besides, high oleic sunflower oil is becoming more popular in Turkey and all over the world (Marquard 1987). However, there is no literature on the difference of refined high oleic sunflower oil degradation quality after multiple uses of the frying oil than refined olive oil and refined linoleic sunflower oil in Turkey. Even the literature search shows that virgin olive oil is also used for frying; refined oils have higher importance for their industrial use. The dominant fatty acid in standard (linoleic type) sunflower oil is a linoleic, polyunsaturated, omega-6 fatty acid, followed by an oleic fatty acid, while the linolenic acid content is always less than 0.3%. The low content of linolenic acid is positive because this fatty acid contributes most to the oxidative instability of the oil (Paunović et al. 2020). Therefore, the main goal of this research was to investigate the effect of repeated process of frying upon two different oils—refined oleic/linoleic sunflower oil and to monitor the physicochemical and chromatic features of these oils after the process of frying degradation has occurred. In addition, the obtained results from refined oleic/linoleic sunflower oil were compared with refined olive oil.
Materials and methods
Material
Refined linoleic sunflower oil (RLSO), refined oleic sunflower oil (ROSO) and refined olive oil (ROO) were purchased from a local market in Kırklareli, Turkey. Frozen French fries were also bought in the local market and stored at − 18 ± 1 °C. The frying process was performed in a thermostatically temperature-controlled fryer (Tefal, Milan, Italy) at 180 °C. All chemical reagents were of chromatographic and spectroscopic grade and were supplied by Sigma Aldrich (U.S.A).
Sample preparation
4 L of each type of oil were placed into separate deep fryer (Tefal, Milan, Italy) with 5 L capacity and heated to 180 °C for 7 h per day for 5 days. Each sample of the potato slices (200 g) was fried for 7-min. After each frying there was made a pause for 35 min. This cycle of frying and pauses were repeated for 10 times during the day for five sequential days. At the end of each day, it was withdrawn sample of the frying oil (15 g). The oil sample was filtered into a closed, dark colored vial and stored at 4 °C until being analyzed.
Fatty acid composition (FAC)
The fatty acid composition of the oil extracts was analyzed by gas chromatography (GC). FAC was measured according to the method of Marquard (1987). The chromatographic separation was done in a Perkin Elmer Auto System XL gas chromatograph fitted with a flame ionization detector (FID), and a fused silica capillary column (MN FFAP (50 m × 0.32 mm i.d.; film thickness 0.25 µm). It was got with the fixed conditions for oven temperature program as 120 °C for 1 min. raised to 240 °C at a rate of 6 °C/min and then kept at 240 °C for 15 min. Injector and detector temperatures were fixed to 250 and 260 °C, respectively. Carrier gas was used at flow rate of 15 cm/s and split ratio was 1/20 mL/min. Methyl esters prepared from fatty acids, including C16:0, C18:0, C18:1, C18:2 and C20:0, as well as a fatty acids methyl ester (FAME) standard mixture, were all purchased from Sigma-Aldrich and used as the standard for interfying fatty acids in the samples. These fatty acids were quantified according to their peak area and expressed as a percentage of total fatty acid content.
Acid value (AV)
The calculation of acid values for all the samples were performed according to the AOCS Official Method Ca-5a-40 (AOCS 1997). The sample (1 g) was thoroughly mixed with ethyl alcohol (15 mL) and it was titrated with potassium hydroxide (0.25 N) solution using phenolphthalein as a color indicator, causes pink coloring. Acid value is defined as the weight of KOH in mg needed to neutralize the organic acids present in 1 g of fat and it is a measure of the free fatty acids (FFA) present in the fat or oil. The AV was calculated by the following formula:
where V is the volume of KOH used for titration of the oil samples, C is KOH concentration, and W is the weight of the oil sample in grams.
Peroxide value (PV)
The peroxide value of the samples was determined by AOCS Official Method cd8-53 (AOCS 2003). The oil sample (nearly 5 g) was weighed into a flask, further was added glacial acetic acid/chloroform (3:2, v:v, 30 mL) and a saturated potassium iodide solution. Then the mix solution was stirred slowly for 1 min and was added distilled water and saturated starch solution. It was titrated with sodium thiosulfate solution (0.01 N) until getting colorless medium. The PV was calculated by the following equation,
Iodine value (IV)
The iodine value of the oil samples was determined following the Standard Methods for the Analysis of Fats, Oils in AOCS Official Method AOCS Cd 1d-92 (AOCS 2005).
Saponification value (SV)
The saponification value was calculated according to the Standard Methods for the Analysis of Fats, Oils in AOCS Official Method AOCS Cd 3d-25 (AOCS 2005).
Anisidine value (AnV)
Anisidine value was determined applying the AOCS Official Method Cd 18–90 (AOCS 1998). The oil samples (0.5 g) were mixed with isooctane (25 mL), which contain p-anisidine reagent (1 mL). The absorbance of this solution at 350 nm was measured by spectrophotometer (Shimadzu 2600, Shimadzu, Kyoto, Japan) and p-anisidine reagent used as blank in the reference cuvette.
Total oxidation value (TOTOX)
The TOTOX value is calculated by the formula AnV + 2PV for determination of total oxidation state of the oils.
where AnV is the anisidine value and PV is the peroxide value (Shahidi and Wanasundara, 2002).
Conjugated diene (K232) and triene (K270) value
The conjugated diene (K232) and triene (K270) analyzes were performed based on the modified version of the AOCS Official Method Ch5-91 (AOCS 2006). In the modified AOCS method, a sample size of 0.03 g was diluted to 50 mL in hexane. The absorbance was measured at 232 nm, 266 nm, 270 nm and 274 nm, using the hexane as a reference, with Shimadzu Spectrophotometer. ∆K and K values at 232 nm and 270 nm were calculated using the following formulas.
where K is the specific absorption value for each wavelength and E is the absorbance value for each wavelength. Also, c is the concentration of solution (g/100 mL) and s is the thickness of the cuvette (cm) (Choe and Min 2005; Smith et al. 2017).
Total polar compounds (TPC)
A Testo 270 instrument (Lenzkirch, Germany) was used to measure the TPC values of the tested oils. The sensor was calibrated using the reference oil having a TPC value of 3.5 ± 0.5% according to the prospectus. Before TPC measurement, the sensor was immersed in the reference oil heated to approximately 50 °C for reference value adjustment (Aydınkaptan and Mazı, 2017).
Statistical analyzes of data
The oil samples were performed in duplicate treatments for all analyzes. Besides, the experiments were evaluated by performing three independent measurements for all treatment and expressed as the mean ± standard deviation (SD). These results were analyzed by variance (ANOVA) and Duncan’s test with 5% significance level (Version 17.0).
Results and discussion
Fatty acid composition
The FAC of ROSO, RLSO and ROO samples were listed in Table 1. Before deep frying process, ROSO contained 75.40% oleic acid, about 41% more than RLSO, and 15.40% linoleic acid, which was about 39% less than RLSO. Besides, ROO contained 68.80% oleic acid, about 7% less than ROSO and 34% more than RLSO. The fatty acids present in a non-radical single form do not react directly with atmospheric oxygen, which exists as diradical in its ground state as triplet oxygen (Min and Boff 2001). Resulting from oxidation reactions, the non-radical fatty acid form is transformed to a free radical by removing labile hydrogen from a carbon atom adjacent to a double bond. The free radical formation of oleic acid (hydrogen atom abstraction from C11) and linoleic acid (C8) requires 75 kcal/mol and 50 kcal/mol energy respectively (Abbas Ali et al. 2014; Smith et al. 2017). Therefore, based on this fact, it should be observed that ROSO has higher oxidative stability compared to RLSO and ROO. Also, as presented in Table 1, the FAC of the oil samples had a significant difference before and after deep frying process, with more profound differences for RLSO than for ROO. The heating process resulted in a reduction in the relative percentage of unsaturated fatty acids (Table 1). It has been verified that heating of oil leads to a fast reduction in USFA (unsaturated fatty acids) than of SFA (saturated fatty acids) (Warner and Mounts 1993; Abbas Ali et al. 2013). In this research, the percentage of linoleic acids was head for decrease, while the percentage of palmitic and stearic acids has raised possibly due to PUFA oxidation products during the heat proceeding. This observation was verified for all samples with compare the area of the peaks at different days. Before deep frying process, linoleic acid was 54.30%, 15.40% and 10.80% for RLSO, ROSO and ROO, respectively. These ratios decreased to 46.50%, 11.05% and 8.30% for them after 5 frying days, when palmitic and stearic acids increased. As presented in Table 1, the value of the C18:2/C16:0 ratio in fresh RLSO was higher than in ROSO due to the large percentage of linoleic acid in RLSO. Also, the lowest value of the C18:2/C16:0 ratio, in fresh oil, was observed in ROO. Besides, the reduction (from the beginning) in C18:2/C16:0 ratio was 4.24 units for RLSO; being higher than that found in ROSO (2.21) and in ROO (0.37). This indicated higher rate of oxidative degradation of RLSO. Some researchers have suggested the C18: 2/C16: 0 ratio as an indicator of PUFA's level of deterioration (Sebedio et al. 1990). The oxidative and thermal stabilities of edible oils appear to be related to oleic and linoleic contents, decreased linoleic and increased oleic content result in increased oil stability. The ratio of C18:2/C16:0 decreased in all oil samples during the heating process. According to our results, it was determined decrease for all samples during heating process. These findings are consistent with many studies, for example, Sebedio et al. (1990) for soybean oil and Abbas Ali et al. 2013 for high oleic sunflower oil found similar results during frying of frozen potatoes. Besides, the oxidation of USFA could cause decrease in C18:2, transformed into primary and secondary oxidation products during the heating process.
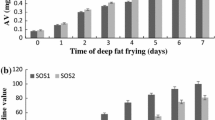
Acid value (AV)
Acid value is related to the content of fatty acids in oils. Free fatty acids (FFA) result from hydrolysis of triacylglycerols and are deterioration products from oxidized triacylglycerols (Xin-Qing et al. 1999). Although the FFA content is considered to be a controversial indicator for the degradation of fried fat, it is related to the quality of fried food. In practice, FFA levels may not affect frying performance or have significant adverse effects on health or sensory evaluation (Xin-Qing et al. 1999).
FFA levels may not affect frying efficiency in practice but they may have significant unfavorable effects on health or sensory evaluation. In our research, there was seen significant increase in AVs of the oils (P < 0.05) during frying process (Table 2). At the end of the procedure, the AV was found to be higher in ROSO (0.67%) than in RLSO (0.59%) but there were subtle differences (P > 0.05). However, AV of ROO (1.40%) had the highest value and there was significant difference compared to ROSO and RLSO (P < 0.05).
Normand et al. (2006) observed the greater rate of formation of FFA in high oleic sunflower oil samples compared with regular sunflower oil samples during 72 h of frying. Warner et al. (1994) found that the higher the oleic acid content of the oil was, the higher the FFA content in the heated oil. Likewise, in this study high oleic sunflower oil samples showed the faster rate of FFA accumulation after 4 h of heating. Also, it is smilar with our research that the highest FFA value detected in samples at the end of heating is still far below the accepted limit of 2% (Abbas Ali et al. 2013).
Peroxide value (PV)
As shown in Table 2, it was observed that peroxide values (PV) increased to the highest value during frying and then decreased to the end of tested frying process. Peak values were as follows: RLSO (41.99 for the second frying day), ROSO (24.51 for the second frying day), and ROO (18.29 for the third frying day). RLSO had highest peak value compared to ROSO and ROO that might due to the decreasing amount of linoleic acids, which is more prone to oxidation, present in the oils (Warner and Mounts 1993).
For peroxides, the data confirms the results showed in early studies (Abdulkarim et al. 2007; Tsaknis and Lalas 2002), with an increase in the peroxides until a maximum is reached, followed by a decrease of those compounds due to their reactions and degradations to other compounds. However, during the heating operation, PVs of the oils analyzed exceeded the acceptable limit and only PV is not enough reliable data for evaluation of heating process. It is more meaningful that explaining together with PV, p-anisidine value (p-AV), total polar compounds and dienes/trienes (Al-Kahtani 1991), they explained under “3.10. Total Polar Compounds (TPC)”.
Iodine value (IV)
IV is used to determine the degree of unsaturation of the fatty acids that are part of triacylglycerol. When fixed amount of iodine solution is added to the oils, iodine molecule reacts with the double bonds in the acyl chain in triacylglycerol. Later on it is titrated with standard sodium thiosulfate until a starch endpoint. The saturation degree of oils can be characterized effectively with IV. Olive oil has rich profile with monounsaturated fatty acids (18:1) and therefore it has an IV in the range of 75 to 90 (g of iodine/100 g of oil). The observed Iodine values vary in the following ranges: palm oil 50–55, corn oil 103–135, soybean oil 120–143, sunflower oil 110–143 and peanut oil 84–105 (Alireza et al. 2010). These values are in accordance with the IV values of our sunflower oil (Table 3). Besides, what is observed is that the IV for ROSO is lower than 110 and also it is more similar to ROO. RLSO samples had the highest reduction of IV (6.83 units) compared to the initial point. This indicates the highest decrease in double bonds due to oxidative loss. The lowest decrease in the IV was determined in the ROO samples, indicating that less oxidative rancidity was occurred in this sample. This was in agreement with the finding of Chebet et al. (2016). There is a tendency for decrease of IV of the oils in the course of deep frying. The decrease in IV in frying may be indication of changes in fatty acids during frying process (Tynek et al. 2001). Although the reduction in IV is due to complex physicochemical changes, it can be an important quality parameter during frying as an indicator of the oxidation degree.
Saponification value (SV)
There was observed increase of saponification values for RLSO (Table 3). The change in saponification values occurred in the range from 193.42 to 196.46 mg KOH/g oil during 5 consecutive days of frying. However, ROO saponification value remained stable, while there was a slight increase in SV of ROSO, while ROO values remained stable.
Anisidine value (AnV)
As emphasized above, only the increase in peroxide value is not enough significant for evaluation of the frying process. The value of AnV should be taken into consideration together with the peroxide value because it is also quite dependable and significant test. After the oxidation of unsaturated fatty acids, hydroperoxides and secondary oxidation products are generated. Determination of hydroperoxide formation is performed by measuring peroxide value (POV) and for the secondary oxidation products by measuring anisidine value (AnV). Hydroperoxides are not stable during deep frying process or heat treatment process and readily dissociate into their mixtures of volatile aldehyde compounds (Yaghmur et al. 2001; Ali et al. 2013). Accordingly, the obtained AnV is considered to be the measure of non-volatile aldehydes (2-alkenal and 2,4-dienal), which are degradation products in oils. Therefore, the anisidine value has a high correlation with secondary oxidation products that are more stable than hydroperoxides (Ali et al. 2013). The AnV differences are presented in Table 4. Before the frying process, there was no great difference in AnV values between the oils. However; after the frying process, it was observed significant differences between AnV values of oils (P < 0.05). At the end of the 35 h frying process, the AnV value reached to 190.48 for RLSO and 82.97 for ROO. For ROO and ROSO, which contain lower amount of PUFA, there was observed increase in AnV (76.58 and 112.73 units respectively, compared with the first obtained values). However, much higher increase in AnV was detected for RLSO (185.37 units from the initial). This indicates that FAC affects the formation of secondary oxidation products from primary oxidation products in oil during heating (Warner and Mounts 1993).
Total oxidation value (TOTOX)
AnV is often used in the industry as a factor associated with PV to calculate total oxidation or TOTOX, using the following equation: TOTOX = 2PV + AnV (Shahidi and Wanasundara 2002). There was significant increase of TOTOX values (P < 0.05). During heating process, the TOTOX values were found to be 268.76 for RLSO, 137.59 for ROSO and 108.86 for ROO (Table 4). The lower TOTOX value of the ROO and ROSO pointed out that they are more stable to oxidative rancidity than the RLSO. RLSO is characterized by highest TOTOX value as well as high percentage of PUFAs.
Conjugated diene/triene (K232/K270) and ΔK values
For checking the oils quality, it was measured their absorbance at 200 and 300 nm. These are frequency values related to conjugated diene (K232) and triene (K270). Higher absorption was detected in this wavelength span for refined oils compared to virgin olive oil.
The hydroperoxides, which indicate primary stage of oxidation, characterized with the absorbance maximum at 232 nm (Choe and Min 2005). Oxidation of polyunsaturated fatty acids occurs by the formation of hydroperoxides. Immediately after the formation of peroxides, the non-conjugated double bonds commonly found in natural unsaturated lipids affects the regeneration of conjugated dienes (K232), which absorbs at 232 nm. Carbonated compounds (secondary oxidation stage) and conjugated trienes (technological treatments) give rise to increase in the absorbance value at 270 nm. When polyunsaturated fatty acids containing three or more double bonds (eg, linolenic acid) undergo oxidation, the conjugation can be sustained to contain another double bond being caused by the formation of conjugated trienes (K270), which absorb at 270 nm. The changes in UV absorbance at 232 and 268 nm as measured by K 232 and K 268 were used as a relative quantification of oxidation (De Abreu et al. 2010). The presence of K232 and K270 is a better oxidation measurement because they remain in the frying oil (Gertz et al. 2000). According to these studies, the instability of peroxide molecules may also indicate the decrease in PV in the advanced stages of rancidity. In fact, fragmentation into smaller molecule compounds would be expected to be associated with the oxidation of lipids. The increase in K 232 and K 268 is comparative to the oxygen uptake and formation of peroxides in early stages of oxidation, also with the degradation rate of linoleic acid. Also, the purity and degree of degradation of oil can be presented by ΔK value, which is another main analyzes performed for estimation of oil quality. There is correlation between ΔK and the state of oxidation by detecting specific oxidized compounds, some of which are generated from secondary oxidation. Besides, ΔK indicates possible adulteration of refined oils which mixed with certain rates (Gertz et al. 2000). Each vegetable oil has a unique fatty acid composition, so the properties of the fatty acids it contains determine the oxidation criteria. For this reason, oxidized compounds will be formed variously when oils are mixed in different proportions. The higher percentage of polyunsaturated acids in the oils, the higher are the levels of conjugated dienes and trienes formed during heating (Gharby et al. 2016). This was the reason why the ROSO, ROO and RLSO, which contained relatively different percentages of polyunsaturated acids (linoleic acid), have accumulated more conjugated dienes and trienes. Changes in the ultraviolet spectrum have been used as a relative measure of oxidation. Besides, it is same for mixed oils, which have different percentages of polyunsaturated acids.
In the Fig. 1 are shown the differences in time for ultraviolet absorption at 232, 266, 270 and 274 nm related to the changes for conjugated dienes/trienes and ΔK values. At the end of the frying process, the lowest level of conjugated dienes and trienes were observed in ROO samples and the highest level was detected in RLSO samples. The lower level of conjugated dienes and trienes is an indicator for good oxidative stability for oils.
Total polar compounds (TPC)
TPC measures directly the degraded components in oil. Table 5 shows changes in polar compounds during deep frying process. The maximum and minimum value of TPC, for commercial frying oils, is accepted as 24 and 27% respectively in several European countries (Freja et al. 1999) There was seen significant rising for the amount of TPC with time in oil samples (P < 0.05) and also there were significant differences for each one during all frying time (P < 0.05) (Table 5). At the 4th day of frying, TPC contents exceed to the limit value, which is 24% based on the standards. These results indicated that the oils used in this study could be used up to 3 days for frying potato slices. If the maximum TPC value for frying oil is accepted to be 24%, then the TPC-based stability sequence of these oils will be as follows: ROSO > RLSO > ROO.
Comparison of frying oil samples showed a gradual increase in acid value over the frying time. There was a significant decrease in iodine value with frying time. It was seen an increase in peroxide value up to the 3th day of frying, but then decreased. During frying, the free fatty acid content progressively increased. Refined oleic sunflower oil and refined olive oil had better quality than the refined linoleic sunflower oil owing to the fatty acid composition and chemical structure. A detailed research was needed for physicochemical properties of these oils with a view to prove the movement in thermal process (Tables 1, 2, 3, 4 and 5). According to the obtained data from different measured parameters, frying conditions applied in our study gave rise to a high amount of deterioration of RLSO. ROSO and ROO have higher stability for frying and its chemical properties were better than RLSO. FFA, which is an important indicator for rancidity of foods, is formed by triglycerides hydrolysis and can also be induced by reaction of interaction of oil and moisture. The frying process causes vigorous humidification leading to advanced deterioration of the fried oil. During the frying process, a very remarkable decrease (P < 0.05) in IV was observed for all samples. This reduction for RLSO was more pronounced than ROSO and ROO because of the unsaturation rate of fatty acids of the triacylglycerol. PV is an indicator for primary lipid oxidation, which is a measurement of peroxides, occurring in fats and oils during oxidation (Abdulkarim et al. 2007). According to our results, a notable rising (P < 0) in PV was seen during frying process until reaching a maximum point, after which was observed a decrease. This decrease in PV might be explained by the occurring reactions of degradation to other compounds. Furthermore, these results have high similarity with previous researches (Warner and Mounts 1993). However, during the heating process, PVs of the analyzed oils were higher (10 mEq O2 /kg) than the accepted by the Guidelines of the Turkish Food Codex for fats and oils. On the other hand, the AnV is more reliable and meaningful test than the PV because AnV evaluates the secondary oxidation products, which are more stable during the frying process (Abbas Ali et al. 2013). K232 and K270 values are expressions of the UV spectrophotometric absorption at 232 and 270 nm. These absorptions indicate about the CD and CT values, respectively. The K232 value, which is indication for CD formation, increases strongly in fried oil.
Finally, thermal procedure leads to remarkable changes in FA composition of sunflower oil. The linoleic acid content was reduced in frying process and, as shown in the previous studies; this decrease explained the rise of palmitic and stearic acids (Sebedio et al. 1990; Warner and Mounts 1993). The sensitivity of the double bounds of linoleic acid, to the simultaneous action of heat and oxygen, would be in charge of for this detecting.
Conclusion
The results of this research indicated that the frying performance of refined sunflower oil, compared to the refined olive oil, was influenced mainly by the content of linoleic acid. In addition, our studies have shown that sunflower oil with high oleic content demonstrate higher frying stability than common oil during deep frying process.
References
Abdulkarim SM, Long K, Lai OM et al (2007) Frying quality and stability of high oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem 105:1382–1389
Abbas Ali M, Bamalli ZM, Nouruddeena I et al (2014) Effect of palm olein addition on the quality characteristics of sunflower oil during deep fat frying. Acta Alim 43(2):288–296
Abbas Ali M, Najmaldien AHA, Latip RA et al (2013) Effect of Heating at Frying Temperature on The Quality Characteristics of Regular and High-Oleic Acid Sunflower Oils. Acta Sci Pol Technol Aliment 12(2):159–167
Aladedunye F, Przybylski R, Niehaus K et al (2014) Phenolic extracts from Crataegus × mordenensis and Prunus virginiana. Composition, antioxidant activity and performance in sunflower oil. LWT 59(1):308–319
Ali A, Najmaldien AH, Latip RA et al (2013) Effect of heating at frying temperature on the quality characteristics of regular and high-oleic acid sunflower oils. Acta Sci Pol Technol Aliment 12(2):159–167
Alireza S, Tan CP, Hamed M et al (2010) Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Food Res Int 17:295–302
Al-Kahtani H (1991) Survey of quality of used frying oils from restaurants. J Am Oil Chem Soc 68:857–862
AOCS (1997) Official methods and recommended practices of the American Oil Chemists Society. AOCS Press, Champaign, Ca-5a-40.
AOCS (1998) Official methods and recommended practices of the American Oil Chemists Society. AOCS Press, Champaign 1998, Cd 18–90.
AOCS (2003) Official methods and recommended practices of the American Oil Chemists Society. AOCS Press, Champaign, Cd 8-53.
AOCS (2005) Official methods and recommended practices of the American Oil Chemists Society. AOCS Press, Champaign, Cd-1d-92.
AOCS (2006) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS Press, Champaign, Ch5-91.
Aydınkaptan E, Mazı IM (2017) Monitoring the physicochemical features of sunflower oil and French fries during repeated microwave frying and deep-fat frying. Grasas Aceites 68(3):202
Brühl L (2014) Fatty acid alterations in oils and fats during heating and frying. Euro Fed Lipid 6:707–715
Chebet J, Kinyanjui T, Cheplogoi PK (2016) Impact of frying on iodine value of vegetable oils before and after deep frying in different types of food in Kenya. J Sci Innov Res 5:193–196
Choe E, Min DB (2005) Chemistry and reactions of reactive oxygen species in foods. J Food Sci 70(9):R142–159
Crosa MJ, Skerl V, Cadenazzi M, Olazabal L, Silva R, Suburu G, Torres M (2014) Changes produced in oils during vacuum and traditional frying of potato chips. Food Chem 146:603–607
De Abreu DAP, Losada PP, Maroto J et al (2010) Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Res Int 43:1277–1282
Freja N, Mozzon M, Lercker G (1999) Effect of free fatty acids on the oxidative stability of vegetable oil. J Am Oil Chem Soc 76:325–329
Gharby S, Harhar H, Matthäus B et al (2016) The chemical parameters and oxidative resistance to heat treatmentof refined and extra virgin Moroccan Picholine olive oil. J Taibah Univ Sci 10:100–106
Gertz C, Klosternmann S, Kochhar SP (2000) Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Tech 102:543–551
Kaya Y, Kaya V, Şahin I et al (2008) The future potential of oleic type sunflower oil in Turkey. In: Proceedings of the 17th International Sunflower Conference, Spain, pp. 791–795
Marquard R (1987) Qualitatsanalytik im Dienste der Ölfflanzenzüctung. Fat Sci Technol 89:95–99.
Min DB, Boff JF (2001) Lipid oxidation of edible oil. In: Akoh C, Min DB (eds) Food lipids. Marcel Dekker, New York, pp 335–363
Normand L, Eskin NAM, Przybylski R (2006) Comparison of the frying stability of regular and high-oleic acid sunfl ower oils. J Am Oil Chem Soc 83:331–334
Paunović DM, Demin MA, Petrović TS et al (2020) Quality parameters of sunflower oil and palm olein during multiple frying. J Agric Sci 65(1):61–68
Rossell JB (2001) Frying: Improving Quality. Woodhead Publishing, Cambridge. https://doi.org/10.1533/9781855736429
Sebedio JL, Bonpunt A, Grandgirard A et al (1990) Deep fat frying of frozen pre-fried french fries: influence of the amount of linolenic acid in the frying medium. J Agric Food Chem 38:1862–1867
Shahidi F, Wanasundara UN (2002) Food lipids: chemistry, nutrition, and biotechnology. In: Akoh CC, Min DB (eds) Methods for measuring oxidative rancidity in fats and oils. Marcel Dekker, New York, pp 465–482
Smith SA, King RE, Min DB (2017) Oxidative and thermal stabilities of genetically modified high oleic sunflower oil. Food Chem 102:1208–1213
Tsaknis J, Lalas S (2002) Stability during frying of Moringa oleifera seed oil variety ‘‘Periyakulam 1”. J Food Compos Anal 15:79–101
Tynek M, Hazuka Z, Pawlowicz R et al (2001) Changes in the frying medium during deep frying of food rich in proteins and carbohydrates. J Food Lipids 8:251–261
Velasco J, Marmesat S, Dobarganes MC (2009) Chemistry of frying. In: Sahin S, Sumnu SG (eds) Advances in deep-fat frying of foods, 1st edn. CRC Press, Boca Raton
Warner K, Mounts TL (1993) Frying stability of soybean and canola oils with modified fatty acid compositions. J Am Oil Chem Soc 70:983–988
Warner K, Orr P, Parrott L, Glynn M (1994) Effects of frying oil composition on potato chip stability. J Am Oil Chem Soc 71:1117–1121
Xin-Qing X, Viet HT, Martin P et al (1999) Chemical and physical analyses and sensory evaluation of six deep-frying oils. J Am Oil Chem Soc 76:1091–1099
Yaghmur A, Aserin A, Mizrahi Y et al (2001) Evaluationof argan oil for deep fat frying. Lebens Technol 34:124–130
Zhang Q, Saleh ASM, Shen Q (2016) Oil during deep-fat frying with different food types. J Am Oil Chem Soc 93:69–81
Zribi A, Jabeur H, Aladedunye F, Rebai A, Matthäus B, Bouaziz M (2014) Monitoring of quality and stability characteristics and fatty acid compositions of refined olive and seed oils during repeated pan- and deep frying using GC, FT-NIRS, and Chemometrics. J Agric Food Chem 62(42):10357–10367
Acknowledgements
The authors give special thanks to Kırklareli University for supporting by the grant project (KLU-BAP-083).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aşkın, B., Kaya, Y. Effect of deep frying process on the quality of the refined oleic/linoleic sunflower seed oil and olive oil. J Food Sci Technol 57, 4716–4725 (2020). https://doi.org/10.1007/s13197-020-04655-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04655-4