Abstract
Blood fruit (Haematocarpus validus) has important bioactive compounds, antioxidant properties and some essential minerals, which plays an essential part in human nutrition and traditional medicine for treating arthritis, jaundice, hypertension, cancer, etc. This work was conducted to optimize the parameters of spray drying process for production of blood fruit juice powder and its quality was compared with freeze dried and tray dried powder. It was observed that powder produced by spray drying techniques resulted in higher yield, solubility and better retention of resveratrol content and was considered to be of superior quality, having a higher degree of reconstitution ratio as compared to powders produced by freeze and tray drying techniques. High performance liquid chromatography study of blood fruit powder showed the presence of resveratrol and other phenolic compounds. Scanning electron microscope was used to study the surface morphology and it revealed that spray-dried powder has uniformity in shape and size as compared to freeze dried and tray dried powder. The present investigation indicated that spray drying results in better, superior quality powders that are easier for packaging, transportation, having better shelf life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood fruit (Haematocarpus validus), locally known as Khoonphal/Tépattang in the state of Meghalaya which belongs to the family of Menispermaceae and is one of the underutilized, promising edible fruits from Garo Hills, Meghalaya, India (Sasikumar et al. 2017, 2019a). Blood fruit has important bioactive compounds, antioxidant properties, and some essential minerals which play a significant role in traditional medicine for treating arthritis, jaundice, hypertension, cancer, etc. (Sasikumar et al. 2019b; Rahim et al. 2015; Raju et al. 2018). The processing of blood fruit juice/pulp by spray drying technique to produce powder is classified among the best processes to increase value and shelf life of the product (Raju and Deka 2018; Sasikumar et al. 2019c).
Blood fruit powder could be prepared by using different drying techniques. In comparison to various drying techniques, spray drying is considered to be the superior one for preserving the quality of the product, increased yield and with better shelf life (Bazaria and Kumar 2018). For spray drying technique various carrier materials were used vis maltodextrin, gum acacia, modified starch, gum arabic, whey protein concentrate, chitosan etc., to produce powders as reported by various authors, such as for bayberry fruit powder by Fang and Bhandari (2012) and gac juice powder as reported by Kha et al. (2010). The spray drying process results in better, superior quality powders that is easier for packaging, transportation and with increased shelf life (Bazaria and Kumar 2018).
Therefore, present work was aimed to optimize the parameters of spray drying process for production of blood fruit juice powder (BFJP) and its quality was compared with freeze dried and tray dried powder.
Materials and methods
Raw material collection and chemicals
Blood fruits were collected from local market of West Garo Hills, Meghalaya. The GPS coordinates of the location are 25.467° North, 91.3662° East, with an altitude of 349 m above sea level, along with Accession No: GUBH18508. Chemicals and solvents used were of laboratory/analytical grade (LR/AR grade).
Blood fruit juice (BFJ) preparation
Ripened blood fruits were sorted, washed thoroughly with 1% KMnO4 solution to remove latex portion and rinsed in running water. Good quality fruits i.e. uniform size, absence of defects and visual wounds were selected for all the experiments. The fruit juices were extracted with the help of a juicer (Model: J398, Philips, India), filtered through a muslin cloth and the filtrate was kept at − 20 °C for 10 h. The frozen juices were thawed at room temperature followed by ultrasonication treatment (20 kHz, 80% amplitude for 15 min and cut-off time 3 s was fixed) to enhance phytonutrients quality of juice (Hasan et al. 2014a) (Table 1). The sonicated juices were filtered through double layer muslin cloth which had a total solid content of 14.0 ± 0.5 g/100 g (w/w) and further juice concentrated up to 20% total soluble solids (0Brix) followed by mixing with β-lactoglobulin in 5:1 (juice: β-lactoglobulin) ratio and pre-heated mixture at 45 °C until complete dissolution and kept at ± 4 °C for further analysis.
Physicochemical properties of blood fruit juice
pH, TSS, titratable acidity and vitamin C
The pH (981.2), TSS (932.12), and titratable acidity (TA) (942.15) of the BFJ were determined as per the methods of AOAC (2010). The ascorbic acid content of the BFJ samples was determined by Dürüst et al. (1997) with minor modification. The prepared juice was diluted in the ratio of 1:5 with 0.4% oxalic acid. The aliquot solution of 10 mL was thoroughly mixed with 10 mL of acetate buffer and added to 80 mL of 2, 6-dichloroindophenol sodium salt hydrate and mixture was observed at 520 nm. The standard ascorbic acid was used and results were expressed as mg ascorbic acid per 100 mL sample. The color properties of BFJ were measured by Hunter Color Lab (Model-4000; UltraScan, USA). The Hunter color values L*(whiteness to brightness), a* (redness to greenness), and b* (yellowness to blueness) of the juice samples were measured.
Determination of phytochemical composition of blood fruit juice
TPC, TFC, TAC and DPPH
The total phenolic content (TPC) was determined Folin Ciocalteu method described by Singleton et al. (1999) with minor modification. The extracted each sample (50 µL) was incubated with Folin Ciocalteu reagent (125 µL/5 min) and added 16% of Na2CO3 (125 µL) to mixture and volume made to 1 mL with distilled water. The sample proceed to measure its absorbance at 760 nm result was designated as mg of gallic acid equivalents (GAE)/100 mL.
Total flavonoids content (TFC) was determined by Mihailović et al. (2018) with slight modified procedure. The extracted sample (50 µL) was mixed 30 µl solution of sodium nitrite with 5% of concentration and adding 60 µl of AlCl3 (10%). Sodium hydroxide solution (200 µL) of a 1 M was added into mixed solution adjusted volume to 1 mL and allowed for 5 min followed by vortexed. The mixture subjected measure its absorbance at 760 nm and result was expressed as mg of Rutin equivalents (RE) per 100 mL.
Anthocyanins were determined according to Chorfa et al. (2016) with minor modifications. The juice sample (1 mL) of each treatment was mixed solution, which contained (HCl–MeOH–H2O in mixer ratio 1:3:16) and allowed for 72 h at 4 °C. The absorbance was measured at two different wave length 653 and 530 nm. Results were expressed as milli grams of cyanidin-3-glucoside equivalent (mg C3GE/100 mL) of juice sample.
Antioxidant activity was measured by methods of diphenyl-1-picrylhydrazyl (DPPH) technique with minor modification (Ozgen et al. 2006). The sample (25 µL) was added into 1 mL of a methanol solution of DPPH (0.04 mM) and incubated for 75 min, followed measured at 517 nm absorbance and the result was expressed as µM of trolox equivalent (TE) per 100 mL.
Emulsion preparation
Takeiti et al. (2010) suggested that the maltodextrin (MD) with 20 DE (Dextrose equivalent) was used as a wall material for fruit beverage powder. The MD was rehydrated separately for overnight and then was heated at 50 ± 1 °C for complete dissolution. Blends of wall material were prepared by mixing directly the components in different concentrations (w/w) until reach to 20% total soluble solid before spray drying and labelled as BFE-1 (5% MD), BFE-2 (8% MD) and BFE-3 (10% MD) respectively, varying maltodextrin concentration from 5 to 10%.
Process parameter optimization for production of blood fruit powder by spray drying (SD) technique
Production of blood fruit powders were carried out by laboratory scale spray dryer (Model: P111, Technosearch, India). An experimental design approach was assessed by taking consideration of the equipment parameters, three of the factors were selected that can affect the production of powders. For determination of optimal process temperature (°C), MD concentration (%) and flow rate (mL/h), Box Behnken design (BBD) was applied for the production of powders by spray drying technique. The temperature (X1), MD concentration (X2) and flow rate (X3) were the chosen independent variables for the study to optimize: (a) powder yield (%), (b) solubility (%), (c) hygroscopicity (g/100 g) and (d) resveratrol content (µg/g). The pressure, air flow rate and peristaltic pump rate of process were maintained at 0.75 MPa, 24 m3/h and 8 mL/min for all the runs carried out. Seventeen experiments (five central points) were generated according to BBD with different levels of temperature (150–170 °C), maltodextrin concentration (5–10%) and flow rate (300–500 mL/h). All the parameters ranges were settled based on preliminary studies (Tonon et al. 2008; Muzaffar and Kumar 2015; Khalilian Movahhed and Mohebbi 2016). Design expert (V-7.0.1.0) was used to produce a response surface plot and its statistical analysis. To analyse the fitted model’s quality, analysis of variance (ANOVA) was conducted. The generalized equation of the polynomial model is given as:
where \(\beta_{0}\), \(\beta_{1 }\), \(\beta_{2 }\) … \(\beta_{33}\) denote the regression coefficients, with \(\beta_{0}\) as the constant term; \(\beta_{1 }\), \(\beta_{2 }\), \(\beta_{3 }\) shows linear effects; \(\beta_{11}\), \(\beta_{22}\), \(\beta_{33}\) demonstrates quadratic effects; \(\beta_{12}\), \(\beta_{13}\), \(\beta_{23}\) represent combined effects and X1 represents inlet temperature, X2 represents MD concentration and X3 represents flow rate. A confidence level of 95% was set for total error criteria, basis of which the test of statistical difference was evaluated. Lastly collected spray dried powders were kept in low density polyethylene pouches and stored at refrigeration temperature.
Freeze drying (FD)
Blood fruit juice was prepared as described in “Blood fruit juice (BFJ) preparation” section. Prepared sample was then kept in a deep freezer (Model-410 Upright, Effendorf, Germany) at − 40 °C for 8 h. After then frozen BFJ was processed in a laboratory scale freeze dryer (Model: Mini-Alpha LD Plus, Martine Christ, Germany) under vacuum at − 48 °C for 12 h. The freeze dried powder (FDP) were collected and stored in an airtight glass bottle for further analysis.
Tray drying (TD)
The sample slurry was prepared as described in “Blood fruit juice (BFJ) preparation” section and subjected to a tray dryer (Model-20, Kencor System Pvt. Ltd, India). Each tray size has 3 mm uniform thickness, in which slurry sample was poured and placed into dryer maintaining temperature at 65 °C for 62 h with 1.5 m/s hot air flow speed. Finally obtained tray dried powder (TDP) were collected and stored in an airtight glass bottle for further analysis.
Yield (%) of blood fruit powder
The blood fruit powder yield was determined by using the following equation:
Hygroscopicity
The hygroscopicity of BFJP was determined by making slight alteration as per the method suggested by Cai and Corke (2000). BFJP (1 g) was weighed in a petri plate and then was kept in a desiccator at room temperature with a saturated sodium chloride solution for a week. After 7 days powders were taken out, weighed and expressed as g of moisture absorbed/100 g dry matter.
Solubility
To evaluate blood fruit powder solubility method suggested by Santhalakshmy et al. (2015) was followed. BFJP (1 g) was mixed with 100 mL distilled water and then for 5 min it was centrifuged at 3000 rpm. After then solution was allowed to settle completely. Later on, 25 mL of supernatant was weighed in as petri plate and subjected to an oven dryer at 105 °C for 5 h. The blood fruit powder solubility (%) was calculated as the weight difference.
Phenolic acids and resveratrol compounds by HPLC detection
For determination of phenolic compounds, HPLC (Waters, e-2695, Germany) was used which is equipped with C18 column and PDA (Photo diode array) detector following the method described by Hasan et al. (2014b) with minor modification. In the HPLC system gradient method was followed with methanol and phosphoric acid in the ratio of 20:80 as solvent A and 80:20 as solvent B respectively. The wavelength range (220–600 nm), column temperature (40 °C), flow rate (1 mL/min) and the injection volume (20 µL) were fixed in an instrument method set. The Empower V-3.0 was used to analyze the data. Regression curve analysis was used to determine the amount of resveratrol content of BFJP, as calculated from the resveratrol peak regions by analyzing the different standard concentrations (Resveratrol, Sigma Aldrich).
Morphology study of BFJP
The microstructure and surface morphology analysis of BFJP was performed as prescribed by Pasukamonset et al. (2011) with minor modification. Scanning electron microscope (SEM) (Model: Sigma 300, Zeiss, USA) was operated at 15 kV and electron beam current of 100 pA and the powder sample were fixed directly on door-metallic specimens (stubs) of 12 mm diameter and then subjected to metallization (sputtering) with a thin layer of gold/palladium in a Sputter Coater SC7620 polaron (VG Microtech, England) at a coverage rate of 0.51 Å/s for 180 s, with a current of 3.5 mA, 1 V and 2 102 Pa. After metallization, the samples were observed with magnifications of 4000, 5000 and 10,000. Image acquisition was performed by the LEO software, version 3.01 was used to examine the morphology and surface appearance of BFJP.
Statistical analysis
Experimental data obtained was analyzed with SPSS Statistics 21.0 software. One way ANOVA followed by DMRT (Duncan’s multiple range test) was used for significance test, considering p ≤ 0.05 statistically significant. Analyzed data were represented as mean ± SD obtained in three replicates (n = 3) from different experiments.
Results and discussion
Physicochemical characteristics of BFJ
The quality attributes of fresh and treated BFJ were determined and shown in Table 1. Blood fruit juice subjected to ultrasonication treatment resulted in increase the phytocompounds and antioxidant activity as compared to fresh juice. The reason behind the increased amount of these phytocompounds is the ease in diffusion of phenolic compounds resulting in rupture of cell wall induced through cavitational pressure (Sasikumar et al. 2019a; Aadil et al. 2013; Raju et al. 2018). Because of the sonocapillarity and sonoporation, permeability of cell membranes is altered and improves the liquid penetration in the sample matrix and thus eases the release of phytocompounds (Medina-Torres et al. 2017).
Process parameter optimization for production of blood fruit powder by spray drying technique
Table 2 shows the experimental data obtained for powder yield (%), solubility (%), hygroscopicity (g/100 g) and resveratrol content (µg/g) for each standard run and estimated regression coefficients for the polynomial model is presented in Table 3.
Yield of BFJP (%)
Figure 1a demonstrates the impact of processing temperature, MD concentration and flow rate on the spray drying process yield. inlet process temperature, MD concentration along with combination showed significant effect (p ≤ 0.05) on powder yield, while the influence of flow rate on the process yield was found to be not significant (p ≥ 0.05). During experimental analysis as noticed, yield of final product initially increased as the temperature rises, which is ascribed to better heat and mass transfer, whereas a further increase in temperature, induced surface of particle to change their state that is heating past their transition temperature (Tg) and therefore becomes sticky in nature and results in lower product yield (Cai and Corke 2000; Papadakis et al. 2006).
The product yield of finished powder initially increases with increase in the maltodextrin concentration and it decreases with further increase in the level of maltodextrin. As reported by Tonon et al. (2008) with an increase in the level of maltodextrin results in reduced process yield which is because of the increased viscosity of the emulsion mixture. Similar result was reported by Saikia et al. (2015) in Khasi mandarin orange, watermelon, carambola and pineapple powder.
Solubility (%)
One of the important characteristic property of powder is solubility which examines the behaviour and response of product in the aqueous phase, so as to be valuable, functional and productive, good solubility properties of food powders are important. Figure 1b demonstrates the impact of processing temperature, MD concentration and flow rate on the solubility of BFJP. Inlet process temperature, MD concentration and their interaction revealed effect on the solubility as significant (p ≤ 0.05), while flow rate did not have any impact on powder solubility (p ≥ 0.05).
The solubility of BFJP increased, as the temperature rises. The probable reason could be as a consequence of processing temperature and because of the residual moisture of finished powder, so as lower will be the finished powder moisture content, less time will be required for dissolution of powder and which indicates better powder solubility (Goula and Adamopoulos 2005). Similarly, when concentration of maltodextrin was increased, it influenced the solubility of blood fruit powder. This alteration could be related to better solubility properties of maltodextrin powder (Cano-Chauca et al. 2005). In the investigation carried out for sweet potato powder (Grabowski et al. 2006) reported that when the percentage of MD increases it positively influenced the water solubility properties of sweet potato powder.
Hygroscopicity (g/100 g)
Figure 1c demonstrates the impact of processing temperature, MD concentration and flow rate on the hygroscopicity of spray dried powder. Inlet process temperature and MD concentration revealed effect on the hygroscopicity as significant (p ≤ 0.05), while flow rate did not have any impact on the hygroscopicity (p ≥ 0.05). It is apparent from the figure that processing temperature influenced hygroscopisity of blood fruit powder. The spray-dried blood fruit powders which were processed at 160 °C and 170 °C (that is at a higher temperature) dominated hygroscopicity and that perhaps cognate to the moisture content (%) of finished blood fruit powder. This is associated with the concentration of water gradient within the ambient air and the product, notably higher for the powder with the least moisture content. Results obtained were in similar to those published by Goula et al. (2004) for tomato pulp powder by spray drying technique.
Concentration level of maltodextrin varied from 5 to 10% and it induced the hygroscopicity of the blood fruit powders. During experimental analysis as observed, hygroscopicity of finished powder was found to be lowest with the highest concentration of maltodextrin and results were quite identical to those quoted by Rodríguez-Hernández et al. (2005) and Cai and Corke (2000) in their investigation carried out for powder prepared from cactus pear juice and for betacyanin pigments powder by spray drying technique respectively.
Resveratrol content (µg/g)
Figure 1d demonstrates the impact of processing temperature, MD concentration and flow rate on the resveratrol content of BFJP. There was significant impact (p ≤ 0.05) of processing temperature and MD concentration on the resveratrol content, while flow rate did not have any impact on the resveratrol content (p ≥ 0.05), but there was significant (p ≤ 0.05) impact of maltodextrin along with feed flow rate on the resveratrol content.
As the processing temperature increased, the resveratrol content decreased and it is directly related to degradation of polyphenols by heat and oxidation when exposed to higher temperature (Rawson et al. 2011). Addition of β-lactoglobulin resulted in retaining the resveratrol content significantly when exposed to high temperature. According to Guo and Jauregi (2018) rise in phenolic content is because of the inclusion of β-lactoglobulin, they suggested its protective property towards degradation of polyphenols specially resveratrol by heat. While quantity of resveratrol increases with increase in MD percentage and this is because MD as a drying agent protected polyphenols from heat and oxidation by forming a active layer and results were in agreement to those published by Bazaria and Kumar (2018) for beetroot juice powder by spray-drying technique.
Optimal solution
For optimized condition, desired goals were allocated for all individual parameters to get the optimum numerical values for the selected responses. All levels of inlet temperature, MD concentration and flow rate were set within their limit and responses (yield, solubility and resveratrol content) were kept maximum except hygroscopicity which was kept minimum. By computational analysis the numerical solution obtained for optimized condition is shown in Table 4.
Quality comparison of spray-dried blood fruit powder with other drying techniques
Physicochemical, bioactive and microstructure analysis of spray dried blood fruit powder were compared with BFJP produced by FD and TD techniques respectively. Changes observed due to different drying techniques in yield, hygroscopicity, solubility and resveratrol content of blood fruit powder is displayed in Table 5.
Maximum yield of blood fruit powder was obtained through SD technique in comparison to FD and TD respectively. This could be as a result of better heat and mass transfer achieved during SD process (Cai and Corke 2000). The hygroscopicity of blood fruit powder produced by spray drying technique was found to be least hygroscopic in nature in contrast to BFJP produced by FD and TD technique. This might be as a result of variation in particle sizes and pulp sugar content, which can significantly induce powder hygroscopicity (Schuck et al. 2012). Another possible reason could be inclusion of MD with blood fruit juice which resulted in lower hygroscopicity and results were in agreement to those published by Rodríguez-Hernández et al. (2005) and Tonon et al. (2008). In case of solubility of blood fruit powder, spray dried powder dominated the solubility with 96.62% as compared to freeze dried (89.87%) and tray dried (76.23%) powder respectively. The high solubility of BFJP could be ascribed to the inclusion of MD and was in agreement to those published by Cano-Chauca et al. (2005). According to Cai and Corke (2000) maltodextrin as a coating material resulted in increased solubility for spray dried betacyanins. The possible reason for the lower solubilities of freeze dried and tray dried powder could be attributed to the cell structure of blood fruit, not getting distorted as a result minute quantity of solids have been dissolved to form part of the supernatant (Caparino et al. 2012).
BFJP produced by SD technique revealed maximum resveratrol content in comparison to freeze dried and tray dried powder. The better retention of resveratrol could be attributed to the addition of β-lactoglobulin to blood fruit juice which resulted in retaining the resveratrol content significantly (Guo and Jauregi 2018). Another possible reason could be inclusion of MD with blood fruit juice, as addition of MD resulted in increase in the resveratrol content, this is because MD as a drying agent protected polyphenols from heat and oxidation by forming an active layer and results were in agreement to those published by Bazaria and Kumar (2018).
Furthermore, pre-treatment of blood fruit juice subjected to ultrasonication influenced the resveratrol content. Because of the sonocapillarity and sonoporation, permeability of cell membranes is altered and improves the liquid penetration in the sample matrix and thus eases the release of phytocompounds (Medina-Torres et al. 2017). The lower resveratrol content of FDP and TDP could be ascribed to the duration of drying methods which is longer for tray drying and freeze drying in comparison to spray drying, but retention was higher for powder produced by FD technique in contrast to TD which is attributed to the lower processing temperature at which powder was produced (Wilkowska et al. 2016). Also better retention could be ascribed to the unbinding of phenolic compounds from the matrix during FD process as it transposes the tissue structure, resulting in easier extraction of phenolic compounds (Pérez-Gregorio et al. 2011).
Effect of phenolic acids and resveratrol content
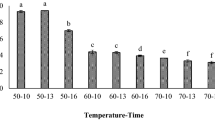
Table 6 shows the detected phenolic acids along with their retention time. Figure 2 shows various phenolic acids detected for blood fruit powder produced by FD, SD and TD technique. The lessening or destruction of phenolic acids was observed in BFJP produced by FD and TD, whereas emergence of new phenolic acids was observed in blood fruit powder produced by spray drying technique. BFJP produced by SD technique revealed maximum resveratrol content in comparison to freeze dried and tray dried powder. The better retention of resveratrol could be attributed to the addition of β-lactoglobulin to blood fruit juice which resulted in retaining the resveratrol content significantly. Moreover, pre-treatment of blood fruit juice subjected to ultrasonication influenced increase in the resveratrol content. Sonication treatment predominantly induces leaching and hydrolysis of both bound and free phenolic acids. The emergence and detection of new phenolic acids possibly be due to the bound phenolics which get released during processing. As phenolic acids composed of both bound and free phenolic acids, in which bound phenolic acids stick to several structural protein and carbohydrate by having ester linkage with lignin or with carboxylic groups by means of their OH groups in acetal bonds or in the aromatic ring (Bhanja et al. 2009).
Microstructure analysis of blood fruit powder by SEM
The spray dried, freeze dried and tray dried blood fruit powders were analyzed by SEM to study the microstructure and surface morphology. Morphology study revealed various shapes and sizes of blood fruit powder (Fig. 3a–c). SEM images of blood fruit powder produced by spray drying technique revealed that particles are agglutinated and amalgamated with one another and also showed variation in sizes which was in agreement to those published by Saikia et al. (2015). It was observed that, when powder was processed at higher temperature particle size of powder in average was smaller concerning the one which is processed at lower temperature and was in accordance to those published by Cai and Corke (2000). Blood fruit powder produced by tray drying technique revealed that particles have non-uniformity in shapes and sizes and have sharp edges, while powder produced by freeze drying revealed that particles tend to clump together, spherical in sizes. As they were similar to powder particles produced by spray drying technique.
Conclusion
The present investigation was conducted to optimize the parameters of spray drying process for production of blood fruit juice powder (BFJP) and its quality was compared with freeze dried and tray dried powder. It was observed that powder produced by spray drying techniques resulted in higher yield, solubility and better retention of resveratrol content and was considered to be of superior quality, having a higher degree of reconstitution ratio in contrast to powders produced by freeze and tray drying techniques. During the processing of the product by spray drying techniques various essential factors in particular drying temperature, compressing pressure, nozzle diameter, flow rate, and carrier agent concentration needs to be considered for better finished product. The present investigation indicated that spray drying results in better, superior quality powders that are easier for packaging, transportation, with increased shelf life.
References
Aadil RM, Zeng XA, Han Z, Sun DW (2013) Effects of ultrasound treatments on quality of grapefruit juice. Food Chem 141:3201–3206. https://doi.org/10.1016/j.foodchem.2013.06.008
AOAC (2010) Official methods of analysis of AOAC international, 20th edn. https://doi.org/10.1007/978-3-642-31241-0
Bazaria B, Kumar P (2018) Optimization of spray drying parameters for beetroot juice powder using response surface methodology (RSM). J Saudi Soc Agric Sci 17:408–415. https://doi.org/10.1016/j.jssas.2016.09.007
Bhanja T, Kumari A, Banerjee R (2009) Enrichment of phenolics and free radical scavenging property of wheat Koji prepared with two filamentous fungi. Bioresour Technol 100:2861–2866. https://doi.org/10.1016/j.biortech.2008.12.055
Cai YZ, Corke H (2000) Production and properties of spray-dried. J Food Sci 65:1248–1252. https://doi.org/10.1111/j.1365-2621.2000.tb10273.x
Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J (2005) Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Technol 6:420–428. https://doi.org/10.1016/j.ifset.2005.05.003
Caparino OA, Tang J, Nindo CI, Sablani SS, Powers JR, Fellman JK (2012) Effect of drying methods on the physical properties and microstructures of mango (Philippine “Carabao” var.) powder. J Food Eng 111:135–148. https://doi.org/10.1016/j.jfoodeng.2012.01.010
Chorfa N, Savard S, Belkacemi K (2016) An efficient method for high-purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem 197:1226–1234. https://doi.org/10.1016/j.foodchem.2015.11.076
Dürüst N, Sümengen D, Dürüst Y (1997) Ascorbic acid and element contents of foods of Trabzon (Turkey). J Agri Food Chem. 45(6):2085–2087. https://doi.org/10.1021/jf9606159
Fang Z, Bhandari B (2012) Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res Int 48:478–483. https://doi.org/10.1016/j.foodres.2012.05.025
Goula AM, Adamopoulos KG (2005) Spray drying of tomato pulp in dehumidified air: II. The effect on powder properties. J Food Eng 66:35–42. https://doi.org/10.1016/j.jfoodeng.2004.02.031
Goula AM, Adamopoulos KG, Kazakis NA (2004) Influence of spray drying conditions on tomato powder properties. Dry Technol 22:1129–1151. https://doi.org/10.1081/DRT-120038584
Grabowski JA, Truong VD, Daubert CR (2006) Spray-drying of amylase hydrolyzed sweetpotato puree and physicochemical properties of powder. J Food Sci. https://doi.org/10.1111/j.1750-3841.2006.00036.x
Guo Y, Jauregi P (2018) Protective effect of β-lactoglobulin against heat induced loss of antioxidant activity of resveratrol. Food Chem 266:101–109. https://doi.org/10.1016/j.foodchem.2018.05.108
Hasan MM, Yun HK, Kwak EJ, Baek KH (2014a) Preparation of resveratrol-enriched grape juice from ultrasonication treated grape fruits. Ultrason Sonochem 21:729–734. https://doi.org/10.1016/j.ultsonch.2013.08.008
Hasan MM, Yun HK, Kwak EJ, Baek KH (2014b) Preparation of resveratrol-enriched grape juice from ultrasonication treated grape fruits. Ultrason Sonochem 21(2):729–734. https://doi.org/10.1016/j.ultsonch.2013.08.008
Kha TC, Nguyen MH, Roach PD (2010) Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J Food Eng 98:385–392. https://doi.org/10.1016/j.jfoodeng.2010.01.016
Khalilian Movahhed M, Mohebbi M (2016) Spray drying and process optimization of carrot-celery juice. J Food Process Preserv 40:212–225. https://doi.org/10.1111/jfpp.12598
Medina-Torres N, Ayora-Talavera T, Espinosa-Andrews H et al (2017) Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 7:47. https://doi.org/10.3390/agronomy7030047
Mihailović NR, Mihailović VB, Kreft S, Ćirić AR, Joksović LG, Đurđević PT (2018) Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J Food Compos Anal 67:1–9. https://doi.org/10.1016/j.jfca.2017.11.007
Muzaffar K, Kumar P (2015) Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol 279:179–184. https://doi.org/10.1016/j.powtec.2015.04.010
Ozgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR (2006) Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem 54(4):1151–1157. https://doi.org/10.1021/jf051960d
Papadakis SE, Gardeli C, Tzia C (2006) Spray drying of raisin juice concentrate. Dry Technol 24:173–180. https://doi.org/10.1080/07373930600559019
Pasukamonset P, Kwon O, Adisakwattana S (2011) Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocolloids 61:772–779. https://doi.org/10.1016/j.foodhyd.2016.06.039
Pérez-Gregorio MR, Regueiro J, González-Barreiro C et al (2011) Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Control 22:1108–1113. https://doi.org/10.1016/j.foodcont.2011.01.006
Rahim MA, Khatun MJM, Rahman MM, Anwar MM (2015) Study on the morphology and nutritional status of Roktogota (Haematocarpus validus)—an important medicinal fruit plant of hilly areas of Bangladesh. Int J Minor Fruits, Med Aromat Plants 1:11–19
Raju S, Deka SC (2018) Influence of thermosonication treatments on bioactive compounds and sensory quality of fruit (Haematocarpus validus) juice. J Food Process Preserv 42(8):e13701. https://doi.org/10.1111/jfpp.13701
Rawson A, Tiwari BK, Patras A et al (2011) Effect of thermosonication on bioactive compounds in watermelon juice. Food Res Int 44:1168–1173. https://doi.org/10.1016/j.foodres.2010.07.005
Rodríguez-Hernández GR, González-García R, Grajales-Lagunes A et al (2005) Spray-drying of cactus pear juice (Opuntia streptacantha): effect on the physicochemical properties of powder and reconstituted product. Dry Technol 23:955–973. https://doi.org/10.1080/DRT-200054251
Saikia S, Mahnot NK, Mahanta CL (2015) Effect of spray drying of four fruit juices on physicochemical, phytochemical and antioxidant properties. J Food Process Preserv 39:1656–1664. https://doi.org/10.1111/jfpp.12395
Santhalakshmy S, Don Bosco SJ, Francis S, Sabeena M (2015) Effect of inlet temperature on physicochemical properties of spray-dried jamun fruit juice powder. Powder Technol 274:37–43. https://doi.org/10.1016/j.powtec.2015.01.016
Sasikumar R, Vivek K, Chakaravarthy S, Deka SC (2017) Effect of post-harvest quality parameters on ultra-sonication treatment of khoonphal (Haematocarpus validus) of Meghalaya, North-East India. J Food Process Technol 8:1–6. https://doi.org/10.4172/2157-7110.1000668
Sasikumar R, Chutia H, Deka SC (2019a) Thermosonication assisted extraction of blood fruit (Haematocarpus validus) juice and process optimization through response surface methodology. J Micro Biotech Food Sci 9:228–235. https://doi.org/10.15414/jmbfs.2019.9.2.228-235
Sasikumar R, Pradhan D, Deka SC (2019b) Effects of thermosonication process on inactivation of Escherichia coli and Saccharomyces cerevisiae and its survival kinetics modeling in khoonphal (Haematocarpus validus) juice to extend its shelf life. J Food Process Preserv. https://doi.org/10.1111/jfpp.14220
Sasikumar R, Vivek K, Deka SC (2019c) Sensory evaluation of ultrasound assisted microwave treated fruit (Haematocarpus validus) juice through fuzzy logic approach. Int Food Res J 26:1229–1236
Schuck P, Dolivet A, Jeantet R (2012) Analytical methods for food and dairy powders, 1st edn. Wiley, New York. https://doi.org/10.1002/9781118307397
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzym 299:152–178. https://doi.org/10.1016/S0076-6879(99)
Takeiti CY, Kieckbusch TG, Collares-Queiroz FP (2010) Morphological and physicochemical characterization of commercial maltodextrins with different degrees of dextrose-equivalent. Int J Food Proper 13(2):411–425. https://doi.org/10.1080/10942910802181024
Tonon RV, Brabet C, Hubinger MD (2008) Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J Food Eng 88:411–418. https://doi.org/10.1016/j.jfoodeng.2008.02.029
Wilkowska A, Ambroziak W, Czyzowska A, Adamiec J (2016) Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Polish J Food Nutr Sci 66:11–16. https://doi.org/10.1515/pjfns-2015-0015
Acknowledgements
The authors grateful to DST-SERB, CRG (EMR/2017000202), Ministry of S&T, Government of India for providing financial assistance for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sasikumar, R., Das, M. & Deka, S.C. Process optimization for the production of blood fruit powder by spray drying technique and its quality evaluation. J Food Sci Technol 57, 2269–2282 (2020). https://doi.org/10.1007/s13197-020-04264-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04264-1