Abstract
As a kind of traditional food, gluten is widely studied for its physical and chemical properties after processing, while little attention is paid to the simulation cooking processing, digestion and safety. In this paper, gluten was heated with microwave to study its structural transformations, nutritional efficiency, and food safety under Chinese home cooking (CHC). After microwave treatment, intermolecular and intramolecular cross-linking of gluten were formed to result in more aggregation. The secondary structure of gluten changed significantly as well as the formation of α-helix and β-turn promoted under the high power input. Treated with 1000 W for 5 min, cross-linking between amino acids increased, leading the reduction of total amino acids, in vitro protein digestibility and the increase of high molecular weight peptides, while the proportion of essential amino acids kept the same. In the simulation of CHC, the highest content of 5-hydroxymethyl furfural was observed after adding all condiments under 1000 W for 5 min. In addition, sugar played a major role in Maillard reaction to promote the formation of melanoidin and fructosamine while salt and oil did not significantly affect these two Maillard products. Vinegar inhibited the reaction due to the acidic condition but provided some melanoidin and fructosamine itself.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gluten plays a major role as a Chinese daily protein source for its high quality and low price, which is mainly composed of gliadins and glutenins. As a natural plant source, gluten is rich in various species of amino acids and carbohydrates that meet the daily intake of human body (Delcour et al. 2012). Microwave heating has vast applications in the field of food processing for several decades due to its high heating rates, uniform heating, safe handling, ease of the operation, and low maintenance (Zielinska et al. 2019). Moreover, microwave heating may change the flavor and nutritional quality of food in a lesser extent than conventional heating (Vadivambal and Jayas 2007).
The effect of heating treatment on protein cross-linking has been widely studied (Lamacchia et al. 2016; Lund and Ray 2017; Verbauwhede et al. 2018). Cross-linking of protein is mainly the covalent bonds, including disulfide and isopeptide bonds, formed among the protein molecular side chains or intermolecular bonds. It has been suggested that heating causes the formation of new enzyme resistant cross-linking within the protein molecules, which reduces the digestibility and biological availability of the amino acids (Hurrell and Carpenter 1977). On the other hand, heating on protein may also induce the formation of isopeptide bond of the ε-amino acid residues of lysine with either the carboxyl residues of aspartic acid or glutamic acid (Rombouts et al. 2011). As the result, protein solubility could be reduced through forming disulfide bond and promoting the polymerization of gluten protein when the temperature increases (Singh and MacRitchie 2004). When the temperature increases to 45 °C, surface hydrophobicity of gluten would change as well as the solubility decreases (Stathopoulos et al. 2008). When the temperature increases further above 80 °C, the content of gliadin decreases whereas glutenin increases, suggesting that high temperature contributes to the polymerization of gliadin with glutenin and the involvement of disulfide bond (Verbauwhede et al. 2018). Regardless of the heating temperature, it always affects the utilization efficiency of protein by affecting the formation of disulfide bonds and isopeptide bonds.
Comparing with the conventional heating, microwave gradually take a place in modern families, while it is widely controversial due to its safety on food (Vadivambal and Jayas 2007). In particular, microwave with high temperature can easily affect the digestibility and nutrition of protein food, which may include the variations of secondary structure, hydrophobic protein or cross-linking and even the occurrence of Maillard reaction (Cabanillas and Novak 2019). It is reported that after microwave heating, the gastric protein digestibility and gastrointestinal protein digestibility of rice protein decreased by 10.3% and 16.8%, respectively, which is higher than high-pressure heating but lower than conventional heating (Liu et al. 2019). In addition, protein physical and covalent aggregation can undermine efficacy of digestive enzymes and compromise the release and bioavailability of peptides and amino acids (Morzel et al. 2006). Besides the disulfide bond, hydrophobic force and other factors, Maillard reaction also have a certain impact on the protein nutritional quality. Several researches have been reported to discuss the effect of microwave on different Maillard reaction. Li et al. (2018) used microwave heating on the glucose-ammonium system, and found the more decrease of pH values, lower whiteness and chroma, stronger glycation and more flavor compounds were recorded compared with oil bath heating. Moreover, some amino acid heterogeneous products such as 5-hydroxymethyl furfural (5-HMF), frutosamine and melanin also be produced in Maillard reaction (Kim and Lee 2008; Song et al. 2019; Zhang et al. 2012). Gluten is rich in protein and carbohydrate, but in the traditional Chinese home cooking (CHC) process, the cooking of gluten is often accompanied by various food additives, in which the related chemical changes have not been studied.
In this paper, gluten was treated with different microwave power and time to study the changes in its secondary structure and amino acid composition, thus determine the effect of microwave heating on protein bioavailability. In vitro digestion was investigated after simulating CHC that adding common condiments into gluten. We also focused on the digestibility and formation of 5-HMF, melanoidin, and fructosamine in digestion products to determine the effect of microwave heating on gluten safety and protein nutrition.
Materials and methods
Materials and chemicals
Gluten flour (Madala Co., Ltd., Fengqiu Henan, China) and water were mixed in the ratio of 1:1.3, and the mixture was placed at 30 °C to allow fermentation for 2 h and acquire the gluten. The component of gluten are as follows: 62.14% moisture, 11.09% starch, 2.13% ash, 23.47% protein, and 0.8% fat. Pepsin (3.4.23.1), trypsin (3.4.21.4), standards and other chemicals agents (analytical or better grade) used in the present study were purchased from Sigma-Aldrich Co., Ltd., US.
Treatment
In brief, 50 g of gluten after cut off was placed in the same position of the microwave instrument. Ten samples were heated at 200, 400, 600, 800, and 1000 W for 1 and 5 min, respectively. Samples with no treatment and stewing were regarded as the control groups. The treated samples were placed in a freezer at − 80 °C after cooling to room temperature. Afterward, the samples were freeze-dried for further analysis.
Physical characterization
Scanning electron microscopy (SEM)
SEM was conducted using the method of Liu et al. (2016a, b) with slight modifications. A small portion of the sample was cut off and stretched to obtain a membrane not thicker than 1 mm, and the membrane was fixed in 2.5% glutaraldehyde at 4 °C for 2 h and then washed for four times with 0.1 mol L−1 phosphate buffer (pH 7.2). The samples were dehydrated using an ethyl alcohol series (50%, 70%, 80%, 90%, each for one time and then 100% three times), where each step lasted for 10 min. The substitution reaction was performed in tert-butanol for 15 min. The sample was observed in a JSM 6701F SEM (JEOL, Japan) at an acceleration voltage of 20 kV after freeze-dried.
Fourier transform infrared (FTIR) for secondary structure characterization
The secondary structures were characterized using FTIR spectra. The dried protein samples were mixed with KBr, ground, and pressed into a pellet. FTIR spectra were obtained in the wave number range of 400–4000 cm−1 during 32 scans with 4 cm−1 resolution by using a Nicolet 5700 FTIR spectrometer (Thermo Nicolet Co., USA). Data were analyzed using Omnic 8.0 software (Thermo Fisher Scientific Inc., Madison, WI) and Peakfit 4.12 (Systat Software, San Jose, CA).
Chemical characterization
Treatment
According to the Chinese Balanced Diet Pagoda, the ratio of gluten to oil to salt to sugar to vinegar is 250:10:3:3:5 (w/w). In brief, 50 g of gluten was added with oil; oil and sugar; oil and salt; oil and vinegar; oil vinegar and salt; oil, vinegar, and sugar; and oil, sugar, salt, and vinegar. These samples were set as the experimental group, which were heated at 200 and 1000 W for 1 and 5 min, respectively. The samples in the stewing treatment were set as the control group. All samples were placed in freezer at − 80 °C after cooling to room temperature, and were freeze-dried for further analysis.
5-HMF
The content of 5-HMF was measured using the method of Porretta and Sandei (1991) with some modifications. In brief, 2 g of grinded sample was placed in a conical flask and added with 25 mL 10% methanol. The sample was stabilized for 10 h and labeled as M after weighing. Then the mixture was ultrasonically treated for 1 h. After that, 10% methanol was added to M, and centrifuged at 5000 rpm for 15 min. The supernatant was filtered using a 0.45 μm film for HPLC. The standard sample of 5-HMF (Solarbio Science & Technology Co., Ltd., Beijing, China) is a 1 ppm solution of 5-HMF dissolved in methanol. HPLC conditions were set as follows: Agilent C18 column with a dimension of 250 mm × 4.6 mm (5 μm), mobile phase consisting of methanol and water at a ratio of 1:9, flow rate at 0.8 mL min−1, column temperature at 30 °C, wavelength at 284 nm, and injection volume of 10.0 μL.
Fructosamine
Fructosamine was measured using Glycosylated serum protein assay kit (Jiancheng Bioengineering Institute, Nanjing, China). OD was measured at 530 nm.
Melanoidin
The sample was measured under the absorbance at 470 nm, whereas the melanoidin content was computed using Lamber–Beer law:
where C is the melanoidin content (mmol L−1), A is the absorbance at 470 nm, V is the volume (mL), e is the molar extinction coefficient of melanoidin (282 L mol−1 cm−1), and b is the thickness of cuvette (0.75 cm).
In vitro digestion
Gluten was digested according to the method of Dupont, et al. (2010) with some midifications. In brief, 10 g of the treated sample was mixed with 250 mL distilled water (40 mg mL−1 [w/v]). The sample was added with 1 mol L−1 HCl to adjust the pH to 2.0 and subsequently preheated for 15 min at 37 °C. Then, 0.5 g of pepsin was added to the mixture (enzyme:substrate = 5:100 w/w), which was then placed in a water bath shaker at 37 °C for 2 h. After pepsin digestion, the mixture was added with 1 mol L−1 NaOH to adjust the pH to 7.5, and 0.5 g trypsin was added (enzyme:substrate = 5:100 w/w). Afterward, the mixture was placed in a water bath shaker at 37 °C for 2 h. 50 mL samples were taken at 1, 2, 3, 4 h during the digestion, respectively, the samples were placed in a boiling water bath for 10 min for enzyme deactivation for further analysis.
Amino acid analysis
The amino acid composition of the samples was studied with the method of Wang et al. (2008) by using an amino acid analyzer (Hitachi L-8900, Japan). 2.5 mL digested sample was hydrolyzed with 10 mL of 6 mol L−1 HCl for 24 h at 110 °C in a 20 mL sealed tube filled with N2. Distilled water was added to 10 mL after hydrolysis. The analysis was conducted in L-8900 amino acid analyzer (Hitachi, Ltd., Japan) with column (4.6 mm × 60 mm) and was carried out at 38 °C, and the detection wavelength 254 nm and flow rate 1.0 mL per minute. Amino acid tryptophan was not determined.
Identification of free amino groups and digestibility
Free amino groups was determined according to the method of Denis et al. (2008) with some modifications. 5 mL of digested sample was added into a beaker with the same amount of distilled water. The mixture was titrated to pH 7 with 0.05 mol L−1 NaOH. Furthermore, 10 mL of 37% (v/v) formaldehyde was added to the mixture, and 0.05 mol L−1 NaOH was titrated to pH 9.2. The consumption after adding formaldehyde was recorded as V1. Distilled water without digestion was set as the control group, and the consumption was recorded as V2.
where CV is the content of free amino groups (mmol L−1), V1 is the consumption of NaOH from pH 7 to 9.2 (mL), and V2 is the consumption of the control group (mL).
Gluten digestibility was determined according the method of Adler–Nissen (1979) by using the free amino acid content.
where D is the digestibility of gluten, CW is the free amino group and broken peptide bond contents (mmol g−1), and Ctot is total peptide bonds of gluten (8.3 mmol g−1).
Molecular weight distributions
Molecular weight was determined using the method of You et al. (2010) with some modifications. The sample was centrifuged at 5000 rpm for 15 min and filtered through a 0.45 µm filter. HPLC conditions were set as follows: Agilent PL aquagel-OH 40 column, mobile phase with ultrapure water, flow rate at 0.5 mL min−1, wavelength at 200 nm during 0–15 min, and injection volume of 10.0 μL.
A molecular weight calibration curve was prepared from the average elution volume of the following standards: bacitracin (1422.71 Da), oxidized glutathione (612.63 Da), reduced glutathione (307.32 Da), and hippuric acid (179.17 Da).
Statistical analysis
All experiments were carried out in triplicate and result were expressed as mean value with ± standard deviation (SD). Statistical analysis was performed using the SPSS® software (SPSS Statistical Software 23, Inc., Chicago, IL, USA). The independent sample t test was used to evaluate the significant differences between two samples (p < 0.05).
Results and discussion
Gluten microstructure
As can be seen in Fig. 1, gluten in control group had a scattered surface structure, whereas the structure of the stewing sample was slightly denser. When treated with the microwave power at 200 W, the gluten structure was firmer than the control. However, a smoother surface structure of gluten was observed in microwave treatment at 200 W for 5 min than at 200 W for 1 min, which might be due to that heat treatment on protein with low temperature may only extend the protein without affecting the bonds (Delcour et al. 2012). The gluten surface structure was significantly rougher, more irregular, and more aggregated than the control when the input power increased from 200 to 1000 W. Microwave may promote the cross-linking of gluten protein molecules, especially the disulfide bonds, thus fracture intermolecular covalent or non-covalent bonds (Lamacchia et al. 2016). Therefore, the roughness of the surface structure may be due to the fractured covalent or non-covalent bonds, while its aggregation may be due to the formation of disulfide or isopeptide bonds. With the increase of both microwave power and heating time, the gluten surface structure became rougher, and the aggregation degree was significantly deeper. This result may be caused by the thermal effects of microwaves on the aggregation. Collisions occur between the polar molecules and thus lead to aggregation. The aggregation of particles was due to the intensified collision under high microwave power.
Gluten secondary structure
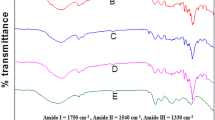
Near 3420 cm−1 is the N–H stretching vibrational absorption band of the protein. The characteristic absorption bands of the protein in the spectrum mainly include amide bands I (1600–1700 cm−1), II (1530–1550 cm−1), and III (1260–1300 cm−1). The infrared spectrum of gluten treated with different microwave powers and treatment times is slightly offset but not significant (Fig. 2a). Amide band I is from the C = 0 stretching vibration, which responds to the secondary structure (Ellepola et al. 2005). First, the coordinates were converted by Omnic 8.0, and then two points baseline correction, smoothing, deconvolution, second derivative spectrum and Gaussian method were performed in the range of 1600–1700 cm−1 with Peakfit 4.12 to minimize the residual R2, and finally peak assignment is performed to calculate the relative percentage of each peak (Zhao et al. 2012). Figure 2b shows the α-helix, β-sheet, β-turn, and the random coil content of each sample. The secondary structure of gluten did not change significantly when the sample was heated at different times at 200 W. When the microwave power was 1000 W, the total α-helix, and β-turn increased with the time increasing, whereas the β-sheet and random coil decreased significantly. The β-sheet and α-helix of the protein molecules are usually found in the interior of polypeptide chains, and β-turn is formed due to the reversal of the polypeptide chain (Lahiri et al. 2018). The result indicates that the microwave heating can change the secondary structure of gluten. The promotion of α-helix and β-turn occurred because microwave heating contributes to the formation of disulfide or isopeptide bonds (Verbauwhede et al. 2018).
Amino acid analysis
As can be seen in Table 1, after stewing and microwave treatments, there is no change in gluten amino acid species. The amino acid sequence of a protein polypeptide chain is the primary structure of the protein, and heat treatment will only change the spatial structure and conformation of the protein without changing the amino acid (Verbauwhede et al. 2018). However, the content of different amino acids after the microwave treatment was significantly changed. The reduction of the cysteine may be due to the formation of intramolecular disulfide bonds or to the conversion of cysteine to dehydroalanine residue (Lamacchia et al. 2016). Moreover, the reduction of arginine and lysine may be due to the cross-linking between the side chains of arginine and lysine or to the participation of the ε-amino groups of lysine and arginine in Maillard reaction (Oh et al. 2018). With the increasing of input power, more cross-linkings were created in gluten that reduced the digestibility of pepsin and trypsin. Interestingly, under the microwave of 1000 W 5 min, the proportion of total essential amino acids almost kept the same as the former treatments although more cross-linkings were generated. In another word, there was no selectivity for the reduction on gluten essential amino acids by microwave.
Free amino groups and molecular weight distributions
As shown in Fig. 3a, the content of free amino acid increased with the extension of digestion time. The control group showed the highest free amino acids content while 1000 W for 5 min showed the lowest. No significant difference was found in the free amino groups during pepsin digestion. After the trypsin digestion, the content of free amino from control and stewing groups were significantly higher than those microwave treatment. On the other hand, the difference among treatments at 200 W for 1 min, 200 W for 5 min and 1000 W for 1 min was insignificant. The free amino content of gluten after the microwave treatment at 1000 W for 5 min was relatively low. This result may be related to the exposure of enzyme active sites of gluten after the different treatments as Asp is the catalytic site for pepsin and His, Asp and Ser are the catalytic active sites for trypsin (Isaksen et al. 2014). Thus the trypsin active sites were hidden to a certain extent that lead to the difference. However, the result of the microwave treatment at 1000 W for 5 min may be due to the high degree of cross-linking polymerization between amino acids, such as the decrease in Asp and Ser (Table 1).
The molecular weight distribution can reflect gluten digestion degree. At the same time, it is related to the activity and functional properties of biopeptides (Liu et al. 2016a, b). As shown in Fig. 3b, the molecular weight distributions of the control and stewing groups were nearly the same. Under the microwave treatment at 200 W, the proportion of digestion products above 1000 Da increased slightly compared with the control. When the heating power was up to 1000 W, the molecular weight distribution changed significantly with the increase in time. After the microwave treatment, the changes in the gluten structure and conformation as well as the formation of isopeptide and cross-linking reduced the active sites of enzyme that hindered enzymatic hydrolysis of the polypeptides. At the same time, the active sites of the enzyme were reduced due to protein cross-linking. Moreover, the peptide content of each molecular weight after microwave had been reduced compared with the control.
Digestibility determination
Protein can be decomposed into small peptides through pepsin acting on phenylalanine, tyrosine, glutamic and aspartic acids, and peptides are furtherly decomposed into amino acids to promote absorption through trypsin acting on lysine and arginine. The digestibility of gluten increased with the extension of digestion time. As shown in Fig. 4, compared with the digestibility of the control group after 4 h digestion was nearly 52%, while the digestibility of group treated at 1000 W for 5 min was only 34%. These results showed that the nutritional value of gluten is not as good as wheat (77%), soybean (78%) and bread (97%) in terms of digestibility (Gilbert et al. 2011). Factors affecting protein digestibility include disulfide bonds, other covalent cross-linkings, isopeptides, changes in the secondary structure, and other components in the food (Cabanillas and Novak 2019). As the microwave power and time increasing, cross-linking of gluten proteins and the formation of isopeptide bonds were promoted by microwave, resulting in the concealment of the pepsin and trypsin active sites (Guan et al. 2011). At the same time, the heat treatment may change the amino acid side chain to form lateral coupling that reduce the contact with digestive enzymes, resulting in the reduction of digestibility (Hurrell 1977). Therefore, low-power heating or high-power microwave with short-time heating are recommended when using microwave.
5-HMF
The retention time of 5-HMF appeared in 14–15 min, and some disturbed peaks were observed near the 5-HMF peak time. Thus, DAD was used to confirm whether it was the target substance. As shown in Fig. 5a, no 5-HMF was observed either after the microwave or stewing treatment except the microwave treatment at 1000 W for 5 min. Moreover, in the microwave group at 1000 W for 1 min (Fig. 5b) and stewing group (Fig. 5c), no 5-HMF was found after the addition of oil, salt with oil or sugar with oil. However, a significant increase of 5-HMF was found after adding vinegar, which is probably because the vinegar itself contains 5-HMF (Falcone and Giudici 2008). In addition, 5-HMF is mainly produced by the decomposition of hexose by heating. Hexose strips down three molecules of water in the acidic conditions to form 5-HMF (Ranoux et al. 2013). Vinegar changed the reaction pH so that the 5-HMF content increased after adding vinegar. Under CHC in Fig. 5d, the 5-HMF content produced by the microwave treatment was higher than stewing. Moreover, the 5-HMF contents were almost the same in the treatment on the basis of oil with vinegar and with sugar or salt no matter under stewing or microwave. This result can be explained by the fact that vinegar is the main source of 5-HMF. When gluten was heated by microwave under the conditions with the addition of all condiments, the 5-HMF content produced under microwave was almost twice than the other groups. The formation of 5-HMF is mainly caused by the dehydration of glucose and fructose to 3-deoxyhexitolone and then the dehydration of 3-deoxyhexone to 5-HMF, during which the presence of metal ions could change the formation of 5-HMF. Metal ions can combine with carbohydrates to form highly active fructofuranosyl cation, and this cation is more effective in decomposing than 3-deoxyhexitolone to form 5-HMF (Fiore et al. 2012).
HPLC of 5-HMF in gluten under different heating treatments (a) and in CHC glutens under microwave (b) and stewing treatment (c). 5-HMF content in CHC gluten under stewing and microwave treatment at 1000 W for 5 min (d). Fructosamine and melanoidin content in gluten under different treatments (e). Fructosamine (f) and melanoidin (g) content in CHC gluten under stewing and microwave treatment at 1000 W for 5 min
Fructosamine
The fructosamine content almost kept the same under the conditions of control, stewing and 200 W treatment (Fig. 5e). However, the content increased significantly when the power input increased to 1000 W. After adding the condiments (Fig. 5f), the content of fructosamine increased under the addition of oil, which may because oil accelerated the heat transfer of the system to provide a stable temperature for Amadori rearrangement (Song et al. 2019). On the basis of addition of oil, we found that the addition of sugar promoted the formation of fructosamine, while vinegar had certain inhibitory effect and salt seemed had no significant effect. Fructosamine is a glycated protein formed by non-enzymatic saccharification of protein, which is formed by Amadori rearrangement of substituted aldehyde amine cyclized from the Schiff base, which is the initial production of Maillard reaction. Amino acids and carbohydrates are the main substances in the Maillard reaction. Thus, the structures and species of amino acids and carbohydrates will affect the reaction rate of Maillard. Alkaline amino acid (Lys, Arg, and His) and other ε-amino or terminated amino acids, as well as saccharides with terminal steric hindrance (such as aldose), were beneficial to the rate of Maillard (Lund and Ray 2017).
Melanoidin
As can be seen in Fig. 5e, the control group had a certain amount of melanoidin. The content of melanoidin gradually increased with the increase of microwave power and time. The melanoidin content increased significantly when the microwave power changed from 200 to 1000 W. As the product in the final stage of Maillard reaction, melanoidin content depends on the extent of Maillard reaction, due to the high rate of Maillard reaction under high power input (Kim and Lee 2008). When simulating CHC, the melanoidin content under the microwave treatment was significantly higher than stewing. Moreover, Maillard reaction is related to temperature and water activity, the rate of Maillard reacition is proportional to the temperature, while inversely proportional to moisture content (Scalone et al. 2015). After the microwave treatment, the moisture content decreased while the temperature increased rapidly, which promoting the Maillard reaction to increase the melanoidin content. In Fig. 5g, the melanoidin content of the sample under stewing or microwave treatment increased slightly after adding oil. The melanoidin content was also increased after the addition of vinegar because vinegar itself contains a certain amount of melanoidin (Falcone and Giudici 2008). However, vinegar changed the pH of reaction system, which may hinder the final stage of Maillard. The amount of melanoidin after adding sugar was significantly higher than salt, because Maillard reaction is related to carbohydrate and protein. The highest content of melanoidin was observed after adding all condiments, which may be due to the fact that the combination of sugar and salt contributed more than the inhibition of vinegar.
Conclusion
We did this research to study the effect of microwave heating on protein bioavailability and safety of gluten under CHC. With the increase of input power and heating time of microwave, the internal structure of gluten changed significantly, including the variation of secondary structure, increase of cross-linking and formation of isopeptide bond. And the cross-linking between some specific amino acids could not be digested, so lead to the reduction of the total amount of available amino acids and digestibility, and the increase of average molecule in the digestion product. When simulating CHC, it was found that the contents of 5-HMF, fructosamine and melanoidin were all increased with the extension of Maillard reaction, and the enhancement of microwave heating on fructosamine and melanoidin was more obvious than stewing. On the other hand, the contents of the three products were mainly affected by vinegar (pH), sugar and salt, respectively.
References
Adler-Nissen J (1979) Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem 27(6):1256–1262
Cabanillas B, Novak N (2019) Effects of daily food processing on allergenicity. Crit Rev Food Sci Nutr 59(1):31–42
Delcour JA, Joye IJ, Pareyt B, Wilderjans E, Brijs K, Lagrain B (2012) Wheat gluten functionality as a quality determinant in cereal-based food products. Annu Rev Food Sci Technol 3:469–492
Denis A, Brambati N, Dessauvages B, Guedj S, Ridoux C, Meffre N, Autier C (2008) Molecular weight determination of hydrolyzed collagens. Food Hydrocoll 22(6):989–994
Dupont D, Mandalari G, Molle D, Jardin J, Léonil J, Faulks RM, Wickham MS, Clare Mills E, Mackie AR (2010) Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol Nutr Food Res 54(6):767–780
Ellepola SW, Choi SM, Ma CY (2005) Conformational study of globulin from rice (Oryza sativa) seeds by Fourier-transform infrared spectroscopy. Int J Biol Macromol 37(1–2):12–20
Falcone PM, Giudici P (2008) Molecular size and molecular size distribution affecting traditional balsamic vinegar aging. J Agric Food Chem 56(16):7057–7066
Fiore A, Troise AD, Ataç Mogol B, Roullier V, Gourdon A, El Mafadi Jian S, Hamzalıoğlu BAI, Gökmen V, Fogliano V (2012) Controlling the Maillard reaction by reactant encapsulation: sodium chloride in cookies. J Agric Food Chem 60(43):10808–10814
Gilbert JA, Bendsen NT, Tremblay A, Astrup A (2011) Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 21:B16–B31
Guan J-J, Zhang T-B, Hui M, Yin H-C, Qiu A-Y, Liu X-Y (2011) Mechanism of microwave-accelerated soy protein isolate–saccharide graft reactions. Food Res Int 44(9):2647–2654
Hurrell RF, Carpenter KJ (1977) Nutritional significance of cross-link formation during food processing. In: Friedman M (ed) Protein crosslinking. Springer, Boston, MA, pp 225–238. https://doi.org/10.1007/978-1-4757-9113-6
Isaksen GV, Åqvist J, Brandsdal BO (2014) Protein surface softness is the origin of enzyme cold-adaptation of trypsin. PLoS Comput Biol 10(8):e1003813
Kim J-S, Lee Y-S (2008) Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem 108(2):582–592
Lahiri P, Verma H, Ravikumar A, Chatterjee J (2018) Protein stabilization by tuning the steric restraint at the reverse turn. Chem Sci 9(20):4600–4609
Lamacchia C, Landriscina L, D'Agnello P (2016) Changes in wheat kernel proteins induced by microwave treatment. Food Chem 197(Pt A):634–640
Li H, Wu C-J, Yu S-J (2018) Impact of microwave-assisted heating on the pH value, color, and flavor compounds in glucose-ammonium model system. Food Bioprocess Technol 11(6):1248–1258
Liu M, Wang Y, Liu Y, Ruan R (2016a) Bioactive peptides derived from traditional Chinese medicine and traditional Chinese food: a review. Food Res Int 89(Pt 1):63–73
Liu TH, Gao X, Li LQ, Du DF, Cheng X, Zhao Y, Liu Y, Li XJ (2016b) Effects of HMW-GS at Glu-B1 locus on the polymerization of glutenin during grain development and on the secondary and micro-structures of gluten in wheat (Triticum aestivum L.). J Cereal Sci 72:101–107
Liu K, Zheng J, Chen F (2019) Effect of domestic cooking on rice protein digestibility. Food Sci Nutr 7(2):608–616
Lund MN, Ray CA (2017) Control of Maillard reactions in foods: strategies and chemical mechanisms. J Agric Food Chem 65(23):4537–4552
Morzel M, Gatellier P, Sayd T, Renerre M, Laville E (2006) Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci 73(3):536–543
Oh M-J, Kim Y, Lee SH, Lee K-W, Park H-Y (2018) Prediction of CML contents in the Maillard reaction products for casein-monosaccharides model. Food Chem 267:271–276
Porretta S, Sandei L (1991) Determination of 5-(hydroxymethyl)-2-furfural (HMF) in tomato products: proposal of a rapid HPLC method and its comparison with the colorimetric method. Food Chem 39(1):51–57
Ranoux A, Djanashvili K, Arends IWCE, Hanefeld U (2013) 5-Hydroxymethylfurfural synthesis from hexoses is autocatalytic. ACS Catal 3(4):760–763
Rombouts I, Lagrain B, Brunnbauer M, Koehler P, Brijs K, Delcour JA (2011) Identification of isopeptide bonds in heat-treated wheat gluten peptides. J Agric Food Chem 59(4):1236–1243
Scalone GLL, Cucu T, De Kimpe N, De Meulenaer B (2015) Influence of free amino acids, oligopeptides, and polypeptides on the formation of pyrazines in Maillard model systems. J Agric Food Chem 63(22):5364–5372
Singh H, MacRitchie F (2004) Changes in proteins induced by heating gluten dispersions at high temperature. J Cereal Sci 39(2):297–301
Song R, Jia Z, Shi Q, Wei R, Dong S (2019) Identification of bioactive peptides from half-fin anchovy (Setipinna taty) hydrolysates and further modification using Maillard reaction to improve antibacterial activities. J Funct Foods 58:161–170
Stathopoulos CE, Tsiami AA, Schofield JD, Dobraszczyk BJ (2008) Effect of heat on rheology, surface hydrophobicity and molecular weight distribution of glutens extracted from flours with different bread-making quality. J Cereal Sci 47(2):134–143
Vadivambal R, Jayas D (2007) Changes in quality of microwave-treated agricultural products—a review. Biosyst Eng 98(1):1–16
Verbauwhede AE, Lambrecht MA, Fierens E, Hermans S, Shegay O, Brijs K, Delcour JA (2018) Thermo-reversible inhibition makes aqualysin 1 from Thermus aquaticus a potent tool for studying the contribution of the wheat gluten network to the crumb texture of fresh bread. Food Chem 264:118–125
Wang X-S, Tang C-H, Yang X-Q, Gao W-R (2008) Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem 107(1):11–18
You L, Zhao M, Regenstein JM, Ren J (2010) Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem 120(3):810–816
Zhang Y-Y, Song Y, Li Q-H (2012) A review on formation mechanism, determination and safety assessment of furfural and 5-hydroxymethylfurfural (HMF) in foods. Food Sci 5:060
Zhao Q, Xiong H, Selomulya C, Chen XD, Huang S, Ruan X, Zhou Q, Sun W (2012) Effects of spray drying and freeze drying on the properties of protein isolate from rice dreg protein. Food Bioprocess Technol 6(7):1759–1769
Zielinska M, Ropelewska E, Xiao HW, Mujumdar AS, Law CL (2019) Review of recent applications and research progress in hybrid and combined microwave-assisted drying of food products: quality properties. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2019.1632788
Acknowledgements
The research was supported by project of the State Key Laboratory of Food Science and Technology, Nanchang University (Project No. SKLF-ZZB-201312), the project of National Natural Science Foundation of China (21466022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiang, S., Zou, H., Liu, Y. et al. Effects of microwave heating on the protein structure, digestion properties and Maillard products of gluten. J Food Sci Technol 57, 2139–2149 (2020). https://doi.org/10.1007/s13197-020-04249-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04249-0